1. Background
Headaches are a common health problem (1). They are also one of the most common reasons patients seek medical attention and the main influential factor in performing diagnostic and medical procedures. International Headache Society (IHS) classification separates headaches into primary and secondary disorders (2, 3). Migraine is the most common primary headache disorder with no specific pathological cause that negatively affects physical, mental, and social health and quality of life (4). Migraine is characterized most often by unilateral head pain that is exacerbated by physical activity. A migraine may also cause nausea, vomiting, fear, and sensitivity to light and sound (5).
According to World Health Organization (WHO), nearly 303 million people worldwide suffer from migraine headaches (6). Women (5 percent to 25 percent) have a greater migraine prevalence than males (2 percent to 10 percent) (7). The prevalence of migraine in Iran is estimated to be 14%, which is comparable to or even greater than the global average (8). Acute or symptomatic therapy and preventative treatment or prophylaxis are the two types of pharmacological treatment for migraine (9). There are several medications used to prevent migraines (10). All medicines have potential side effects and contraindications. On the other hand, if there are comorbidities, they can interfere with the patient's compliance with medication therapy or the medication regimen complexity. They may not even have the necessary effectiveness, reducing the patient's willingness to accept migraine prophylaxis therapy (11). Furthermore, using alternative methods and medicinal herbs in the treatment and prevention of migraine has long been studied (12). Many medicinal herbs were found to be more appealing to patients due to their lower side effects, lower cost, and availability. Nowadays, they are considered part of the treatment of diseases, for instance, reducing migraine attacks (13).
Zingiber officinale Rosc. is a popular spice in many countries (14). This plant belongs to the Zingiberaceae family and is native to Asia (15). It is now cultivated in Africa, India, and other tropical countries. The presence of a group of phenolic components termed gingerols, of which 6-gingerol is the most prevalent, gives fresh ginger its citrusy taste. The spiciness of dried ginger is in terms of the presence of shogaols, which are dehydrated compounds of gingerol (16-18). Numerous studies have shown the antioxidant, anti-inflammatory, antimicrobial and anti-cancer effects of ginger (19). The bioactive components of ginger, such as gingerdione and shogaol, have pharmacological effects similar to non-steroidal anti-inflammatory drugs, which inhibit arachidonic acid metabolism, and ultimately synthesize prostaglandins; therefore, they act more effectively as an anti-inflammatory agent than conventional anti-inflammatory drugs with fewer side effects (20). 6-Shogaol has analgesic effects via inhibiting substance P (SP) release. It appears to interfere with the arachidonic acid cascade, leading to the inhibition of cyclooxygenase as well as the inhibition of prostaglandin synthesis (21). Martins et al. studied the effectiveness of ginger (Z. officinale Rosc.) in the treatment of migraine prophylaxis in 2020. The number of days with severe pain, analgesic usage for acute migraine, and migraine attack length all decreased in both groups, with no significant difference between the ginger and placebo groups (22).
2. Objectives
As far as the researchers of the present study are concerned, only one study has so far been conducted toward the effect of ginger on migraine prevention; thus, given the importance of this issue, the present study was performed with the aim of investigating the efficacy of ginger consumption in headache prophylaxis in the patients with migraine.
3. Methods
3.1. Design and Participants
This randomized, double-blind clinical experiment was conducted at the Golestan Hospital's specialist neurological clinic in Ahvaz, Iran. Those who met the following criteria were included in the research out of the total number of migraine patients: People aged 18 and 50 years who, according to IHS standard, were diagnosed with a history of migraine aura and migraine without aura for at least a year, diagnosis of moderate to severe migraine (which is defined as the number of headache attacks 2 - 15 times a month, or attacks with less intensity and duration that cause inability and disruption in daily activities) and the patients who had not previously received any preventive treatment. Exclusion criteria included non-migraine headaches, an allergy to ginger, other neurological diseases (stroke, Alzheimer's disease, etc.), patients with severe gastrointestinal disorders, people taking anticoagulants, and pregnant and lactating women. At last, 110 patients were recruited and randomly allocated either to the intervention (55 patients) or the control (55 patients) groups. According to the data obtained from a similar study (22), with alpha equal to 0.05 and beta 0.2, T90 in the control group was reported as equal to 57 ± 5. In the intervention group, T90 was reported equal to 54 ± 6 at the end of their study compared to the beginning of the study, and considering that the consumed dose was increased from 600 to 1000 mg in the mentioned study, we reduced the mean of the intervention group by 0.5. As a result, the sample size in each group was 55 patients that was calculated using the following formula:
3.2. Ethical Considerations
A randomized, double-blind, placebo-controlled clinical trial was used in this investigation. The trial was authorized by the Ethical Committee of Ahvaz Jundishapur University of Medical Sciences (IR.AJUMS.REC.1398.738). With the identifier IRCT20200126046263N1, this research was also filed with the Iranian Registry of Clinical Trials. Written informed consent was signed by all participants.
3.3. Randomization
Random allocation of the patients to the study groups was performed as follows. The first patient was randomly assigned to one group and then the next patient to the other group, and this sequence was repeated until the completion of 110 patients. As a result, the patients were separated into two groups of 55 patients randomly. Furthermore, neither the patients nor the researchers knew which group they were in or what sort of intervention they had got.
3.4. Interventions
The patients in the intervention group received 500 mg of ginger tablet and 20 mg of propranolol tablet twice a day for three months. Similarly, the control group received placebo tablets containing 500 mg starch and 20 mg of propranolol tablet. The ginger tablet (Vomigone, IRC9406633051781240) was produced by Dineh pharmaceutical supplements company) Qazvin, Iran) and the placebo was provided by the School of Pharmacy, Ahvaz University of Medical Sciences. The herbal medicine intervention used in this trial was a dry extract of Z. officinale Rosc. Each tablet contained 500 mg of Z. officinale rhizome (equivalent to 5 mg volatile oil or 25 mg Gingerol). The placebo tablets used in this trial were identically sized tablets made with starch powder and colored (with food coloring) to match the ginger tablets. The drugs were delivered to the participants in identical and packed boxes. Patients were also asked not to eat any foods containing ginger and its components during the study and to follow their previous diet and avoid any changes in their current diet and physical activity habits. It is worth noting that dosages of less than 2 g of ginger extract containing 5% gingerols are perfectly safe for people (23).
3.5. Measures
Demographic data (age, sex, marital status, history of diseases, and type and amount of drugs used), headache impact (the number and duration of migraine attacks and headache severity) were obtained by face-to-face interviews. Anthropometric data (weight, height, waist circumference (WC), hip circumference (HC), waist-to-hip ratio (WHR), and computed body mass index (BMI) were achieved before and after the intervention. Headache effect was assessed by the Visual Analogue Scale (VAS) (24) and the migraine disability test (MIDAS) (25) questionnaires. Three-day food recalls were performed to assess dietary consumption, and the dietary intake of patients was assessed using the Nutritionist IV software (26). At the beginning and end of the trial, we employed the International Physical Activity Questionnaire (IPAQ) to measure physical activity. Using established criteria, data from the IPAQ were translated to metabolic equivalent-minutes each week (27). Furthermore, anxiety, which affects the severity and frequency of migraine headaches, was assessed using the BAI questionnaire (28).
3.6. Statistical Methods
In order to statistically analyze the data, SPSS software version 26 was used. First, the Kolmogorov-Smirnov test was used to analyze the normal distribution of all variables. The mean score of the quantitative data was compared between the two groups at the beginning and end of the research using an independent sample t-test or a Mann-Whitney U test. The paired sample t-test or Wilcoxon test was employed to compare the mean score of the quantitative variables before and after the intervention. The qualitative variables were examined using the chi-square test, while the modified variables were studied using analysis of variance (ANOVA). A P < 0.05 was considered significant.
4. Results
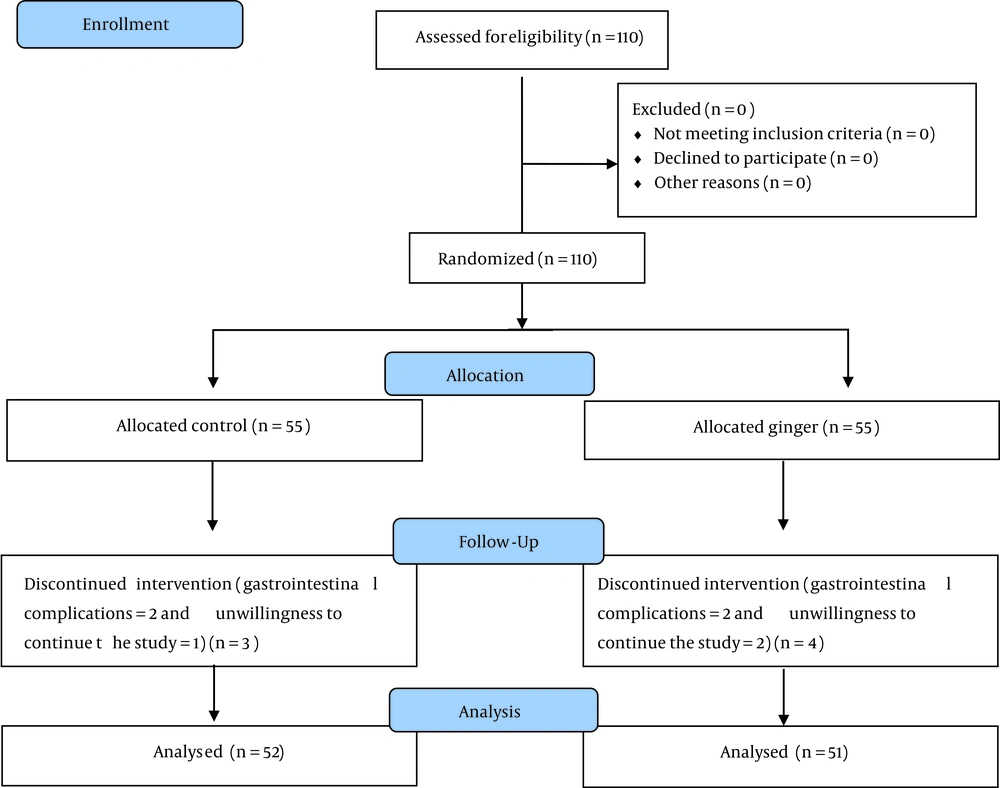
A total of 110 patients were enrolled in the trial, who were evenly divided into two groups of 55subjects. At the end of the trial, the ginger group had 51 individuals, and the control group had 52. The research group and experimental design are shown in Figure 1.
The demographic characteristics of patients at the beginning of the study are provided in Table 1. A comparison of these characteristics shows that there was no significant difference in age, gender, and marital status of the patients between the two groups. The majority of the patients were women (72.8%), which is consistent with the fact that the prevalence of migraine is higher in women than men (29). The mean age of the patients was 31.62 ± 7.1 years. According to studies, 90% of migraine patients experience their first headache in the first four decades of their life (29).
a Chi-square test.
b Mann-Whitney test.
Table 2 shows the physical activity, anxiety, and BMI at the baseline and post-intervention. There were no significant differences between the two groups at the beginning and end of the study (P > 0.05). After adjusting the effect of intervening variables (energy intake and physical activity), BMI between the two groups indicated a significant difference at the end of the study (P < 0.05). Intra-group comparison of the mentioned variables showed that in the group receiving ginger, weight, BMI, WC, HC, and WHR at the end of the study significantly decreased in comparison with the beginning of the study (P < 0.05).
| Variables | Ginger (n = 51) | Placebo (n = 52) | P-Value a | P-Value b |
|---|---|---|---|---|
| Activity (met/min/week) | ||||
| Baseline | 1525.4 ± 512.6 | 1550 ± 500.3 | 0.93 | - |
| T90 | 1581.3 ± 624.4 | 1609.6 ± 599.3 | 0.52 | - |
| P-value c | 0.08 | 0.07 | ||
| Anxiety | ||||
| Baseline | 17.4 ± 7.4 | 17.5 ± 5.9 | 0.97 | - |
| T90 | 17.2 ± 7 | 17.1 ± 5.9 | 0.84 | - |
| P-value c | 0.1 | 0.2 | ||
| Weight (kg) | ||||
| Baseline | 74.2 ± 1 | 73.3 ± 9.2 | 0.58 | 0.61 |
| T90 | 71.8 ± 9.8 | 73 ± 93 | 0.24 | 0.53 |
| P-value c | 0.001 | 0.13 | ||
| BMI | ||||
| Baseline | 26.3 ± 1.7 | 26.1 ± 0.9 | 0.46 | 0.59 |
| T90 | 26.1 ± 0.9 | 26 ± 1 | 0.07 | 0.03 |
| P-value c | 0.001 | 0.06 | ||
| WC (cm) | ||||
| Baseline | 91.2 ± 10.9 | 91.6 ± 8 | 0.84 | 0.64 |
| T90 | 89.3 ± 10.9 | 91.4 ± 7.8 | 0.24 | 0.3 |
| P-value c | 0.001 | 0.16 | ||
| WH (cm) | ||||
| Baseline | 104.3 ± 10.9 | 104.7 ± 7.2 | 0.8 | 0.59 |
| T90 | 102.3 ± 10.9 | 104.6 ± 7.1 | 0.2 | 0.23 |
| P-value c | 0.001 | 0.25 | ||
| WHR | ||||
| Baseline | 0.87 ± 0.013 | 0.87 ± 0.021 | 0.69 | 0.83 |
| T90 | 0.87 ± 0.014 | 0.87 ± 0.024 | 0.67 | 0.23 |
| P-value c | 0.001 | 0.5 |
Abbreviations: T90, 90-day treatment; BMI, body mass index; WC, waist circumference; HC, hip circumference; WHR, waist-to-hip ratio.
a Mann-Whitney U test.
b ANCOVA.
c Wilcoxon test.
The food intakes of the participants in this study were investigated at the beginning and at the end of the study (Table 3). At the beginning and end of the trial, there was no statistically significant difference in calorie intake or macronutrient levels between the two groups (P > 0.05). Also, after controlling for the influence of the intervening variable (physical activity), there was no statistically significant difference in calorie intake or macronutrient levels between the intervention and control groups at the beginning and end of the trial (P > 0.05).
| Variables | Ginger (n = 51) | Placebo (n = 52) | P-Value a | P-Value b |
|---|---|---|---|---|
| Energy | ||||
| Baseline | 2574 ± 345.1 | 2515.9 ± 317.4 | 0.58 | 0.61 |
| T90 | 2503.3 ± 334.6 | 3496.2 ± 329.1 | 0.74 | 0.87 |
| P-value c | 0.48 | 0.39 | ||
| Carbohydrate | ||||
| Baseline | 356.5 ± 48.3 | 352.2 ± 44.4 | 0.58 | 0.61 |
| T90 | 353.9 ± 51.4 | 353.9 ± 51.4 | 0.93 | 0.9 |
| P-value c | 0.62 | 0.62 | ||
| Fat | ||||
| Baseline | 90.5 ± 12.2 | 89.4 ± 11.2 | 0.58 | 0.54 |
| T90 | 90.3 ± 12.3 | 88.9 ± 12.3 | 0.57 | 0.61 |
| P-value c | 0.22 | 0.37 | ||
| Protein | ||||
| Baseline | 73.4 ± 10.4 | 74.8 ± 9.8 | 0.76 | 0.5 |
| T90 | 71.1 ± 10.05 | 73.6 ± 10.3 | 0.22 | 0.22 |
| P-value c | 0.88 | 0.42 |
Abbreviation: T90, 90-day treatment.
a Mann-Whitney test.
b ANCOVA.
c Wilcoxon.
The migraine clinical indicators in both groups were investigated at the beginning and end of the study. Table 4 summarizes the findings. The mean MIDAS score, headache intensity, headache duration, and headache frequency were not statistically significant between the two groups (P > 0.05) at the beginning of the trial in the ginger and placebo groups (after excluding the influence of anxiety as an intervening variable). The findings revealed that at the end of the trial, the mean MIDAS score, headache intensity, and headache duration were substantially different in both groups (P 0.05). Despite a considerable reduction in the frequency of migraine episodes in the ginger group at the end of the research, there was no statistically significant difference between the two groups in general (P = 0.208).
| Variables | Ginger (n = 51) | Placebo (n = 52) | P-Value a | P-Value b |
|---|---|---|---|---|
| MIDAS | ||||
| Baseline | 22.8 ± 4.3 | 23.1 ± 4.1 | 0.78 | 0.96 |
| T90 | 11.3 ± 3.9 | 20.5 ± 5.9 | 0.001 | 0.001 |
| P-value c | 0.001 | 0.14 | ||
| Severity (0 - 10) | ||||
| Baseline | 7.4 ± 1.9 | 7.5 ± 2 | 0.73 | 0.82 |
| T90 | 3.6 ± 1.8 | 6.9 ± 2.5 | 0.001 | 0.001 |
| P-value c | 0.001 | 0.09 | ||
| Frequency (number of migraine attacks per month) | ||||
| Baseline | 10.18 ± 2.6 | 10.02 ± 2.2 | 0.87 | 0.66 |
| T90 | 8.9 ± 2.7 | 9.6 ± 2.1 | 0.2 | 0.11 |
| P-value c | 0.001 | 0.18 | ||
| Length (h) | ||||
| Baseline | 17.3 ± 2.4 | 17.04 ± 2.6 | 0.76 | 0.56 |
| T90 | 10.3 ± 2.8 | 15.8 ± 4.3 | 0.001 | 0.001 |
| P-value c | 0.001 | 0.25 |
Abbreviations: MIDAS, migraine disability assessment test; T90, 90-day treatment.
a Mann-Whitney test.
b ANCOVA.
c Wilcoxon.
5. Discussion
People with migraine headaches are recommended to consider an effective preventive treatment to avoid headaches and to reduce the severity of pain. As far as the researchers of the present study are concerned, only one study on preventive treatment of ginger in migraine patients was found. Martins et al. in 2020 investigated 107 patients with migraine. For three months, patients received 200 mg of dried extract of ginger (5 percent active component) or placebo (cellulose) capsules three times a day. The number of days with severe pain, analgesic usage for acute migraine, and migraine attack length all decreased in both groups, with no significant difference between the ginger and placebo groups (22). There are limited studies on the effects of ginger on the treatment of acute migraine. Martins et al., in 2019, conducted a study on 60 patients. To treat a migraine attack, participants were randomly assigned to one of two groups: 400 mg of ginger extract or placebo with one intravenous medication (100 mg of ketoprofen). They kept a headache journal before, 0.5 hours, 1 hour, 1.5 hours, and two hours after taking the drug. The degree of the pain, functional status, migraine symptoms, and treatment satisfaction were all taken into consideration. Patients given ginger had a substantially superior clinical response after 1 hour (P = 0.04), 1.5 hours (P = 0.01), and 2 hours (P = 0.04), according to the findings. In addition, ginger medication resulted in a decrease in discomfort and an increase in functional status at all times (30). Maghbooli et al. performed a study on 100 migraine sufferers who did not have an aura in 2014. For one month, they were randomly assigned to either ginger powder (250 mg) or sumatriptan (50 mg) at the beginning of their headache. Patients documented the time of headache onset, intensity, the time gap between headache onset and medicine administration, and patient self-estimation of response for five following migraine bouts. According to the findings, ginger powder is statistically similar to sumatriptan in the treatment of common migraine episodes, although the clinical side effects of ginger powder are fewer than sumatriptan (31). Cady et al. conducted a study on 60 migraine sufferers in 2011. Sublingual feverfew/ginger or placebo were given to the subjects in a 3: 1 ratio. At the outset of a headache, the participants were instructed to utilize them for one month. The findings revealed that sublingual feverfew/ginger is a helpful and safe pain reliever for migraine patients who regularly have mild headaches before moderate to severe headaches develop (32). Although there was no significant difference in the number of migraine attacks per month, the ginger group showed significant superiority to the placebo group in reducing their MIDAS score, headache severity, and duration of headache, which indicates the effectiveness of ginger in improving and preventing migraine.
Regarding the limitations of this research, it can be mentioned that due to financial and equipment limitations, it was not possible to examine the potential effects of ginger on inflammatory biomarkers, such as tumor necrosis factor, calcitonin gene-related peptide, and C-reactive protein in this study. Hence, more investigations to describe the effects of ginger on the level of inflammatory factors in patients with migraine are suggested.
5.1. Conclusions
The findings of this study revealed that ginger supplementation can help reduce headache severity, duration, and MIDAS score. However, there was no evidence of this positive effect in terms of frequency. According to the results of this study and the positive effect of ginger supplement on the recovery of patients with migraine, it seems that ginger can be added to FDA approved treatments in order to prevent prophylaxis; due to its affordability, availability, and brief side effects, it is possible to take advantage of ginger’s beneficial effects.