1. Background
One of the purposes of root canal therapy is to eliminate bacteria from the root canal system (1). A common failure in deciduous root canal treatment is the propagation of microorganisms in the sophisticated root canal system, especially the apical or periapical parts of the pulp-treated teeth (2). The oral cavity hosts numerous bacterial, fungal, and protozoan species (3). A load of microorganisms in root canals is directly correlated with the duration of microbial exposure in pulp and periapical inflammations. Studies have shown that the severity of pulp and periapical inflammations is directly linked to a load of microorganisms in the root canals and the time of microbial exposure (4). Hence, root canal treatment aims to eliminate bacterial populations and their products from the canals through mechanical and chemical cleaning procedures (5). Therefore, appropriate irrigants should overcome such anatomical limitations (6). Technically, optimal irrigants should exert the best antibacterial effects and the least toxic effects on periapical tissues (7).
Chlorhexidine gluconate (CHX) (C22H30Cl) is a non-allergic canal irrigant that has strong cationic and antimicrobial characteristics (7). Although CHX is routinely used as the final irrigant in root canal therapy, it has toxic effects (8). Natural herbals have been increasingly used in recent years, especially in pharmaceutical companies, due to their strong antimicrobial activities (9). Satureja khuzestanica, an herb with more than 90% carvacrol, has potent antimicrobial, anti-inflammatory, and pain-relieving characteristics (10). This medicinal plant has a significant antibacterial ability due to a wide range of secondary metabolites such as carvacrol, pinene, beta-bisabolene, and coumarin derivatives. Carvacrol and beta-bisabolene are the most effective compounds in biofilm inhibition (11). Chamomile is another medicinal plant with antimicrobial characteristics (7). This plant is a widespread short-size flower with white petals and a golden center, including inflorescence as the most valuable part of the flower (8). Although the exact mechanisms of the antibacterial effects of chamomile have not been well described, they might be linked to the chemically active constituents and α-bisabolol, which is effective at low concentrations on various pathogenic bacteria such as Escherichia coli, Staphylococcus aureus, Bacillus subtilis, Streptococcus faecalis, and Pseudomonas aeruginosa, currently resistant bacteria to typical antimicrobial agents (12). Recent studies have revealed that chamomile can solve digestion problems and relieve the pain of growing deciduous teeth in children (8). Natural antimicrobials have been increasingly used in recent years due to their relatively low costs and few side effects (6).
2. Objectives
This study aimed to find alternate root canal irrigants with the best antibacterial activities and fewer toxicities. Thus, the antibacterial effects of CHX alone and mixed with two other herbal irrigants were assessed in primary tooth root canal disinfection. Response surface methodology (RSM) based on central composite design (CCD) was used to optimize the disinfection of the primary tooth root canals.
3. Methods
3.1. Microorganism and Culture Preparation
A pure culture of E. faecalis ATCC 29212 was inoculated onto Mueller-Hinton agar plates (Himedia, Mumbai, India) and incubated at 37°C overnight. Then, the culture was adjusted to 0.5 MacFarland on an optical densitometer (Densichek Plus, bioMerieux, France) using tryptone yeast extract broth (Himedia, Mumbai, India).
3.2. Experiment Solution Preparation
Briefly, 2% CHX, 1% chamomile essence, 1% CHX, 1% S. khuzestanica, and 1% CHX were prepared for the study. Phosphate-buffered saline (PBS) was used as a negative control. Chamomile essence was purchased from GolDaru, Isfahan, Iran. The aerial parts (leaves and flowers) of S. khuzestanica were collected during the flowering stage from Khorramabad, Lorestan province, Iran. Harvested flowering aerial parts were dried at room temperature. Dried plant materials were powered (100 g) and subjected to hydrodistillation (1000 mL distilled water) for 3 h using a Clevenger-type apparatus. Samples were dried using anhydrous sodium sulfate and then stored in amber vials at 4°C until use. For the preparation of 1% S. khuzestanica solution, 1 g of S. khuzestanica powder was diluted in 100 mL of PBS. Chamomile essence was provided commercially (Merck, Germany).
3.3. Tooth Specimen Preparation
The study was carried out on 80 extracted first primary molars with no visible resorptions of more than one-third of the roots. Teeth were extracted due to enamel damages, parents' unwillingness to receive complicated treatments, and management reasons. The crowns of the teeth were horizontally cut from the cementoenamel junctions (CEJ), and canals were cleansed to prepare roots for the experiments. Canals were washed after changing each file using sterile normal saline. The apical foramen was sealed with cyanoacrylate to prevent bacterial leakage. Teeth were horizontally mounted and sterilized at 121°C for 20 min at 15 psi using an autoclave.
3.4. Grouping and Assessment Protocols
A total of 80 specimens were randomly divided into five major groups as follows: Group 1, 2% CHX (n = 20); group 2, 1% chamomile essence and 1% CHX (n = 20); group 3, 1% S. khuzestanica and 1% CHX (n = 20); group 4, infected dentine tubes as positive controls (n = 10); and group 5, sterile dentine tubes as negative controls (n = 10).
Pure E. faecalis suspensions containing 108 colony-forming units (CFU)/mL were injected into the roots using insulin syringes under aseptic conditions to generate infections in groups 1, 2, 3, and 4. Dental infection by the bacteria was verified using the cultivation of the root specimens. Dentine chips were removed from the canals using sequential sterile low-speed round burs at the experimental times of 0, 7, 14, 21, and 28 days of incubation. After 5 min, the solution was removed using sterile paper tips. Specimens were incubated at 37°C for 28 days. The number of bacterial colonies was calculated using a colony counter (Funke-Gerber, Germany). The data were analyzed using the analysis of variance and covariance (ANOVA) to show differences between the experimental groups and the positive control.
3.5. Optimization Analysis
After selecting the best solution from the previous step, factors including CHX concentration (A), chamomile essence concentration (B), and contact time of the irrigating solution with the external surface (C) were selected to optimize the disinfection of primary tooth root canals. Ranges for each parameter were chosen based on a study by Gomes et al. (10). Symbols of these parameters and their ranges are shown in Table 1.
| Parameters | Name | Minimum | Maximum |
|---|---|---|---|
| A | Chlorhexidine conc. (%) | 0.1591 | 1.84 |
| B | Chamomile conc. (%) | 0.1591 | 1.84 |
| C | Contact time (min) | 1.27 | 14.73 |
3.6. Statistical Analysis and Model Validation
Data from the central composite experiment were subjected to multiple regression analysis using least squares to build the regression models. Experimental design, data analysis, interaction plotting, and optimization of factors were carried out with Design Expert 13 Statistical Software and Excel 2019.
4. Results
4.1. Assessment of the Best Solution
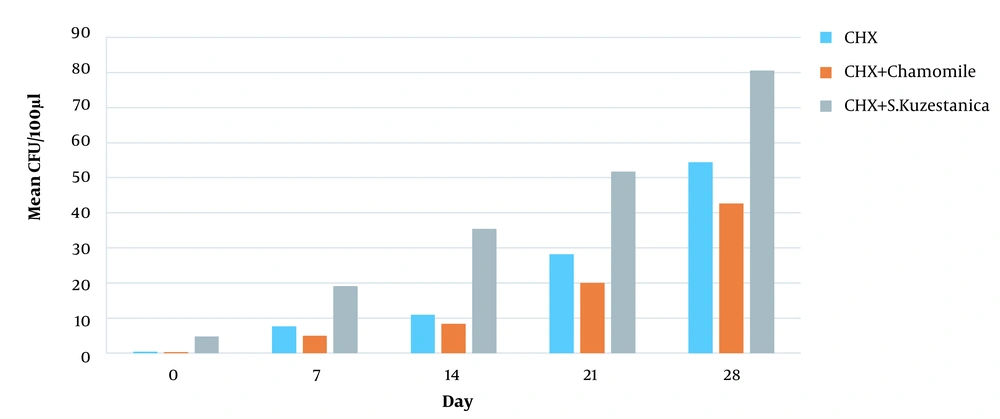
An approximate estimate of the number of viable bacteria that penetrated the dentinal canals at various layer depths was represented by the CFU. The number of CFU in the three experimental groups was minimum on day 0 (first cultures). On day 0, the CHX group showed the most effective antibacterial activity. However, the 1% chamomile essence and 1% CHX group showed the most effective antibacterial activity on days 7, 14, 21, and 28 (P < 0.05). No bacterial growth was seen in negative controls, while bacteria were propagated well in positive controls, indicating the method's efficiency (Table 2).
| Irrigant | Day 0 | Day 7 | Day 14 | Day 21 | Day 28 |
|---|---|---|---|---|---|
| 1% Satureja khuzestanica and 1% CHX | 4.70 ± 3.68 B | 19.11 ± 4.05 A | 35.40 ± 6.99 A | 51.80 ± 13.11 A | 80.55 ± 5.30 A |
| 2% CHX | 0.30 ± 2.97 A | 7.68 ± 2.34 A | 10.90 ± 2.75 A | 28.20 ± 3.22 A | 54.48 ± 5.55 A |
| 1% chamomile essence and 1% CHX | 0.40 ± 0.69 A | 5.00 ± 3.74 B | 8.40 ± 4.53 B | 20.00 ± 5.32 B | 42.66 ± 4.35 B |
Abbreviation: CHX, chlorhexidine.
a Values in a column with different capital letters (A – B) are statistically significant.
Based on Figure 1, the minimum colony count belonged to 1% chamomile essence and 1% CHX solution, while the maximum colony count belonged to 1% S. khuzestanica and 1% CHX solution with significant differences in the number of colonies (P < 0.001).
4.2. Investigation of Parameter Effects
In total, 20 experiments were carried out to assess the effects of three significant independent parameters on the antimicrobial process. The factors included the CHX concentration, chamomile essence concentration, and contact time of the irrigating solution with the external surface (Table 3). Process optimization was carried out based on the RSM. All the experiments were carried out to report the optimal conditions and assess the effects of operating parameters on the disinfection process.
| No. | Parameters | Response | ||
|---|---|---|---|---|
| A | B | C | ||
| 1 | 1 | 1 | 8 | 5 |
| 2 | 1.8 | 1 | 8 | 2.6 |
| 3 | 1.5 | 1.5 | 12 | 0.9 |
| 4 | 0.5 | 1.5 | 12 | 10 |
| 5 | 1 | 0.16 | 8 | 19.25 |
| 6 | 0.5 | 1.5 | 4 | 21.32 |
| 7 | 1.5 | 0.5 | 12 | 15.77 |
| 8 | 0.16 | 1 | 8 | 25.12 |
| 9 | 0.5 | 0.5 | 4 | 40.1 |
| 10 | 1 | 1 | 8 | 4.7 |
| 11 | 1 | 1 | 8 | 3.2 |
| 12 | 1 | 1 | 1.27283 | 20.3 |
| 13 | 1.5 | 0.5 | 4 | 12.24 |
| 14 | 1 | 1 | 14.7272 | 0.3 |
| 15 | 1 | 1 | 8 | 9.66 |
| 16 | 0.5 | 0.5 | 12 | 38.12 |
| 17 | 1 | 1 | 8 | 6.3 |
| 18 | 1 | 1.8 | 8 | 2.12 |
| 19 | 1 | 1 | 8 | 15.2 |
| 20 | 1.5 | 1.5 | 4 | 2.1 |
Abbreviations: No., number of the experiments; A, chlorhexidine concentration (0.1591% - 1.84%); B, chamomile concentration (0.1591% - 1.84%); C, contact time (1.27 - 14.73 min); Response, mean colony numbers for Enterococcus faecalis (CFU).
4.3. Effects of CHX Concentration
The CHX concentration was set as 0.15% - 1.84%. The P < 0.0001 indicated that the increasing or decreasing parameter significantly affected the final results. In general, converting the sum of squares into mean squares via dividing it by the degree of freedom (df) allowed a comparison of these ratios and showed whether there were significant differences due to the CHX concentration. The larger this ratio was, the greater the treatments affected the results. In this case, 951.45 was large enough (Table 4).
| Source | Sum of Square | df | Mean Square | F-Value | P-Value |
|---|---|---|---|---|---|
| Model | 2167.12 | 9 | 240.79 | 1648.39 | < 0.0001 a |
| A) CHX conc. | 951.45 | 1 | 951.45 | 6513.32 | < 0.0001 a |
| B) Chamomile conc. | 704.73 | 1 | 704.73 | 4824.37 | < 0.0001 a |
| C) Contact time | 137.92 | 1 | 137.92 | 944.15 | < 0.0001 a |
| AB | 55.65 | 1 | 55.65 | 380.97 | < 0.0001 a |
| AC | 36.55 | 1 | 36.55 | 250.22 | < 0.0001 |
| BC | 20.80 | 1 | 20.80 | 142.40 | < 0.0001 a |
| A2 | 166.64 | 1 | 166.64 | 1140.78 | < 0.0001 a |
| B2 | 73.05 | 1 | 73.05 | 500.08 | < 0.0001 a |
| C2 | 67.43 | 1 | 67.43 | 461.58 | < 0.0001 a |
| Residual | 1.46 | 10 | 0.1461 | ||
| Lack-of-fit | 0.4608 | 5 | 0.0922 | 0.4608 | 0.7924 b |
| Pure error | 1.0000 | 5 | 0.2000 | ||
| Cor total | 2168.58 | 19 |
Abbreviations: df, degree of freedom; Cor, corrected total of sum of squares.
a Significant.
b Not significant.
4.4. Effects of Chamomile Concentration
The chamomile concentration was reported as 0.15% - 1.84%. The P < 0.0001 showed that the increasing or decreasing parameter significantly affected the final results. The mean squares of chamomile concentration (704.73) showed good effects of this parameter on the disinfection process of primary root canals (Table 4).
4.5. Effects of Contact Time
The contact time of the irrigating solution with the external surface was reported as 1.27 - 14.73. The p values < 0.0001 revealed that the increasing or decreasing parameter significantly affected the final results. The mean squares of contact time (137.92) showed relatively good effects of this parameter on the disinfection process of primary root canals (Table 4). Values and coefficients of the parameters in the model equation indicated the effects of the parameter values and types on the response. The ANOVA results for the model for each response are presented in Table 4. The importance of each parameter was assessed using F-values and P-values. In this model, all the parameters with values less than 0.05 were significant. Lack-of-fit is another critical parameter that expresses a fraction of the sum of squares remaining, which occurred due to the inefficiency of the model.
4.6. Effects of Interactions Between Various Parameters on Disinfection Efficiency
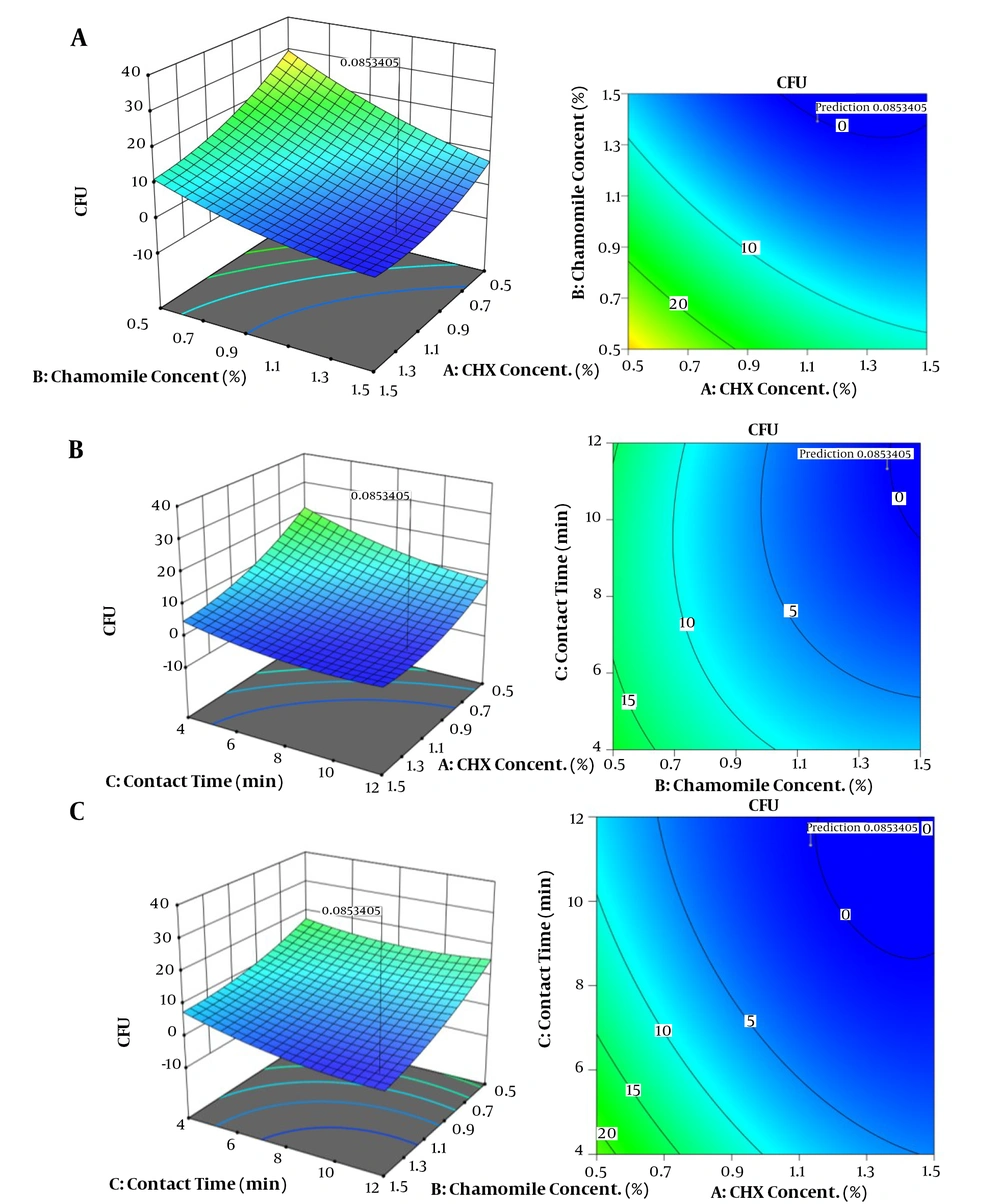
For the response, three-dimensional (3D) and contour plots were used to represent interactions between the parameters. As presented in Figure 2A, the response decreased when CHX and chamomile concentrations increased. Contour plots of the parameters verified the 3D plots with the lowest response at a CHX concentration of 1.5 and chamomile concentration of 0.5. Figure 2B shows the positive effects of chamomile concentration and contact time on responses. The contour plot of the parameters expressed the lowest response at a contact time of 12 min. Figure 2C represents 3D and contour plots of CHX concentration and contact time effects on the response. Results demonstrated that increasing reaction time and initial concentration of CHX increased disinfection efficiency.
Three-dimensional (right) and contour plots (left) for the effects of independent variables on disinfection of primary tooth root canals. A, Chlorhexidine concentration and chamomile concentration; B, Chamomile concentration and contact time, and C, Chlorhexidine concentration and contact time.
4.7. Adequacy of the Model
Statistical parameters verifying the adequacy of the quadratic model are shown in Table 5. The anticipated model was well-fitted with data in this model, as shown by excellent correlation coefficients (0.9993 for the response). The correlation between R2Adj and R2Pred was in line with the model accuracy, and their closeness to one indicated that the experimental and anticipated outcomes were in good agreement. The response included an adequate precision of 69.37 > 4, demonstrating a good signal-to-noise ratio.
| Model | R2Sqared | R2Adj | R2Pred | PRESS | SD |
|---|---|---|---|---|---|
| Quadratic (response) | 0.9993 | 0.9987 | 0.9976 | 8.50 | 0.3822 |
Abbreviations: PRESS, predicted residual sum of squares; SD, standard deviation.
4.8. Optimization of Independent Variable and Validation Experiment
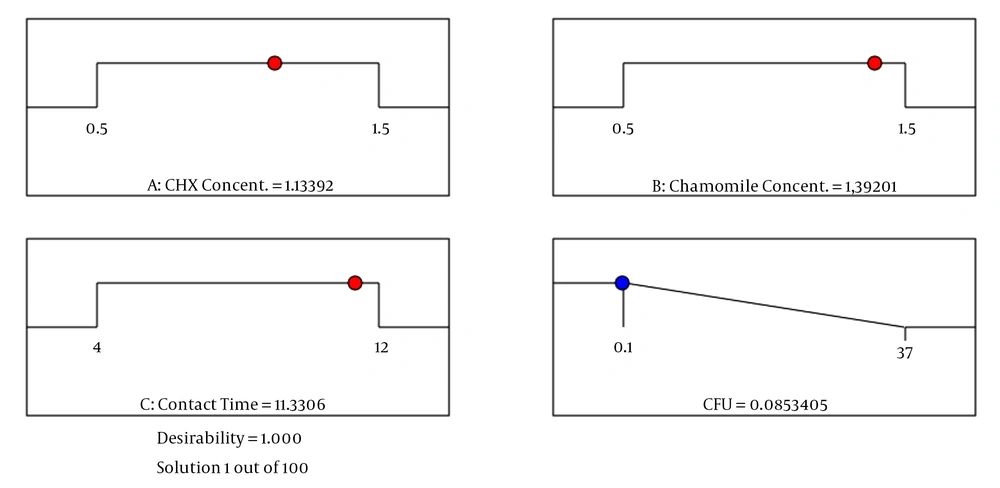
Optimization of antimicrobial parameters (initial concentration of CHX, initial concentration of chamomile, and contact time) was carried out using a numerical technique based on the predicted model and factors within their critical ranges as constraints. Independent parameters used in numerical optimization, including the concentration of CHX and concentration of chamomile, were set within the range between low (0.5) and high (1.5), while the disinfection efficiency was set to the maximum value. In optimum circumstances (CHX concentration = 1.13%, chamomile concentration = 1.39%, and contact time = 11.3 min), the antimicrobial efficiency of the primary root canals was maximum (0.083 CFU) with an overall desirability value of 1.00 (Figure 3).
5. Discussion
An optimum root canal irrigant solution must include maximal tissue dissolving and antibacterial effects while having low toxic effects. In this study, E. faecalis was used to investigate the antibacterial efficiency of root canal irrigants in vitro (10, 13). The variations and anatomical limitations in milk teeth, especially in milk molars, are further described (14). Generally, E. faecalis is one of the most resistant bacteria to disinfectants in root canals (9). Filing the root canals results in debridement and helps irrigants penetrate the apical part of the root canals more easily. Furthermore, the irrigation time can affect antimicrobial chemical activities (14). Normal saline is the most commonly used irrigant for deciduous root canals, which is not an actual disinfectant. Indeed, CHX is a broad-spectrum antimicrobial agent (15). The CHX gluconate is a tissue solvent with a broad antibacterial spectrum. The chemical has been effective against E. faecalis in deciduous teeth (14, 16). Technically, endodontic irrigants must be nontoxic to periapical tissues, which is especially critical in young patients. Overflow of irrigating solutions through the apical regions in primary teeth may harm the underlying permanent teeth due to possible resorption zones (4).
Recently, researchers have become further interested in medicinal plants as herbal medicines, which have good antimicrobial activities and natural origins (17). Gotze et al. investigated differences between the antibacterial effects of aroeira-da-praia (Schinus terebinthifolia) and quixabeira (Syderoxylum obtusifolium) and their abilities to remove smear layers compared to sodium hypochlorite. The results showed that all compounds could eliminate E. faecalis, while none could remove smear layers (18). Ghanavati Nasab et al. demonstrated that thyme essence could be similar to CHX in antimicrobial characteristics (19). However, studies have suggested that herbal irrigants should be used in further concentrated doses to compete with chemical irrigants. For example, Jieeryin (Salvadora persica) solutions with 30%, 15%, and 50% concentrations could include similar effects to those of chemical irritants (20).
In the present study, the antibacterial properties of CHX alone and mixed with chamomile and S. khuzestanica were assessed within 28 days. The 2% CHX solution had the most effective antibacterial activity in the first culture. This result agrees with that of Schafer and Bossman's study, reporting that 2% CHX solutions were more effective than less concentrated CHX solutions, with quicker E. faecalis elimination (21). In another study, Oncag et al. detected that 2% CHX gluconate had greater antibacterial effects and fewer hazards than 5.25% sodium hypochlorite (4). However, CHX and chamomile antibacterial activity increased rapidly, indicating their abilities to be adsorbed to hydroxyapatite with prolonged gradual releases at therapeutic levels. In agreement with the current study findings, Venkataram et al. demonstrated that chamomile was superior to 2.5% NaOCl alone in removing the smear layer (22). In contrast, CHX and S. khuzestanica included a small antibacterial property.
The mean CFU was statistically lower in CHX and chamomile solution than in other solutions at all experimental times, except for the first time. In the optimization step of this study, the disinfection process with CHX and chamomile solutions was carried out using CCD. To the best of the authors' knowledge, CCD was used for the first time to assess and optimize various antibacterial parameters of a chemical-herbal irrigant solution for primary root canals. These parameters included CHX concentration, chamomile concentration, and contact time. The CCD is the most commonly used fractional factorial design in response surface models (23). However, this approach results in the use of some progressive tests to assess several factors simultaneously and identify the key factors quickly (23). The correlation coefficient (R2Squared) measures how well the anticipated model fits the experimental data. It is a more accurate estimate of model efficacy than the R2Squared since it represents the df. The adequate precision in the model was 69.37, showing a good signal-to-noise ratio, and a low SD (0.38) demonstrated the high accuracy of the predicted model (23).
5.1. Conclusions
This study demonstrated that the mixture of CHX and chamomile is more effective than CHX alone and the mixture of CHX and S. khuzestanica in the enhanced disinfection of the root canal system. Besides, to the best of our knowledge, this study, for the first time, utilized a CCD to optimize the variables affecting the disinfection process, including disinfectant concentration and reaction time. Based on the results, increasing disinfectant concentration and reaction time drastically increased the efficiency. The optimum disinfection occurred at a reaction time of 11.3 min, the CHX concentration of 1.13%, and the chamomile concentration of 1.39%. This result is clinically important.