1. Background
Diseases are an inseparable part of human life, and we may contract various diseases over our lifetime. Fifty-two percent of all deaths worldwide and 46% of diseases are due to non-communicable diseases. The statistics show an increase in the prevalence of these diseases. Non-communicable diseases constitute a wide range of diseases and cause many disorders in the human body, such as hyperglycemia (1, 2).
Diabetes mellitus is the most common endocrine disease, a group of metabolic disorders associated with hyperglycemia, characterized by impaired insulin secretion and function, or both (3). Long-term complications of diabetes include atherosclerosis and its complications (4), retinopathy, nephropathy, and neuropathy, which are followed by problems such as coronary heart disease, cataracts, blindness, kidney failure, foot ulcers, and amputation (3, 5). Common methods of treating diabetes today include exercise and traditional therapies, diet, insulin, and antidiabetic drugs. Diabetes medications such as biguanides, sulfonylureas, and meglitinides have various side effects, including decreased blood glucose, liver failure, and increased blood coagulation (6). Because the treatment of people with diabetes is unsatisfactory and patients complain of delayed recovery, studies on new drugs to lower blood glucose with fewer side effects are ongoing. Leech therapy has been used by Egyptian, Indian, Greek, and Persian physicians for thousands of years (7).
Over the past two decades, leech therapy has been increasingly used for medicinal purposes. It has shown successful results in various treatments, including preventing vascular disturbance after microsurgery and skin reconstruction (7, 8), neurological disorders, degenerative joint disease, priapism, and many skin conditions. Additionally, leech therapy has been effective in reducing the complications of diabetes, particularly gangrene and the need for amputation (9). There are various protein compounds in leech saliva; however, only a few have been identified at the molecular and functional levels. Among those which have been studied, hirudin and gelin can prevent blood clotting in mammals by preventing thrombin formation (10, 11), which is similar to the function of synthetic drugs such as aspirin, clopidogrel, prasugrel, and ticagrelor (12).
Recently, extensive research has been conducted on a variety of salivary bioactive peptides and some peptide proteins with different molecular weights, including antithrombin, antiplatelet (13), factor Xa inhibitor, active coagulation factor (Lifaxin), antibacterials (theromacin and theromyzin), and other substances (14). These proteins and peptides derived from leeches make them good candidates for developing novel and potent antiplatelet agents. This ringworm can also treat many chronic and abnormal disorders, such as cardiovascular problems, cancer, metastasis, and infectious diseases (9, 14). In leech therapy (hirudotherapy), leech salivary gland cells containing many biologically active proteins and peptides are transferred to the host while the leech is fed from the host's blood. This has various effects, such as suppressing inflammation, reducing pain intensity, and inhibiting blood coagulation in the host (15).
According to previous cases, some potential side effects of leech therapy include allergies, local infections with Aeromonas bacteria, swelling of local lymph nodes, itching and bleeding in the area, and transmission of some viral, fungal, and parasitic diseases (15-17). The most important species of leeches used for treatment purposes are in Europe (Hirudo medicinalis) and America (Hirudo decora) (15, 18). Other species include Hirudinaria manillensis (13), Hirudo nipponia (19, 20), Hirudo verbena, Hirudo orientalis (Asian leech) (21), and Haementeria depressa (22, 23). For therapeutic purposes, the European leech species (Hirido medinalis) is preferred to other species due to its small incision in the prey's skin and lower blood consumption (24). Studies have shown that substances with anticoagulant properties delay homeostasis at the site of leech bites, and histamine and serotonin in leech saliva cause local allergic reactions around leech bites (16-18). In leech therapy, patients are usually treated with 2 to 6 leeches at a time. However, the concentration of compounds in saliva that are transferred locally to the site or directly into the systemic bloodstream during feeding is still unknown (15).
2. Objectives
Since no study has been done on the effect of leech salivary secretions on serum biochemical changes, the present study was performed to investigate leech therapy in diabetic and nondiabetic rats.
3. Methods
3.1. Experimental Design
This experimental study used 28 adult male albino Wistar rats aged 5 to 6 months weighing 200 ± 20 g, purchased from Pasteur Institute in Tehran. The animals were kept randomly and in pairs in polycarbonate cages under standard conditions at 20 to 22°C and 12-hour light-dark cycle with free access to standard water and food at the Pathology Research Center of Islamic Azad University, Shahrekord Branch. To adapt to the new environment, all experiments were performed at least two weeks after the establishment of rats. All laboratory methods were approved by the Islamic Azad University Ethics Committee, Shahrekord Branch, and all ethical principles related to the animals tested were observed during this study.
3.2. Induction of Diabetes in Rats
To induce experimental diabetes in rats, alloxan (manufactured by Sigma Aldrich, Germany) was injected twice (100 mg/kg body weight) in saline solution subcutaneously in the back of the neck once a day for two consecutive days. Blood sugar above 220 mg/dL indicated the rat was diabetic (25).
3.3. Detection and Maintenance of Leeches
Around 150 leeches of the Hirudo medicinalis species, ranging in size from 4 to 7 cm and weighing approximately 0.4 to 0.6 g, were purchased from the Rastak Leech Production, Storage, and Distribution Center in Isfahan, Iran. These leeches were green hermaphrodites with black stripes on both sides of the abdomen. After confirmation by a zoologist at the Biology Section of the Department of Veterinary Medicine, Islamic Azad University, Shahrekord Branch, they were kept in special polycarbonate containers with a volume of 6 liters of non-chlorinated water at room temperature of 10 ± 5°C, 12-hour light-dark cycle. The water was replaced manually every three days. The leeches were quarantined at a constant temperature for 75 to 80 days after being fed freshly coagulated cow blood until they were treated (26).
3.4. Method of Leech Therapy
After inducing anesthesia in rats using chloroform inhalation inside the desiccator, the rats were placed on their abdomens on the operating table. The back hair (the central part of the spine) was then shaved with an electric razor. The area was then rinsed with saline, and a few punctures were made in the back of the lumbar skin by a lancet for droplets of blood to come out. After using a 5 ml syringe with a separated head, two leeches were placed on the site for 5 minutes once every 5 days (27). The leeches used for each rat were not used in later stages and were euthanized with 70% ethanol.
3.5. Animal Treatments
Rats were randomly divided into four groups (n=7): Healthy control group (negative), Alloxan-induced diabetic group (Diabetic control group), and two treatment groups containing control and diabetic rats treated with Hirudo medicinalis, respectively. For a duration of 28 days, the day of leech treatment was considered the first day of the experimental procedure. During these 28 days, on days 1, 6, 11, 18, and 25, each group underwent a leech treatment process after shaving the hair in the back of the spine and washing it with sterile physiological serum (Figure 1).
At the end of week 4 of this experimental study and 48 hours after the last stage of leech treatment in rats and following one night of fasting (although they still had access to water), they were anesthetized with chloroform, their chest cavities were opened, and blood samples were taken from the heart. Blood was transferred into tubes without anticoagulant, and the serum was separated by a Hitachi centrifuge (Germany) at 3000 rpm for 20 minutes. It was then immediately sent to a biochemical laboratory to measure biochemical parameters in the presence of an ice pack.
3.6. Histopathological Examination
The liver, kidneys, and pancreatic tissues of each rat were removed from the abdominal area and fixed in formalin buffer 10% for 24 hours, and then embedded in paraffin, sectioned into 4-6 µ slices, and stained with hematoxylin and eosin (H&E; Sigma Aldrich). Histopathological changes were assessed by a light microscope.
3.7. Blood Drawing for Enzymatic Assays
Serum glucose, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), urea, creatinine (Cr), total cholesterol (TC), triglyceride (TG), and low-density lipoprotein (LDL) levels were measured using commercial assay kits (Pars Azmoon, Iran) and an autoanalyzer (BT3000, Italy). The measurements were conducted according to the recommended instructions at the end of the study period. Also, the deposition method was used to measure high-density lipoproteins (HDL). In the first stage, low-density and very-low-density lipoproteins (VLDL) were precipitated using a precipitating reagent. Then, a clear supernatant containing HDL was obtained. Cholesterol in this transparent layer was extracted from the color intensity using a digital spectrophotometer. The amount of light absorption at 520 nm was then compared with the standard solution using the enzymatic method (28).
3.8. Statistical Analysis
Statistical analyses were performed using analysis of variance (ANOVA) with the Tukey test. Differences were considered significant when P < 0.05. The results were expressed as mean ± Standard Error of the Mean (SEM).
4. Results
4.1. Effect of Diabetes and Leech Therapy on Glucose Serum
Blood glucose levels significantly increased in the diabetic group and those diabetics treated with leeches compared to the control group (P = 0.001). Also, serum glucose significantly increased in the diabetic group treated with leeches compared to the diabetic group (P = 0.001) (Table 1).
| Groups | CG | DG | CHTG | DHTG | P-Value |
|---|---|---|---|---|---|
| Glucose, mg/dL | 121.83 ± 7.13 A | 290.60 ± 50.52 B | 114.5 ± 10.87 A | 319.50 ± 60.37 C | 0.001 |
| TG, mg/dL | 72.83 ± 22.30 C | 75.00 ± 8.86 C | 69.25 ± 28.92 C | 70.66 ± 15.98 C | 0.97 |
| Cholesterol, mg/dL | 60.33 ± 6.56 C | 58.00 ± 8.15 C | 62.25 ± 8.61 C | 62.83 ± 12.81 C | 0.84 |
| HDL, mg/dL | 25.63 ± 1.81 C | 19.19 ± 2.89 B | 28.57 ± 1.32 C | 19.46 ± 1.55 B | 0.001 |
| LDL, mg/dL | 20.58 ± 2.10 B | 33.88 ± 4.57 C | 21.00 ± 2.04 B | 24.68 ± 2.05 B | 0.001 |
Abbreviations: CG, control group, CHTG, control hirudotherapy group, DG, diabetic group; DHTG, diabetic hirudotherapy group.
a Data presented as mean ± SEM. The number of samples in each group is n= 6. Non-similar letters in each row indicate statistically significant differences (P ≤ 0.05). Dissimilar letters in each row indicate a significant difference.
4.2. Effect of Diabetes and Leech Therapy on Lipid Profile Serum
The serum TC levels of HDL significantly decreased in diabetic and diabetic groups treated with leeches compared to the control and control groups treated with leeches (P < 0.05). The level of LDL significantly increased in the diabetic and diabetic groups treated with leeches compared to the control group (P = 0.001 and P = 0.024, respectively) (Table 1).
4.3. Effect of Diabetes and Leech Therapy on AST, ALT, ALT, and LDH Activities
The level of AST significantly increased in rats treated with leeches compared to the control group (P = 0.001), diabetics treated with leeches compared to diabetics (P = 0.005), and diabetics treated with leeches compared to the control group treated with leeches (P = 0.004) (Table 2). The level of ALT was significantly increased in diabetic rats treated with leeches compared to the control group (P = 0.025) (Table 2). The level of ALP in the diabetic and diabetic groups treated with leeches significantly increased compared to the control group (P = 0.02 and P = 0.005, respectively) (Table 2). Serum LDH levels significantly increased in the leech-treated diabetic group compared to the control, leech-treated, and diabetic groups (P = 0.001) (Table 2).
| Group | CG | DG | CHTG | DHTG | P-Value |
|---|---|---|---|---|---|
| AST, IU/L | 83.33 ± 12.69 A | 92.40 ± 19.56 A | 104.50 ± 17.54 A,B | 136.83 ± 33.40 B | 0.005 |
| ALT, IU/L | 44.50 ± 3.20 A | 74.20 ± 26.17 A,B | 50.75 ± 10.90 A | 96.00 ± 62.09 B | 0.046 |
| ALP, IU/L | 426.00 ± 62.29 A | 955.40 ± 516.59 A,B | 701.50 ± 72.49 A,B | 1075.00 ± 429.81 B | 0.024 |
| LDH, IU/L | 732.16 ± 248.27 A | 559.20 ± 168.71 A | 619.50 ± 229.14 A | 1421.00 ± 262.3 B | 0.001 |
| Cr, mg/dL | 0.76 ± 0.05 A | 1.04 ± 0.11 A,B | 0.95 ± 0.36 A,B | 1.08 ± 0.26 B | 0.049 |
| Urea, mg/dL | 40.33 ± 4.76 C | 72.00 ± 5.61 A | 44.75 ± 8.61 C | 89.16 ± 12.43 B | 0.001 |
| Ca, mg/dL | 10.46 ± 0.23 B | 10.18 ± 0.73 B | 10.72 ± 0.82 B | 10.58 ± 0.6 B | 0.57 |
| P, mg/dL | 5.58 ± 0.27 A | 7.14 ± 1.89 A,B | 7.90 ± 1.19 A,B | 8.66 ± 1.04 B | 0.003 |
Abbreviations: CG, control group, CHTG, control hirudotherapy group, DG, diabetic group. DHTG, diabetic hirudotherapy group.
a Data presented as mean ± SEM. The number of samples in each group is n = 6. Non-similar letters in each row indicate statistically significant differences (P ≤ 0.05). Dissimilar letters in each row indicate a significant difference.
4.4. Effect of Diabetic and Leech Therapy on Creatinine and Urea Activities
The serum Cr significantly increased in the diabetic and diabetic groups treated with leeches compared to the control group (P = 0.05) (Table 2). The serum urea showed a significant increase in diabetic rats compared to the control group (P = 0.001), diabetic rats compared to leech-treated diabetics (P = 0.001), and leech-treated diabetic rats compared to healthy rats (P = 0.001) (Table 2).
4.5. Effect of Diabetic and Leech Therapy on Calcium and Phosphorus Serum
Serum phosphorus levels significantly increased in the rats of the healthy group treated with leeches compared to the control group (P = 0.001). The amount of blood phosphorus significantly increased in the diabetic group compared to the control group (P = 0.047) and in the diabetic group treated with leeches compared to the diabetic group (P = 0.05) (Table 2).
4.6. Histopathological Findings
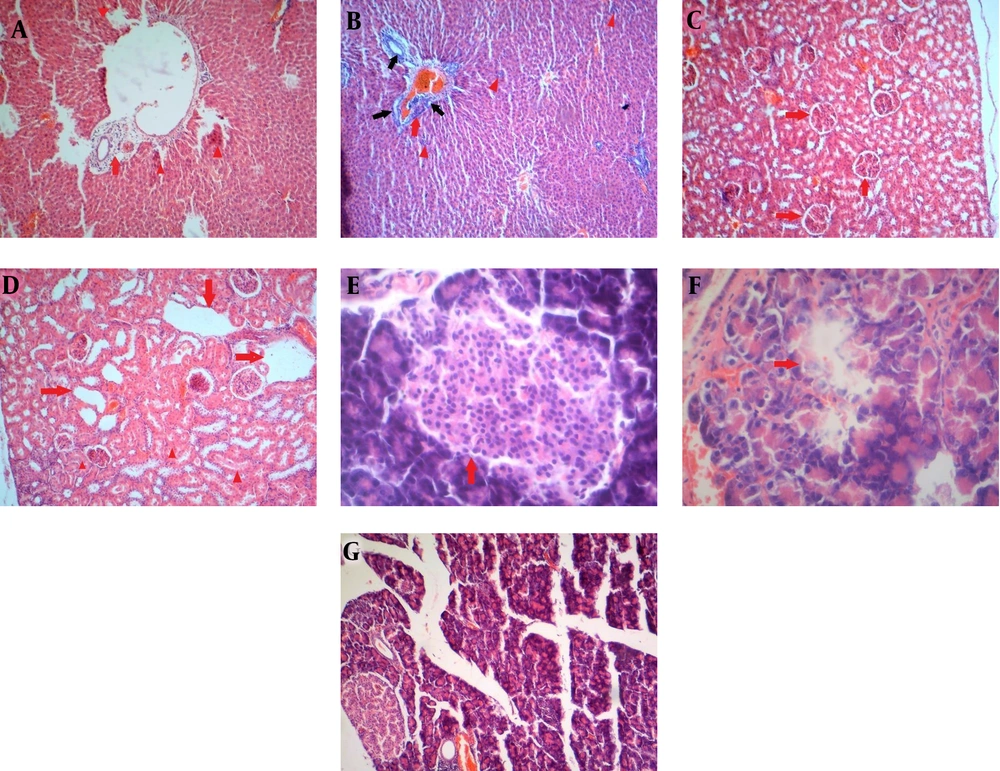
In the microscopic evaluation in the control group, polyhedral hepatic cells are arranged in cords that radiate from the central vein to the portal area. In the portal area, branches of the liver arteriole, portal vein, bile ducts, and connective tissue are surrounded by hepatocytes. The cytoplasm of liver cells appeared pink with eosin, and some liver cells had two nuclei, while others had more than two nuclei. Liver tissue in the diabetic rat group showed dilatation of the central hepatic vein, degeneration of the hepatocytes irregularly around the hepatic vein, dilatation of the hepatic sinusoids, and damage to the hepatic venous endothelial cells. Liver tissue of healthy leeches treated with leeches showed cytoplasmic changes in liver cells, nucleated hyperchromia, the disappearance of cells near the central hepatic vein, and damage to vascular endothelial cells (Figure 2A). Diabetic rats treated with Hirudo medicinalis exhibited more severe liver damage than the other groups. This was characterized by hyperchromia and condensation of the nucleus, eosinophilic cytoplasm, disruption of liver cell arrangement, dilation of sinusoids, necrotic foci, increased bile ducts in the portal area, and infiltration of inflammatory cells around the bile ducts (Figure 2B). Histopathologic findings of renal tissue in control (normal saline) rats showed a Bowmann capsule and normal glomerular structure (red arrow) (Figure 2C). However, the diabetic and diabetic groups treated with leeches showed progressive tubular dilation, leading to cyst formation, degeneration, and renal tubular necrosis (Figure 2D). Microscopic studies of pancreatic tissue in the control group showed the natural structure of beta cells in the islets of Langerhans (Figure 2E). In alloxanized rats, we observed severe beta cell damage, decreased islets of Langerhans, and cell death (Figure 2F). No abnormal pathological changes were observed in the pancreatic tissue of the healthy group treated with leeches (Figure 2G).
(A) The liver of healthy male rats treated with leeches, mononuclear inflammatory cells in the portal area (red arrow), and scattered necrosis foci (red arrowhead) (H&E × 100). (B) Liver of diabetic male rats treated with leeches. Focal exudation of inflammatory cells (red arrowhead) and increase of mononuclear inflammatory cells (red arrow) and bile duct hyperplasia (black arrow) in the portal tract (H&E × 100). (C) The tissue kidney of healthy rats (H & E × 100). (D) The tissue kidney of diabetics treated with leeches, squamous metaplasia of the renal tubular cells due to tubular dilation (red arrow), and necrosis of renal tubules (red arrowhead) (H & E × 100). (E) Pancreatic tissue of control group rats. The structure of the natural Langerhans Islands (red arrow) (H&E × 400). (F) Pancreatic tissue of leech-treated diabetic rats, Langerhans Islands necrosis (red arrow). (H&E × 400). (G) Pancreatic tissue of healthy male rats treated with leeches (H&E × 100).
5. Discussion
The present study showed that three days after the second subcutaneous injection of alloxan monohydrate at a dose of 100 mg/kg body weight in the studied rats induced diabetes and hyperglycemia, which was observed until the end of the study period. The rapid uptake of alloxan by beta cells of the pancreatic islets of Langerhans in the pancreas, and the subsequent production of oxygen-free radicals, result in islet necrosis in the pancreas. Beta cells are more sensitive to oxygen-free radicals than other tissues, such as the liver. This is why alloxan is considered a diabetic compound (29, 30). Studies have shown that alloxan-induced hyperglycemia causes the overproduction of free radicals, oxidative stress, and inactivation of antioxidant enzymes such as catalase, superoxide dismutase, and glutathione peroxidase (31).
The enzymes ALT, AST, LDH, and ALP are commonly used as clinical, functional indicators of hepatotoxicity (32). Increased AST, ALP, LDH, and ALP activity in leech-treated diabetic male rats compared to male diabetic rats indicates the presence of known and somewhat unknown compounds in leech saliva that can cause liver damage in rats. Cytoplasmic aminotransferases flow into the sinusoidal bloodstream when the integrity of the hepatocyte membrane of the liver is damaged. Enzyme escape can be caused by both cell destruction and increased cell membrane permeability (33). These results demonstrate that hirudotherapy in diabetic rats can produce several hepatocyte changes. Leeches are a source of prostaglandin-like substances with biological activity similar to prostacyclin (destabilase complex) with a molecular weight of 391 kDa but a completely unknown chemical structure that prevents platelet aggregation (33, 34). Noda et al. have shown that prostacyclin (PGI2) and prostaglandin analogs reduce mortality in groups treated with galactose amine poisoning, which suggests that changes in prostacyclin-induced blood flow may be responsible for the protective effects of liver cells (35). Therefore, it seems that the compounds in salivary secretions that enter the blood vessels where leeches come into contact can increase the dilation of blood vessels and cannot be responsible for liver damage. Also, Sahu et al. showed that oral administration of androstenedione in female rats two weeks before mating and throughout pregnancy at doses of 5, 30, and 60 mg/kg body weight did not cause liver damage (32). Therefore, such compounds found in leech saliva secretions do not appear to cause liver damage. Boada et al. showed that intraperitoneal injection of androgenic steroids at a dose of 5 mg/kg intraperitoneally for 4 days, 60 days, and 90 days caused abnormal changes in the nucleus and cytoplasm of liver tissue (36). Therefore, the presence of anabolic-androgenic steroid compounds and other unknown compounds appears to be responsible for exacerbating liver damage and other enzymatic changes in leech-treated diabetic rats. Also, these histological and serum changes may be due to the increase in the duration of leech treatment at each turn or the duration of leech treatment intervals in diabetic male rats treated with leeches.
In male diabetic rats treated with leeches, the amount of secreted leech saliva is not controlled based on micrograms per kilogram. Our findings showed that the use of leech therapy in diabetic rats not only does not improve hypoglycemia compared to male diabetic rats but also significantly increases blood glucose compared to the diabetic group (Table 1), which is contrary to Mohammed's findings (37). According to previous findings, leech therapy can only heal foot ulcers in diabetics 30 days after leech treatment due to the anti-inflammatory action of bdellins and eglins in leech saliva (11), improving blood circulation and reducing anemia. The presence of carboxypeptidase, histamine-like substances, and acetylcholine accelerates the healing process (24, 38-40).
The present study also showed that leech therapy in diabetic rats reduces LDL compared to the diabetic group but has no effect on TG, cholesterol, and HDL (Table 1). Also, LDL is one of the most important lipoproteins in the blood and esterifies high plasma cholesterol levels into tissues, which is common in diabetic patients. The most common pattern of dyslipidemia is an increase in LDL and TG and a decrease in HDL (41). In the present study, alloxan-induced diabetic rats showed an increase in LDL and a decrease in HDL (Table 1). Increased levels of LDL may be associated with decreased levels of LDL receptors (42). Wu and Yang reported that leeches might affect the expression of ACAT-2, Fas, and HMGCR genes in liver tissue and change cholesterol synthesis, fatty acids, cholesterol transfer, and fat regulation (43).
In pathological studies of renal tissue in male control rats, renal structures, including glomeruli, renal tubules, and blood vessels, were normal (Figure 2C). However, in diabetic male rats, glomerular lesions, such as glomerular degeneration, and various renal tubular changes, including tubular swelling, necrosis, and increased connective tissue between renal tubules, were observed (Figure 2D). These findings are consistent with Kamble and Bodhankar (44).
Our results showed that urea and serum creatinine levels were significantly elevated in diabetic rats and diabetic rats treated with leeches, compared to the control group and control group treated with leeches. This increase in urea and creatinine levels can be attributed to hyperglycemia, decreased insulin secretion, and subsequent metabolic acidosis, which are known to cause renal failure in diabetic rats (Table 2). Therefore, elevated urea and creatinine levels can be considered indicators of renal failure (45, 46).
Elevated serum phosphorus in leech-treated diabetic rats may be due to renal failure, the inability of the kidneys to excrete phosphate, secondary parathyroid hormone, and bone disorders (47, 48). It is suggested that future studies determine the activity of oxidative stress parameters and the activity of serum insulin in diabetic and diabetic rats treated with leeches.
In conclusion, leech treatment alone cannot be considered a standalone treatment method. However, it could be used in conjunction with other treatment methods. Also, clinical monitoring and laboratory tests (blood count, biochemistry routine test, and fibrinolytic system) are strongly recommended. However, even with all its beneficial effects, leech therapy could be harmful in some situations. One of the main complications of hirudotherapy in diabetic rats is hepatotoxicity and acute renal failure (ARF) due to endopeptidases, aminopeptidases, and phosphatases in leech saliva secretions (48-50).