1. Background
Physical dependence and addiction are still the main dilemmas in the clinical application of opioid analgesic drugs (1). Many endeavors have been made to resolve the problem of opioid dependence by focusing on its underlying physiopathological mechanisms (2-5). In this regard, it has been demonstrated that benzodiazepine/GABA receptors (6), calcium currents (7, 8), oxidative/nitrosative stress (9, 10), and glial cell-activated neuroinflammation (11) play an essential role in the pathophysiology of opioid dependence. Accordingly, several investigations have indicated that applying naturally occurring compounds, including herbal medicines, might be worthwhile for managing dependence on opioid agents by targeting the aforementioned mechanisms (12-15).
Ganoderma lucidum, a member of the Ganodermataceae family, is a significant source of several natural medicinal mushrooms with a long history of therapeutic applications for the management of many diseases (16-18). Ganoderma lucidum has suitable therapeutical value for the isolation of new bioactive compounds. Triterpenoids and polysaccharides are the primary bioactive metabolites isolated from G. lucidum and have a vital role in its pharmacological effects (19). A 12.4 kDa immunomodulatory protein (Ling Zhi-8) have described from G. lucidum mycelia with biological activity (20). Ganoderma lucidum has also shown anti-tumor, antimicrobial, and anti-aging activities (21-23).
Several pharmacological effects have been known for G. lucidum. For instance, evidence demonstrates that G. lucidum exhibits sedative and sleep-promoting properties (24-26) through activating benzodiazepine/GABAA receptors (27). Furthermore, polysaccharides extracted from G. lucidum represented an inhibitory effect on intracellular calcium accumulation in hippocampal neurons (28). Moreover, G. lucidum extract and its major constituents, including polysaccharides and triterpenes, have an inhibitory effect against oxidative/nitrosative stress (29-31). Besides, recent studies demonstrated the inhibitory effect of G. lucidum on glial cells and inflammatory cytokines (31-33).
2. Objectives
Considering the pharmacological effects of G. lucidum on benzodiazepines/GABAA receptors, calcium currents, oxidative/nitrosative stress, and neuroinflammation and the fact that these mechanisms contribute to the induction of opioid dependence, it was hypothesized that G. lucidum might attenuate morphine dependence by targeting the aforementioned mechanisms. Thus, the present study aimed to clarify the effects of G. lucidum hydroalcoholic extract on the induction of morphine dependence and the expression of morphine abstinence syndrome in mice.
3. Methods
3.1. Animals
Adult male NMRI mice with a weight of 20 to 30 g, which were purchased from the animal house of Shahid Beheshti University of Medical Sciences (Tehran, Iran), were used in this study. The mice were housed under standard conditions at a temperature of 23 ± 2°C and a 12 h light/dark cycle. The animals were fed commercial food pellets and had free access to tap water, except during the experiments. All the procedures performed on the animals, including handling, restraining, and injecting, were carried out under the institutional guidelines for laboratory animal care and use.
3.2. Process of Drug Preparation
3.2.1. Extraction Method
The percolation route was chosen to produce a hydroalcoholic extract of G. lucidum. Hence, 50.2 g of the fine powder of the biomass was moved into a glassy percolator, 5 cm in diameter and 30 cm in height. Then, 150 mL of methanol/water (7:3) solvent was transferred to the percolator. After 24 h, its valve was opened, and the filtered extract was carried out at one droplet per second. This process was repeated another three times
3.2.2. Condensation and Dehumidification of the Extract
The extract was condensed using a rotary evaporator (Heidolph, Germany) at 40°C. The final condensed extract was dried with a lab heater (Heidolph, Germany) at 40°C. The dried extract was stored at 4°C for subsequent steps.
3.3. Drugs
Morphine sulfate and naloxone hydrochloride were purchased from Temad Co. (Iran) and Tolidaru Co. (Iran), respectively. Normal saline was used as the vehicle to solve morphine and naloxone. All the injection solutions were prepared freshly and administered through the intraperitoneal (i.p.) route (injection volume: 0.1 mL/ 20 g body weight). In the negative control group, the same volume of normal saline was administered i.p. in mice. Diazepam (Caspian Co., Iran) was used as the positive control.
3.4. Assessment of Ganoderma lucidum Effect on Morphine Dependence
A 19-day administration schedule was implemented to induce morphine dependence. First, the mice were given morphine sulfate, i.p., once daily over nine days in an increasing dose of 10, 20, and 40 mg/kg so that each dose was administered for three days. Then, the i.p. injection of morphine at 40 mg/kg was continued over the next 10 days, i.e., days 10 to 19 (34).
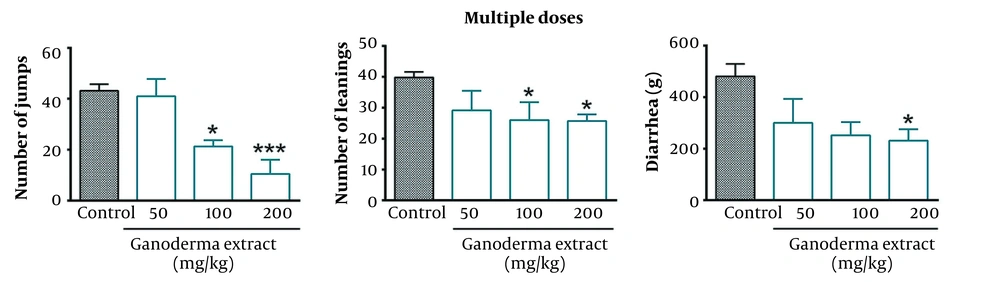
To evaluate the effect of G. lucidum on the development of morphine dependence, we divided the mice into four groups (six in each group), including one control group and three G. lucidum-treated groups. In the treatment groups, the mice received different doses of G. lucidum hydroalcoholic extract (12.5, 25, and 50 mg/kg, i.p.), while in the control group, normal saline was injected into the animals from days 10 to 18.
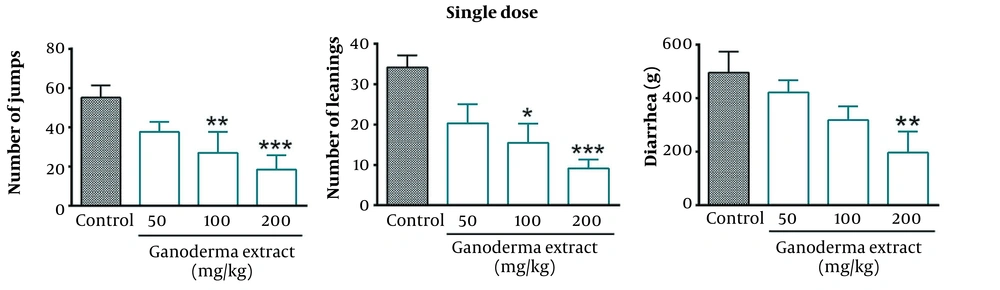
To evaluate the effect of G. lucidum on the expression of morphine dependence, we divided the mice into four groups (six in each group), including a control group and three G. lucidum-treated groups. In the treatment groups, three single doses of G. lucidum (50, 100, and 200 mg/kg, i.p.) were administered to the mice, while in the control group, normal saline was given to the animals on the 19th day.
One hour after the injection of the last dose of morphine on the 19th day, all mice received a single dose of naloxone (3 mg/kg, i.p.) and were immediately placed in a Plexiglass box. Then, the symptoms of morphine abstinence syndrome, including jumping, leaning, and diarrhea, were assessed in each mouse for 30 min (14).
3.5. Statistical Analysis
The data were reported as mean values ± SEM for six mice per group and analyzed using one-way ANOVA, followed by the multiple comparison test of Tukey. If the P-value was less than 0.05, the result was considered significant.
4. Results
4.1. Yield of Extraction
When a solid material comes in contact with a solvent, the soluble components in the solid material move into the solvent (35); thus, the final extract had a 4.51 g weight, and the efficiency of the extracting process was 8.98%.
4.2. Effects of Ganoderma lucidum on Morphine Dependence
The administration of multiple doses of morphine over 19 days resulted in a remarkable dependence in the control group's mice. This outcome was confirmed by the symptoms of morphine withdrawal syndrome, which was precipitated by injection of naloxone (3 mg/kg, i.p.) one hour after the last dose of morphine on the 19th day.
Treatment of mice with single doses of G. lucidum extract (100 and 200 mg/kg, i.p.) one hour before the injection of naloxone on the 19th day significantly decreased the number of jumps (P < 0.01 and P < 0.001, respectively) and leanings (P < 0.05 and P < 0.001, respectively). The high dose of G. lucidum extracts significantly decreased diarrhea in mice subjected to morphine dependence (P < 0.01) (Figure 1).
The repeated administration of G. lucidum extracts with doses of 25 and 50 mg/kg, i.p., for nine days significantly attenuated the number of jumps (P < 0.05 and P < 0.001, respectively) and leanings (P < 0.05) in morphine-dependent mice (Figure 2).
As the positive control, administering single or repeated diazepam doses (0.25 mg/kg, i.p.) significantly suppressed the symptoms of morphine withdrawal syndrome, including jumps, leaning, and diarrhea in morphine-dependent mice (Figures 1 and 2).
5. Discussion
The present study demonstrated for the first time that G. lucidum extracts inhibited the induction of morphine dependence and suppressed the morphine abstinence syndrome in mice. The attenuating effect of G. lucidum on withdrawal syndrome was as effective as the standard medication, diazepam, which was applied as the positive control.
In the present study, the chronic administration of increasing doses of morphine for 19 days induced apparent physical dependence in mice which was evident by the symptoms of withdrawal syndrome elicited by naloxone injection. This protocol is one of the most fundamental ways of inducing morphine dependence in mice and rats by chronically administering morphine (34, 36). Although the acute administration of morphine in a three-day injection schedule is commonly applied to induce morphine dependence (14, 15), the long administration pattern further resembles the pathophysiological process of morphine dependence. In the present study, the treatment of mice with G. lucidum for 10 days significantly attenuated the symptoms of morphine withdrawal syndrome. Furthermore, the injection of single doses of G. lucidum on day 19 considerably decreased the naloxone-precipitated morphine withdrawal syndrome. The effect of both single and multiple doses of G. lucidum on morphine dependence in mice was dose-dependent and comparable to diazepam.
Evidence indicates that G. lucidum exhibits sedative and sleep-promoting properties (24-26, 37, 38). These studies suggested that the sedative-hypnotic effects of G. lucidum might be attributed to an agonistic effect on benzodiazepine/GABAA receptors (27). It has been demonstrated that GABAergic neurons are essential in modifying opioid dependence. For instance, benzodiazepines as the positive modulators of benzodiazepine receptors and the GABAA receptor agonist, muscimol, can prevent morphine dependence and attenuate the signs and symptoms of deprivation syndrome in morphine-dependent rodents (6, 39-41). Considering the involvement of benzodiazepines and GABAA receptors in the therapeutic effects of G. lucidum, it appears that the attenuating effects of G. lucidum on morphine dependence and the prevention of morphine withdrawal syndrome are associated with its stimulatory effect on benzodiazepine/GABAA receptors. Our result is consistent with previous studies indicating that GABA-modulating medicinal herbs efficiently attenuate morphine dependence in rodents (42-44).
Furthermore, the contribution of calcium currents and the role of intracellular calcium has been confirmed in opioid dependence (7, 8, 45). Evidence indicates that polysaccharides in G. lucidum have an inhibitory effect on the accumulation of intracellular calcium in epileptic hippocampal neurons (28). Thus, it can be argued that the attenuating effect of G. lucidum on morphine dependence is exerted at least in part through its inhibitory effect against calcium accumulation inside the neurons.
Ample evidence shows that G. lucidum extract and its major constituents, particularly polysaccharides and triterpenes, possess a potent inhibitory effect against oxidative/nitrosative stress through enhancing the antioxidant enzyme activities, preventing lipid peroxidation, scavenging free radicals, and inhibiting nitric oxide (NO) production (29-31, 46-48). Evidence indicates that ethanol extract of G. lucidum increases cellular antioxidant potential via activation of Nrf2 (49). Phytochemical analysis has shown ganoderic acids A, B, C, and D, ganodermanontriol, lucidenic acid B, and fatty acid amides (oleamide, hexadecanamide, and 9-oxo-10 Octadecadienoic acid) are major chemicals that function as antioxidant agents in G. lucidum extracts (47). Several studies demonstrated that oxidative and nitrosative stress is vital in the induction and development of opioid dependence (9, 10, 50). Align with these reports, it has been demonstrated that antioxidant medications, including medicinal herbs, have a promising effect in treating opioid dependence (51, 52). Accordingly, it seems that the suppressive effect of G. lucidum on morphine dependence partly refers back to its inhibitory effects on oxidative/nitrosative stress.
Recently, Torkzadeh-Mahani et al. described the contribution of morphine to glial cell-activated neuroinflammation in the CNS, which may result in morphine dependence (53). Also, Zhang et al. argued that activated astrocytes and microglial cells could promote mechanisms that underlie opioid dependence and addiction (54). There is evidence indicating that inflammatory cytokines secreted from astrocytes and microglial cells induce the process of central sensitization and consequently reduce the therapeutic effects of morphine (55). The inflammatory cytokines seem essential in acquiring physical dependence on morphine and morphine deprivation syndrome (11). Based on the importance of glial cell-activated neuroinflammation in eliciting opioid dependence, it is conceivable to accept that the inhibition of neuroinflammation in the CNS through suppressing activated glial cells and inhibiting inflammatory cytokines is a feasible strategy for attenuating opioid dependence. In this regard, several studies demonstrated the anti-inflammatory effect of G. lucidum (33) and confirmed that it has an inhibitory effect on astrocytes and microglial cells, as well as inflammatory cytokines (31, 32, 56). Ethnopharmacological studies showed that triterpenoids and steroids, including ganoderic acid C and 3-oxo-5α-lanosta-8,24-dien-21-oic acid, are the main components of G. lucidum with anti-inflammatory properties (57, 58). Thus, it appears that G. lucidum can attenuate morphine dependence, at least in part, by inhibiting glial cells and inflammatory cytokines in the CNS.
Recently, it has been reported that G. lucidum extracts attenuated memory impairment induced by morphine and diminished conditioned place preference score in morphine-addicted mice (59). The present study, as many research works discussed earlier, deals with the therapeutic aspect of G. lucidum on morphine dependence by applying behavioral experiments in mice. However, the molecular mechanisms of G. lucidum action on morphine dependence should be further elucidated in future studies.
5.1. Conclusions
Overall, it is concluded that G. lucidum extract attenuates the induction of morphine dependence and inhibits the withdrawal syndrome symptoms in mice.