1. Context
According to the World Health Organization, three out of 10 pregnancies end in induced abortion, and nearly 40 - 50 million abortions occur annually. Therefore, access to legal, safe, and comprehensive abortion care is essential for achieving the highest possible level of reproductive health (1). Induced abortions occur in all societies regardless of medical, socioeconomic, and religious status (2). In countries with restricted rules for induced abortion, women may be forced to have an illegal abortion that is harmful, particularly for young, poor, and low educated ones (3), possibly leading to maternal mortality (4). Indeed, there is a possibility that the placental and/or fetal tissue remains in the uterus after abortion, which is called incomplete abortion, and the remained tissue stands for the retained products of conception (RPOC) (5) that may cause prolonged vaginal bleeding, endometritis, and occasionally intrauterine adhesions and impairment of future fertility.
Nowadays, early pregnancy termination can be managed medically with misoprostol and/or mifepristone because they are low-cost alternatives to surgery and have higher than 80% of success and great satisfaction (6). However, they have some side effects: bloating, nausea, diarrhea, abdominal cramps, and headaches (7). Moreover, in some instances, such as high doses of the drug, a history of cesarean section, or uterine surgery, it can lead to uterine rupture (8). Therefore, special attention has been paid to traditional medicine as an alternative for chemical agents.
Medicinal plants have been commonly used in Iran and other traditional countries from the distant past (9). Recognizing the plants for inducing abortion and expulsion of retained products of conception can reduce the medical and surgical side effects of abortion. On the other hand, it may enhance the awareness and understanding of the toxic effects of these drugs, which prevent their improper or unknowing use during pregnancy (10). At the same time, most pregnant women assume that herbal medicine does not interfere with fetal and maternal action, but it may cause specific fetal and maternal side effects or drug interactions (11).
Traditional Persian medicine (TPM) has a rich clinical experimental background, which has been passed down orally and in written forms. The present study aimed to overview the medicinal plants used to induce abortion or manage incomplete abortion based on the review of traditional Persian textbooks and evidence of such plants in the electronic databases to find their effects, potential mechanism, and side effects. This may help us propose clinical trials to investigate the safety and efficacy of these drugs in abortion.
2. Evidence Acquisition
2.1. Manual Search
In this review, the most authoritative and well-known textbooks of Persian traditional medicine from the ninth to 18th century were investigated, such as Al-Qanun Fi at-Tibb (The Canon of Medicine) written by Ibn-e-Sina in 1025 Common Era (CE), Tuhfat-al-Momenin written by Mohammad Tonekaboni in 1670 CE, and Makhzan-ul-advia (Storehouse of Medicaments) written by Aghili Khorasani in 1771 CE (12-14). An essential search keyword was medicinal plants with abortifacient activity, which was equal to the Arabic term "mosqete janin." Other keywords, such as inducing menstruation, were also considered, equivalent to "modere heiz." After identifying the plants, their generic names were extracted from popular medicinal plants of Iran and rechecked in Google to confirm the accuracy.
2.2. Electronic Search
In the next step, electronic databases such as PubMed, Scopus, Web of Science, and Google Scholar were searched between January 1970 and April 2021. The main search keywords were "induced abortion" or "emmenagogue" and "herbal plants" in the title and abstract. In this search strategy, the articles in non-English languages were excluded.
Nonetheless, the overlapped plants between hand search and electronic search were briefly reviewed, and the scientific names of the plants, plant families, plants extracts, and active components and their efficacy and safety in animal and human studies were described.
3. Results
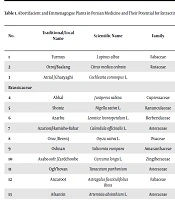
Totally 88 plants with abortifacient and emmenagogue activity were identified by the review of the traditional Persian medicine textbooks, 47 of which were helpful in the management of incomplete abortion or retained products of conception. They are categorized as abortifacients, emmenagogues, fetus extractors, and placenta extractors in Table 1.
| No. | Traditional/Local Name | Scientific Name | Family | Activity | |||
|---|---|---|---|---|---|---|---|
| Placenta Extractor | Emmenagogue | Fetus Extractor | Abortifacient | ||||
| 1 | Turmus | Lupinus albus | Fabaceae | ● | ● | ● | |
| 2 | Otroj/Baalang | Citrus medica cedrata | Rutaceae | ● | ● | ● | |
| 3 | Atrial /Ghazyaghi | Cochlearia coronopus L. | Brassicaceae | ● | |||
| 4 | Abhal | Juniperus sabina | Cupressaceae | ● | ● | ||
| 5 | Shoniz | Nigella sativa L. | Ranunculaceae | ● | ● | ● | ● |
| 6 | Azarbu | Leontice leontopetalum L. | Berberidaceae | ● | ● | ||
| 7 | Azarion/Hamishe-Bahar | Calendula officinalis L. | Asteraceae | ● | ● | ||
| 8 | Oroz/Berenj | Oryza sativa L. | Poaceae | ● | |||
| 9 | Oshnan | Salicornia europaea | Amaranthaceae | ● | ● | ||
| 10 | Asabe-sofr/Zardchoobe | Curcuma longa L. | Zingiberaceae | ● | |||
| 11 | Ogh’hovan | Tanacetum parthenium | Asteraceae | ● | ● | ||
| 12 | Anzaroot | Astragalus fasciculifolius Bioss | Fabaceae | ● | |||
| 13 | Afsantin | Artemisia absinthium L. | Asteraceae | ● | ● | ||
| 14 | Imar-anootali | Verbena supina L. | Verbenaceae | ● | |||
| 15 | Barzad | Ferula gummosa Boiss | Apiaceae | ● | ● | ● | ● |
| 16 | Artanisa | Cyclamen purpurascens Miller | Primulaceae | ● | ● | ||
| 17 | Bakhur-al-akrad | Peucedanum officinale L. | Apiaceae | ● | ● | ||
| 18 | Jawshir | Opopanax chironium L. | Apiaceae | ● | ● | ||
| 19 | Jaavars/Arzan | Panicum miliaceum L. | Poaceae | ● | |||
| 20 | Jasjas | Pulicaria crispa Cass. | Asteraceae | ● | ● | ● | |
| 21 | Joze-al-sherk | Aframomum granum-paradisi K. Schum | Zingiberaceae | ● | ● | ||
| 22 | Hanzal | Citrullus colocynthis | Cucurbitaceae | ● | Fetus killer | ● | |
| 23 | Hemmas /Nokhod | Cicer arietinum L. | Fabaceae | ● | ● | ||
| 24 | Khass /Kahoo | Lactuca sativa | Asteraceae | ● | ● | ||
| 25 | Henna | Lawsonia inermis L. | Lythraceae | ● | |||
| 26 | Darcini | Cinnamomum aromaticum Nees. | Lauraceae | ● | ● | ||
| 27 | Darfelfel | Longum piper | Piperaceae | ● | ● | ||
| 28 | Zabib-al-jabal /Mavizak | Delphinium staphisagria L. | Ranunculaceae | ● | |||
| 29 | Doghos | Athamanta cretensis L. | Apiaceae | ● | ● | ||
| 30 | Sarakhs | Dryopteris filix-mas L. | Dryopteridaceae | ● | ● | ||
| 31 | Samsam /Konjed | Sesamum indicum L. | Pedaliaceae | ● | ● | ||
| 32 | Sodab | Ruta graveolens L. | Rutaceae | ● | ● | ||
| 33 | Sharife /Kaj | Annona squamosa L. | Annonaceae | ● | |||
| 34 | Bumadaran | Achillea millefolium | Asteraceae | ● | ● | ● | ● |
| 35 | Shitaraj | Plumbago rosea L. | Plumbaginaceae | ● | |||
| 36 | Zayyan | Jasminum arborescens Roxb. | Oleaceae | ● | ● | ||
| 37 | Ghaar | Laurus nobilis L. | Lauraceae | ● | ● | ||
| 38 | Gentiana | Gentiana lutea L. | Gentianaceae | ● | ● | ● | |
| 39 | Karafs | Apium graveolens L. | Apiaceae | ● | ● | ||
| 40 | Photrasaliyoun | Carum petroselinum | Apiaceae | ● | ● | ● | ● |
| 41 | Komaphytus | Ajuga chamaepitys | Lamiaceae | ● | ● | ● | |
| 42 | Loof | Arum italicum | Araceae | ● | ● | ● | |
| 43 | Foodanaj /Pooneh | Mentha longifolia L. | Lamiaceae | ● | ● | Fetus killer | ● |
| 44 | Narjes | Narcissus tazetta L. | Amaryllidaceae | ● | ● | ● | |
| 45 | Hoofarighoon | Hypericum perforatum | Hypericaceae | ● | ● | ● | |
| 46 | Baboonaj/Babooneh | Matricaria chamomilla | Asteraceae | ● | |||
| 47 | Saatar/Avishan-Shirazi | Zataria multiflora Boiss | Lamiaceae | ● | |||
| 48 | Aslagh | Vitex agnus-castus | Lamiaceae | ● | |||
| 49 | Ratbe /Yonje | Medicago sativa | Fabaceae | ● | |||
| 50 | Marmazad/Chay-kouhi | Stachys lavandulifolia | Lamiaceae | ● | |||
| 51 | Halyoun/Marchoobe | Asparagus officinalis | Asparagaceae | ● | |||
| 52 | Hendeba/Casnii | Cichorium intybus | Asteraceae | ● | |||
| 53 | Marzanjoosh | Origanum vulgare | Lamiaceae | ● | |||
| 54 | Bad-avard | Carduus benedictus | Asteraceae | ● | |||
| 55 | Darshishaan | Calicotome spinosa | Fabaceae | ● | |||
| 56 | Kornob | Brassica oleracea L. | Brassicaceae | ● | |||
| 57 | Morr | Commiphora myrrha | Burseraceae | ● | |||
| 58 | Moshketaramashie | Mentha aquatica L. | Lamiaceae | ● | ● | ||
| 59 | Abu khalsa | Arnebia euchroma | Boraginaceae | ● | ● | ||
| 60 | Armanin | Salvia viridis L. | Lamiaceae | ● | |||
| 61 | Oshagh | Dorema ammoniacum | Apiaceae | ● | |||
| 62 | Tin /Anjir | Ficus carica L. | Moraceae | ● | ● | ||
| 63 | Jazar /Havij | Daucus carota L. | Apiaceae | ● | |||
| 64 | Hasha | Coridothymus capitatus | Lamiaceae | ● | ● | ● | |
| 65 | Kheiri | Cheiranthus cheiri L./Erysimum cheiri L. | Brassicaceae | ● | ● | ● | |
| 66 | Saghmoonia | Convolvulus scammonia | Convolvulaceae | Fetus killer | |||
| 67 | Salikheh | Cinnamomum iners | Lauraceae | ● | ● | ||
| 68 | Tarraghiyoun | Pimpinella trgium L. | Apiaceae | ● | ● | ||
| 69 | Farasiyoun | Marrubium vulgare L. | Lamiaceae | ● | ● | ||
| 70 | Ghost | Costus speciosus | Costaceae | ● | ● | ● | ● |
| 71 | Felfel | Piper nigrum L. | Piperaceae | ● | |||
| 72 | Ghantoriyoun-kabir | Centaurea centaurium L. | Asteraceae | ● | ● | ||
| 73 | Ghantoriyoun-saghir | Centaurium minus L. Centaurium erythraea | Gentianaceae | ● | ● | ||
| 74 | Ghaysoom | Artemisia vulgaris L. | Asteraceae | ● | ● | ||
| 75 | Kashem/Anjedan roomi/Golpar | Heracleum persicum | Apiaceae | ● | ● | ||
| 76 | Kondosh | Veratrum album L. | Melanthiaceae | ● | ● | ||
| 77 | Ladan | Cistus villosus L. | Cistaceae | ● | ● | ● | |
| 78 | Loze-al-morr/Badam-Talkh | Amygdalus amara (C.F.Ludw.) Hayne | Rosaceae | ● | ● | ● | |
| 79 | Loobia | Phaseolus vulgaris L. | Fabaceae | ● | ● | ● | |
| 80 | Fovvah | Rubia tinctorum L. | Rubiaceae | ● | ● | ● | |
| 81 | Kherva /Karchak | Ricinus communis L. | Euphorbiaceae | ● | ● | ||
| 82 | Parsiavoshan | Adiantum capillus-veneris | Pteridaceae | ● | ● | ● | |
| 83 | Anisoon | Pimpinella anisum L. | Apiaceae | ● | ● | ● | |
| 84 | Sowm/Seer/Garlic | Allium sativum | Amaryllidaceae | ● | ● | ||
| 85 | Holbeh | Trigonella foenum-graecum L. | Fabaceae | ● | ● | ||
| 86 | Folus | Cassia fistula | Fabaceae | ● | Facilitation of delivery | ||
| 87 | Zaafaran/Saffron | Crocus sativus | Iridaceae | Facilitation of delivery | |||
| 88 | Harmal/Esfand | Peganum harmala | Nitrariaceae | ● | Fetus killer | ||
After removing duplicated studies in electronic search, the search of the electronic databases found 14 plants with abortifacient or emmenagogue activity. Of them, six plants, including Sesamum indicum L. (Sesame), Commiphora myrrha (myrrh), Lawsonia inermis L. (Henna), Opopanax chironium L. (Jooshir), Plumbago rosea (Shitraj or Stumbag), and Juniperus sabina (Abhal), were also found in the manual search. The abortifacient or emmenagogue activity and other properties of all 14 plants are described in the following.
3.1. Heracleum persicum
Heracleum persicum is a flowering plant of the Apiaceae family. This plant grows in Iran, Iraq, and Turkey (15). It is known as “Golpar” in Iran (16). Various natural chemicals such as volatile (terpenes, aliphatic esters, phenyl propenes, and carbonyls) and non-volatile (furanocoumarins, alkaloids, flavonoids, and tannins) constituents and several minerals were identified during phytochemical analysis (16). Dried fruits are used as contraceptive, lactagogue, emmenagogue, and pain killer agents. A study showed that H. persicum L. is teratogenic with abortifacient activity in mice (17). Therefore, it should be taken with caution during pregnancy.
3.2. Origanum vulgare
Origanum vulgare L. (Marzanjosh) is an important medicinal herb of the Lamiaceae family. Origanum vulgare was traditionally used to treat dysmenorrhea (18). The active chemicals are volatile (essential oil) and non-volatile phenolic compounds (phenolic acids and flavonoids). Others biologically active compounds are terpenoids, tannins, and sterols (19). It has been shown that high dosages of O. vulgare extract can increase the rate of abortion and fetal malformations in the fetus of mice (20).
3.3. Zataria multiflora Boiss
Zataria multiflora Boiss (Shirazi thyme) is an aromatic perennial shrub from the Lamiaceae family, which is native to Iran, Afghanistan, and Pakistan. It is full of phytochemical components and biological activities. Zataria multiflora is called "Avishane Shirazi" in Persian and is used as a seasoned flour in many foods in Iran. The chemical components of Z. multiflora include β-sitosterol, luteolin, apigenin, linalool, 6-hydroxyluteolin, thymol, carvacrol, γ-terpinene, and p-cymene (21). Zataria multiflora is effective in the treatment of primary dysmenorrhea (22). It is enriched by gamma-terpinene that may damage DNA (23, 24). However, thyme consumption in early pregnancy may lead to abortion due to its adverse effects on the placental diameter. Therefore, its use would be with caution during pregnancy (25).
3.4. Sesamum indicum L.
Sesamum indicum L. is from the Pedaliaceae family. It has been well known for over 5,000 years as an oilseed crop. Sesamum indicum L. is widely used in traditional medicine as oily seeds with emmenagogue activity (26) and facilitated delivery (27). Also, in the cases of oligomenorrhea with menstruation retard for more than two weeks, it may be an effective choice in inducing menstruation with ignorable side effects compared to hormonal therapies (28). In a clinical trial by Aghababaei et al., Sesame was efficient for removing retained products of conception with minor vaginal bleeding and pain than the control group (29).
3.5. Commiphora myrrha
Commiphora myrrha (Nees.) Engl. is a large shrub or small tree belonging to Sapindales, a family of Burseraceae and genus Commiphora, found in abundance in northeast Africa and the Middle East (30). Preliminary research has indicated that myrrh contained about 3 - 8% essential oil, 25 - 40% resins, and 30 - 60% water-soluble gum. It contains several bioactive metabolites such as flavonoids, terpenoids, steroids, lignans, carbohydrates, and long-chain aliphatic alcohol derivatives (31). It is a uterine stimulant with emmenagogue activity (32). Vafaei et al. assessed the efficacy of myrrh in patients with incomplete abortion. Meanwhile, the rate of successful complete abortion was 82.9% compared with 54.3% in the placebo group that was significantly different (33).
3.6. Stachys lavandulifolia
Stachys lavandulifolia belongs to the Lamiaceae family and grows worldwide, although it is endemic in Iran. They are well known for their flavoring and therapeutic effects. It contains hydroxyl and phenolic compounds such as polyphenols, tannins, steroids, flavonoids, and terpenoids (34). Due to the presence of flavonoid compounds, it changes the function level of the hypothalamic-pituitary-gonadal axis. This plant can reduce the progesterone level by more than 20%; therefore, it leads to miscarriage and inability to preserve the fetus and risk of abortion depending on dosage in animals (35). Abortion can be caused by the use of S. lavandulifolia during pregnancy; therefore, it should be considered a contraindication or used with caution (35). Also, it is used to control premenstrual syndrome (PMS) and primary dysmenorrhea symptoms (36) and can be suggested as add-on therapy or even an alternative remedy to non-steroidal anti-inflammatory drugs (NSAIDs) with fewer side effects (37).
3.7. Peganum harmala
Peganum harmala L. from the Zygophyllaceae family is well-known for its seeds, root, and bark in traditional Persian medicine and is known as "Esfand," "Espand," and "Harmal" in Iran. The pharmacological and therapeutic effects of P. harmala are mainly from alkaloids (38-40).
An in-vivo study found that the hydroalcoholic extract of P. harmala had contractive effects on the uterus and stripped the myometrium via the external calcium flow by the voltage-dependent calcium channels (41). Quinazoline alkaloids vasicine and vasicinone have been attributed to the abortifacient activity (42, 43). Therefore, it is contraindicated during pregnancy (40).
3.8. Ruta graveolens L.
Ruta graveolens belonging to the Rutaceae family is an ever-green shrub distributed worldwide (44). Some of its chemical constituents are glycosides (flavonoids) and alkaloids (quinolones: coquisagenine, skimmianine, and graveoline) (45). Dried aerial parts of R. graveolens induce abortion through the mechanism of multiple organ damage and death. There is no estrogenic effect; however, it can interfere with implantation time (46). The aqueous extract interferes with the pre-implantation phase in mice (47).
In a study examining the abortion and estrogenic activity of ethanolic extract of Sodab on different days of pregnancy in female rats, abortion or stillbirth was not observed on one to six days, but fetal malformation was observed after use on 7 - 9 days of gestation (47). Besides, DNA replication and mitosis can also be prevented by Psoralens in Sodab (R. graveolens L.) (47). The alkaloids in the plant can reduce the number of fetal cells and decrease the transmission of fetuses from the oviduct to the uterine branches.
3.9. Crocus sativus
In Persian medicine, C. sativus, known as Saffron, has a hard, circular, meaty onion covered with thin, brown membranes (48). It contains several active and potent biological compounds, including crocin and croctin as the most active ones. An experimental study by Hosseini et al. (49) assessed the abortifacient and teratogenic effects of different doses of C. sativus in female mice. Their results showed that the numbers of resorbed and demised fetuses were more significant than in the control group. Saffron may affect embryonic implantation that results in abortion.
Indeed, high doses of Saffron may interfere with organogenesis that mostly happens in the second gestational trimester and leads to abnormalities such as decreased tail length and placenta weight and diameter in animal studies (49).
Tafazoli et al. evaluated the effect of Saffron on abortion and its side effects on BALB/c mice and indicated that the percentages of absorbed and abnormal embryos increased significantly in the Saffron group (50). In a prospective case-control study on pregnant female farmers during the harvesting season of Saffron, the abortion rate was significantly higher among female farmers who had Saffron exposure (51). In a clinical study, Darooneh et al. investigated the effect of C. sativus (Saffron) on cervical ripening and the progress of labor in primiparous women. Their results showed that Saffron could shorten the average length of the first and second labor stages and induce stimulation and intensification of uterine contractions, labor facilitation, and lower oxytocin use (52).
3.10. Opoponax chironium
The genus O. chironium is a member of the Apiaceae family and grows in the Mediterranean. It is yellow and consumed as food and medicine. It contains abortifacient activity, and its mixture with honey is used as a vaginal suppository, which is very effective as feticide and for expelling the product of conception (53).
3.11. Lowsonia inermis
Lowsonia inermis is known as Henna in Persian medicine (54). This plant contains quinones, phenylpropanoids, flavonoids, terpenoids, phenolic compounds, and fatty acids. Apigenin is a flavonoid that exists in aromatic vegetables like Henna. Lowsonia inermis may be teratogen and should be used cautiously during pregnancy (55). Jafarzadeh et al. investigated the teratogenicity of this plant, showing that the L. inermis plant could create abnormalities in mice, and its teratogenic effects were dose-dependent (56). Also, Esteki and Miraj investigated the abortifacient effects of hydroalcoholic extract of L. inermis on female mice. Their results showed that L. inermis could significantly increase estrogen and decrease progesterone levels; besides, induced abortion was significantly lower in the experimental groups (57).
3.12. Plumbago rosea
Plumbago rosea L., mostly known as Rakta Chitrak, is mainly growing in the wild and abundantly in India. Quinones, polyphenols, alkaloids, and flavonoids are the significant phytochemicals reported from the plant. Plumbagin, hydroxy-1, 4-napthaquinone, sitosterol glycoside, fatty alcohol, and tannins are the active constituents in this plant. Sheeja et al. worked on the antifertility activity of P. rosea stem in female albino rats using different solvent extracts like petroleum ether, chloroform, acetone, and ethanol, confirming the significant estrogenic and anti-estrogenic activity of acetone extracts (58).
The plant's roots possess anti-tumor, anti-teratogenicity, anti-fertility, and uterine activities (59). The extracts of this plant can be further explored for contraceptive use (59). The possible presence of the utero-active compound in this plant was indicated by inhibiting oxytocic agents causing uterine motility. Furthermore, unwanted pregnancy can be avoided by supporting the accredited traditional use of pronounced fetotoxic and mild abortifacient potentials observed at higher doses in pregnant mice (60).
3.13. Juniperus sabina
Juniperus sabina is called Abhal in Arabic and Savin in English. This plant is one of the plants used for abortion in the past (61). Its extract or infusion is used as a medicine to increase menstruation. In Persian medicine, its essential oil and extract are used for their abortive and laxative qualities (62). It also contains lignans such as deoxypodophyllotoxin and terpene. The major components of the terpene part include sabinyl acetate and Sabinene. Sabinyl acetate is a terpenoid compound, comprising about 50% of this plant and accounting for its abortive and presumably poisonous qualities.
Oils rich in sabinyl acetate are considered dangerous (63). The abortive qualities are generally attributed to essential oils, particularly sabinyl acetate, which effectively prevents implantation. A study conducted by Mortazavi Gazar et al. assessed the effects of alcoholic extracts of J. sabina on oogenesis in mice. They showed that J. sabina could cause corpus luteum and atrophic follicles to increase and Graafian follicle and primary follicle to decrease, which can have undesirable effects on implantation and the number and size of the ovum (61).
3.14. Matricaria chamomilla
Chamomile is from the Asteraceae family, the primary source of which is the Mediterranean, but nowadays, it is widespread in Europe, the temperate regions of Asia, and even the United States (64). Their flowers include multiple phenolic compounds, primarily the flavonoids apigenin, quercetin, patuletin, luteolin, and their glucosides terpenoids α-bisabolol and its oxides and azulenes, including chamazulene.
In an experimental study, Mirzakhani assessed the abortive effect of this plant in 80 adult female rats and showed a significant increase in the number of aborted fetuses and follicle atresia and decreases in the serum levels of estrogen, progesterone, luteinizing hormone (LH), and follicular stimulation hormone (FSH) (65). It also causes uterine contraction invoking miscarriage in pregnant women and decreasing the labor pain intensity (66). The abortifacient medicinal plants that overlap in traditional and modern medicine are listed in Table 2.
| No. | Scientific Name | Family | Study Type (Animal or Human) | Study Results | Active Ingredients | Reference |
|---|---|---|---|---|---|---|
| 1 | Heracleum persicum | Umbelliferae | Mice | Increasing estrogen and reducing progesterone by abortion induction | (18) | |
| 2 | Origanum vulgare | Lamiaceae | Mice | Increasing fetal abnormalities and inducing abortion | (19) | |
| 3 | Zataria multiflora Boiss | Lamiaceae | Mice | Lack of abortion-reducing the diameter of the pair | (20) | |
| 4 | Sesamum indicum | Human (clinical trial) | Hot water extract | |||
| 5 | Commiphora myrrha | Human (clinical trial) | ||||
| 6 | Stachys lavanduliflora | Lamiaceae | Mice | Abortive effects | (18) | |
| Experimental-mice | Abortive | Aqueous-alcoholic | (21) | |||
| 7 | Peganum harmala | Nitrariaceae | Mice | |||
| 8 | Ruta graveolens L. | Rutaceae | Library study | Effective on the reproductive system, especially in men; its effect on abortion induction has not been proven; influencing infertility | (22, 23) | |
| Experimental-mice | No effect on the fetus | Edible extract | (24) | |||
| Experimental-hamster | No effect on the fetus and fertility process | Edible extract and powder from root mil | (25) | |||
| 9 | Crocus sativus | Iridaceae | Mice | Effect on abortion and preterm childbirth | (26, 27) | |
| Experimental-mice | Strong abortive | Aqueous extract | (28) | |||
| Descriptive-analytical human | Abortive | Contact or long proximity to saffron | (29) | |||
| Experimental-mice | No effect on the fetus | Edible extract | (30) | |||
| Experimental-mice | Abortive | Extract | (31) | |||
| 10 | Opoponax ferula galbanifulas | Apiaceae | ||||
| 11 | Lawsonia inermis | Lythraceae | Mice | Increasing fetal abnormalities | (32) | |
| Experimental-mice | Abortive | Aqueous-alcoholic extract | (33) | |||
| 12 | Plumbago rosea | Plumbaginaceae | Experimental mice | Abortive | Alcoholic extract of the root | (34) |
| 13 | Juniperus sabina L. | Cupressaceae | ||||
| 14 | Matricaria chamomilla L. | Asteraceae | Experimental-mice | Impact on abortion | Bloom extract | (35) |
| Clinical trial-human | Abortive | Aqueous-alcoholic extract | (36) |
4. Discussion
Traditional medicinal plants have been considered a reliable resource for healing in local communities worldwide for thousands of years, and more than three-quarters of the world population trust traditional herbal medicine for health care. There is an increasing interest in traditional herbal remedies due to their safety, efficacy, cost-effectiveness, eco-friendliness, ready availability, cultural acceptability, and fewer side effects than synthetic drugs (67). They are also the source of drug discovery and may even be considered the origin of modern medicine (68).
According to the WHO report, women comprise a group with the most frequent use in complementary and alternative medicine therapy (69). During pregnancy, especially in developing countries, women tend to self-medication with herbs as natural and safe substances. However, the lack of knowledge of side effects and the interactions of herbal medicines with chemical drugs may cause carcinogenic or toxic compounds in the body (61).
This study tried to assemble the available evidence about the effect of medicinal plants on abortion in Persian and conventional medicine references (12, 13), with different mechanisms including estrogenic activity, increased menstrual flow, induced abortion, uterine stimulation, increased bleeding risk, uterine contraction, uterotrophic activity, and stimulant action on uterine muscles (70). Some medicinal plants with abortive effects introduced in Persian medicine can decrease progesterone levels, such as M. chamomilla (57, 65). Progesterone is a critical hormone in early pregnancy. A low serum progesterone level is associated with threatened miscarriage (71).
Minimal human studies have been done, primarily in-vitro or in-vivo. Two clinical trials assessed the effects of medicinal plants (S. indicum L. (Sesame) and C. myrrha) on incomplete abortion management. Both studies showed that these plants are effective (29, 33). A prospective case-control study found that the miscarriage rate was significantly higher among female farmers with Saffron exposure (51).
The present study showed that some studied plants are emmenagogue (eg, M. chamomilla, Z. multiflora, and S. lavandulifolia) or delivery facilitator (not abortifacient, eg, C. sativus (Saffron)) (12, 13). Many of these plants are present in the daily diet, including Cicer arietinum L. (Hemmas, Nokhod), Phaseolus vulgaris L. (lubya), rice bran (first or outer skin of rice), Oryza sativa L. (oroz, Berenj), L. albus, Cinnamomum aromaticum Nees., Apium graveolens L., S. indicum L. (Konjed), Daucus caruta L., Piper nigrum L.
Studies on the abortifacient activity of some plants, such as C. sativus, revealed controversial results at different doses (49). Our findings revealed that the abortifacient activity of L. inermis L. (Henna), J. sabina (Abel or Abhal), and S. indicum L. (Konjed) had been proven in both traditional medicine and recent studies (29, 57, 61).
Studies carried out on P. harmala have shown that the consumption of this plant interrupts fetal growth, and it is an abortifacient plant (43). In Persian medicine, P. harmala is also used as feticide (12, 13). Crocus sativus agents share common effects on cell division and DNA cycles, leading to abortion. The impact of C. sativus on abortion is more approved, while in Persian traditional medicine, it is used as a placenta extractor and a delivery facilitator (12, 13). Also, R. graveolens L. has no abortion induction effect (47), while in Persian medicine texts, it is also known as an abortifacient to menstruation and emmenagogue (12-14).
Based on traditional medicine contexts, even though pineapple does not affect pregnancy, embryos, and abortion, recent studies have proven these effects (72). Anchusa italic has an abortive effect, while there is nothing mentioned in Persian medicine contexts in this regard (73). Some of the plants studied in new medicine are not effective or have poor effects, which can be related to factors such as part of the plant used (leaf, root, etc.), preparation method (aqueous, water extract, alcoholic, etc.), or medication rout of use (oral, topical, suppository, or water).
The present study results can be used as an outline for future studies about effective plants in conventional and complementary medicine. Further studies are needed to understand better the effects of medicinal plants on incomplete abortion management. Further pharmacological and clinical studies are recommended to evaluate the efficacy of all Persian medicine plants in abortion and their possible action mechanisms. Plants such as Citrus medica cedrata, Mentha longifolia L., Achillea millefolium, Carum petroselinum, Narcissus tazzeta L., Hypericum perforatum L., Cheiranthus cheiri L., Arum italicum L., Rubia tinctorum L., Ferula gummosa Boiss, Nigella sativa L. (Shoniz), and L. albus have three or four effects and high potency and frequency in Persian medicine (12, 13). Therefore, using these herbs in future clinical studies is proposed for incomplete abortion management.
4.1. Conclusions
The abortifacient activity of L. inermis L. (Henna), O. chironium L. (Jooshir), P. rosea (Stumbag, Shitraj), and J. sabina (Abel or Abhal) have been proven in both traditional medicine and recent studies. The properties of many traditional plants with abortifacient activity are unknown in modern medicine; however, they should not be used in pregnant women. However, it may have the power to be entered into modern medicine. Identifying the pharmacology and their action mechanisms may be helpful to introduce them as a potential alternative for chemical agents in the management of induced or incomplete abortion with possibly lower side effects.