1. Background
Mercury (Hg), as a naturally occurring heavy metal, has a wide variety of industrial applications, such as barometers, thermometers, and dental fillings. Consequently, exposure and environmental release of this element may typically occur (1). According to the agency for toxic substances and disease registry (ATSDR), Hg is the third most dangerous heavy metal in the world (2). Exposure to Hg is highly hazardous, especially in regions with high levels of pollution in air, water, and food. In addition, Hg exerts negative impacts on the reproductive system of male rats (3). Inorganic Hg compounds (I-Hg) can be found as salts in monovalent or divalent forms (4).
The mechanism of tissue damage, caused by mercuric chloride (HgCl2), is associated with the promotion of oxidative stress as the main factor of tissue damage. In fact, HgCl2 enhances the formation of reactive oxygen species (ROS, including hydrogen peroxides, lipid peroxides, and highly reactive hydroxyl radicals) (5), which may cause cell membrane damage and cell destruction (6). HgCl2 depletes protective antioxidants (eg, glutathione) and inhibits the activities of free radical scavenging systems, including catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx) (7-9).
Previous studies have shown that the livers are the most affected organs by HgCl2 exposure (10, 11). Oxidative stress induced by high levels of exposure to HgCl2 has an important contribution to the molecular mechanism and pathogenesis of liver damage (12, 13). The molecular mechanisms responsible for tissue damage by HgCl2 have not been completely clarified. However, suggestions on the type of damage in HgCl2-induced cellular toxicity are as follows: interference with antioxidant enzymes, alterations in endogenous thiol-containing molecules, and enhanced lipid peroxidation (14).
Growing evidence suggests that endogenous antioxidants (eg, vitamins, trace minerals, antioxidant enzymes, tripeptides, and reductants) protect cellular homeostasis against oxidative disruption via reactive molecules, generated through molecular oxygen reduction (15). Several studies have revealed that phenolics possess radical scavenging properties. The antioxidant (radical scavenging) activity of phenolics is mainly attributed to their potent ability to scavenge ROS (eg, superoxide anions, hydrogen peroxides, hydroxyl radicals, and hypochlorous acids) (16, 17).
Gallic acid (3, 4, 5-trihydroxybenzoic acid, GA) exhibits the most important antioxidant activities due to its radical scavenging capacity (18). Green tea, walnut, grapes, berries, mango, and some other fruits and vegetables are rich sources of natural gallic acid derivatives (19). Furthermore, GA is generally described as an excellent free radical scavenger and an inducer of programmed cell death (apoptosis) in tumor cells (1). Given this antioxidant effect, GA-containing plant extracts show antiviral, antifungal, and anticancer effects and reduce oxidative liver and kidney damage (2, 4).
2. Objectives
In the current study, we examined the protective role of oral gallic acid against HgCl2-induced oxidative stress in the liver tissues of rats.
3. Methods
3.1. Chemicals
GA, HgCl2, thiobarbituric acid (TBA), 5, 5-dithiobis (2-nitrobenzoic acid, DTNB), reduced glutathione (GSH) (20), trichloroacetic acid (TCA), bovine serum albumin (BSA), and Bradford reagent were purchased from Sigma-Aldrich chemical company (St. Louis, MO, USA). All used chemicals and reagents were of analytical grade.
3.2. Animals
Adult male Wistar rats (weight, 180 - 200 g) were obtained from the animal house of Ahvaz Jundishapur University of Medical Sciences. The rats were kept in polypropylene cages and received standard rat chow and water ad libitum. The animals were maintained in a 12-hour light/dark cycle at controlled temperature (20 ± 2°C). The study was performed according to the guidelines for the use of experimental animals, issued by the animal ethics committee of Ahvaz Jundishapur University of Medical Sciences.
3.3. Experimental Design
The animals were randomly divided into 5 experimental groups (7 rats per group). Group 1 received normal saline (2 mL/kg) for 28 days, while group 2 received HgCl2 (0.4 mg/kg) for 28 days. Groups 3 and 4 received GA at doses of 50 and 200 mg/kg body weight, respectively. One hour before treatment with GA, groups 3 and 4 received HgCl2 (0.4 mg/kg for 28 consecutive days). Group 5 received only GA (200 mg/kg) for 28 consecutive days. All the administrations were performed through oral gavage. The doses of HgCl2 and GA were selected based on previous studies (4, 21).
3.4. Sample Collection
Twenty-four hours after the final administration, the animals were anaesthetized with a combination of ketamine and xylazine (60/6 mg/kg, ip), and blood samples were collected from the jugular vein. The serum was separated via centrifugation for 10 minutes at 3000 rpm and stored at -20°C until further analysis. The animals were sacrificed by decapitation. Next, their liver tissues were isolated and quickly washed with saline. For histological studies, a part of the liver tissue was fixed in 10% phosphate-buffered formalin. For biochemical estimations, the second part of the liver tissue was homogenized (1/10 w/v) in ice-cold tris-HCl buffer (0.1 M; pH, 7.4). The protein content of the homogenates was measured by the Bradford method (22), using crystalline BSA as the standard.
3.5. Serum Analysis
The colorimetric analysis of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) (23) was performed through measuring the amount of pyruvate or oxaloacetate, produced by the formation of 2, 4-dinitrophenylhydrazine, according to the method proposed by Reitman and Frankel (24). Moreover, alkaline phosphatase (ALP) was assayed using the kits provided by Randox Laboratories Co. (Antrim, UK), according to the methods described by Belfield and Goldberg (25).
3.6. Oxidative Stress Markers
3.6.1. Lipid Peroxidation Assay
Lipid peroxidation was determined by measuring the amount of malondialdehyde (MDA) via thiobarbituric acid (TBA) color reaction, using the method described by Buege and Aust (26). In brief, 0.5 mL of the homogenate was mixed with 2.5 mL of TCA (10%, w/v), and the samples were centrifuged at 3000 rpm for 10 minutes. Afterwards, 2 mL of each supernatant sample was transferred to a test tube, containing 1 mL of TBA solution (0.67%, w/v). The mixture was kept in boiling water for 10 minutes until forming a pink-colored solution. The mixture was then cooled down immediately, and absorbance was measured at 532 nm by a spectrophotometer (UV-1650 PC, Shimadzu, Japan). The concentration of MDA was calculated based on the absorbance coefficient of TBA-MDA complex (ε, 1.56 × 105 cm-1.M-1).
3.6.2. GSH Assay
The level of GSH in the tissue homogenate was measured, using the method expounded by Ellman (2), based on the formation of a yellow-colored complex with the Ellman’s reagent (DTNB). The homogenates were immediately precipitated with 0.1 mL of 25% TCA, and the precipitate was removed after centrifugation. Free endogenous GSH was assayed in a 3-mL glass by adding 2 mL of 0.5 mM DTNB (prepared in 0.2 M phosphate buffer; pH, 8) to 0.1 mL of the supernatant. Then, the developed yellow color was read at 412 nm using a spectrophotometer (UV-1650 PC, Shimadzu, Japan). The standard curve was plotted over a GSH concentration range of 1 - 10 µM. The GSH content was expressed as nmol/mg protein.
3.7. Enzymatic Antioxidant Status
3.7.1. CAT Activity Assay
CAT activity in tissues was assayed using the procedure described by Aebi (27). In a cuvette containing 200 µL of phosphate buffer and 50 µL of tissue supernatant (obtained after centrifugation of tissue homogenate at 12000 g for 20 minutes at 4°C), 250 µL of 0.066 M H2O2 was added, and reduction in optical density (OD) was measured at 240 nm for 60 seconds. It should be noted that 1 unit of activity is equal to the degraded moles of H2O2 per minute, divided by the milligrams of protein in the tissue supernatant. The molar extinction coefficient of 43.6 M-1.cm-1 was used to determine CAT activity.
3.7.2. SOD Activity
The tissue supernatant was obtained after centrifugation at 12000 g for 20 minutes at 4°C. Spectrophotometric measurements were performed by calculating the inhibition rate of hematoxylin autoxidation for the SOD assay, according to the method described by Martin (28); it was expressed as unit/mg protein.
3.7.3. GPx Assay
The GPx activity was measured with the GPx kit (Randox Labs, Crumlin, UK).
3.8. Histopathological Examination
For the histological examination, a small section of the liver was fixed in 10% phosphate-buffered formalin, embedded in paraffin. Afterwards, it was sectioned at 5-µm thickness and stained with hematoxylin and eosin (H and E) for light microscopic observations. In each section, 25 fields were analyzed.
3.9. Statistical Analysis
The results are reported as mean ± SD. The statistical analyses were conducted using one-way analysis of variance in SPSS v. 20. The group differences were calculated using Tukey’s post hoc test. For all the tests, P value < 0.05 was considered statistically significant.
4. Results
4.1. Effects of GA and HgCl2 on Serum Markers
The results showed that the serum AST, ALT, and ALP levels in the HgCl2-treated group significantly increased, compared to the control group. Pretreatment with GA (50 and 200 mg/kg) significantly decreased the levels of these markers, compared to the HgCl2 group (P < 0.05) (Table 1).
4.2. Effects of GA and HgCl2 on MDA and GSH Levels
The results revealed a significant rise in the liver MDA level of rats, exposed to HgCl2 (an index of lipid peroxidation), compared to the control group (P < 0.05). As presented in Table 2, a significant decrease in MDA level was observed in livers pretreated with 200 mg/kg of GA. The GSH level remarkably decreased in the liver of rats, exposed to HgCl2. Pretreatment with GA (200 mg/kg) significantly inhibited the HgCl2-induced reduction in GSH content. Moreover, there was no significant change in MDA and GSH levels in the group only receiving GA (200 mg/kg).
4.3. Effects of GA and HgCl2 on Enzymatic Antioxidant Status
As presented in Table 3, HgCl2 significantly decreased SOD, CAT, and GPx activities, compared to the control group (P < 0.05). Pretreatment with GA at doses of 50 and 200 mg/kg significantly increased SOD, CAT, and GPx activities in comparison with the HgCl2 group (P < 0.05). There was no significant change in SOD, CAT, and GPx activities in the liver tissues of rats, which only received GA (200 mg/kg).
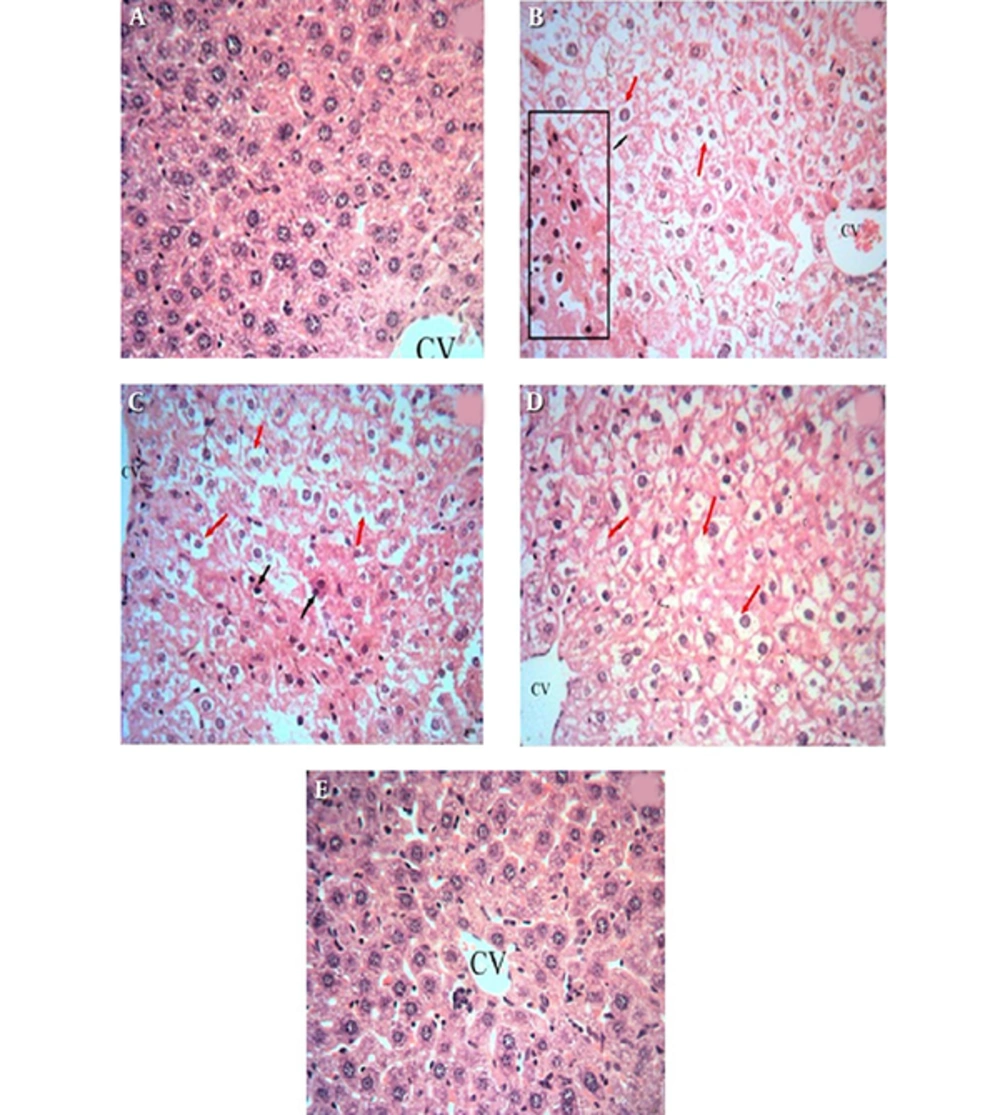
4.4. Light Microscopic Findings
The histopathological results are presented in Figure 1. There was no abnormality or histological changes in the livers of the control and GA (200 mg/kg) groups (Figures 1A and 1E). In the groups treated with HgCl2 and GA 50 mg/kg plus HgCl2 (Figures 1B and 1C), some hepatocytes indicated necrosis and disappearance/disarrangement of hepatic lobule structures. Furthermore, changes in the central vein exhibited congestion with hemorrhagic blood. Figure 1D demonstrates the effects of simultaneous administration of GA 200 mg/kg and HgCl2. The high dose of GA (200 mg/kg) resulted in the protection of liver tissues against the damage induced by HgCl2 in comparison with the control group. The liver tissues of the GA group showed a normal histological structure (Figure 1E).
Histological changes in the liver of rats: A, a control liver with a normal architecture; B, rats treated with HgCl2 with prominent inflammation and hepatocyte vacuolation; C, rats treated with GA (50 mg/kg) and HgCl2, with hepatocytes showing necrosis and disappearance/disarrangement of hepatic lobule structures; D, rats treated with GA (200 mg/kg) and HgCl2, which reversed all abnormalities induced by HgCl2; and E, rats treated with GA (200 mg/kg). The liver sections were analyzed via H and E staining (magnification, × 400).
5. Discussion
Hg, as a ubiquitous pollutant, is the third most dangerous heavy metal, derived from both natural sources and human activities (29). Accidental and occupational Hg exposure may result in the toxicity of livers as vital organs of waste excretion. Accumulation of Hg leads to various adverse changes and induces toxicity in tissues (30). Previous research shows that HgCl2 intoxication leads to free radical formation and oxidative stress, implicated in the cardiovascular pathogenesis affecting livers, kidneys, and lungs.
HgCl2 bonds with GSH were once identified in the cells. In the present experimental study, the levels of antioxidant properties reduced in the liver of Hg-intoxicated rats. The level of GSH, which is the primary line of cellular protection against toxic agents and major intracellular conjugation factors, reduced and displayed damaged function in Hg toxicity (31, 32). Binding of HgCl2 to GSH reduces GSH level in the cells and decreases the antioxidant capacity of the cells (33).
Numerous studies have recommended that antioxidant nutrients and/or medicines play a protective role in human health (34). The main purpose of the current study was to assess the protective effect of gallic acid on liver toxicity induced by HgCl2 as a result of reduced activities of antioxidant enzymes in the livers of rats. In addition, the GSH content increases the susceptibility of rats to oxidative stress. In the present study, HgCl2-treated rats were exposed to some morphological changes in the livers, including congestion in blood sinusoids, degeneration, vacuolation, and loss of the structural pattern of hepatic tissues in the liver (Figure 1).
The HgCl2-induced increase in the serum AST, ALT, and ALP levels was related to hepatic structural damage, since these enzymes, as important markers of hepatocellular damage, are usually localized in the cytoplasm. Eventually, they are released into circulation after the occurrence of cellular damage (35). This hepatic damage is in accordance with the cellular damage and loss of structural pattern in the liver tissues of HgCl2-treated groups (Figure 1). The results of previous studies support our findings, indicating the increase in liver enzymes. According to these studies, inorganic Hg causes hepatotoxicity (4, 32, 36, 37). All these changes on liver function tests in HgCl2-treated animals substantiated evidence on hepatotoxicity induced by inorganic Hg.
The present study showed that oral administration of GA significantly reduced the HgCl2-induced serum ALT, AST, and ALP levels as hepatic enzyme markers (Table 1). Furthermore, GA dramatically improved Hg-induced liver damage, as evidenced by the restored, almost normal architecture of hepatocytes, hepatic lobules, and portal tracts (Figure 1D). These findings are similar to some previous reports, which showed that GA reversed the increase in serum enzymes in lindane-, sodium fluoride-, carbon tetrachloride-, and ferulic acid-induced liver damage (38-40).
Different ROS species (•OH, O2-, RO, ROO, and NO) play a critical role in boosting chemical-induced cellular damage in liver tissues. The induced oxidative stress due to ROS may lead to the initiation and progression of some diseases, such as cardiovascular diseases, diabetes, and neurodegenerative disorders (38). The human body is equipped with defense mechanisms against free-radical damage, induced by nonenzymatic antioxidants (eg, GSH) (20) and endogenous antioxidant enzymes (eg, GPx, SOD, and CAT) (41). Therefore, high levels of ROS or any disturbance in the oxidant-antioxidant status can result in oxidative damage to macromolecules (eg, DNAs, proteins, and lipids), tissues, or organs (42).
In view of the presented results, the GSH level and antioxidant enzyme (SOD, CAT and GPx) activities significantly decreased in the liver tissues of HgCl2-treated rats in comparison to the control group (P < 0.05). This finding indicated that HgCl2 could cause severe oxidative stress. These results are parallel to several previous studies, which reported the significant depletion of GSH, as well as a significant decrease in the activities of SOD, CAT, and GPx after HgCl2 intoxication, corroborating the oxidative stress status (4, 37, 43, 44).
MDA is one of the most common markers of lipid peroxidation. Lipid peroxidation is a well-known mechanism of cellular damage in the human body. MDA is an extremely reactive 3-carbon dialdehyde and the main oxidative product of unsaturated fatty acids in the membranes with toxic attributes. High quantities of MDA have been attributed to different disorders in humans (45). Since HgCl2 toxicity produces reactive oxygen metabolites in many tissues, measurement of MDA level in the livers can be valuable in the diagnosis of hepatotoxicity, induced by HgCl2 (19).
The present investigation showed that 1 week after intoxication of rats by HgCl2, the MDA levels in liver tissues significantly increased, compared to the control rats. Consequently, oxidative stress may be one of the main contributing causes of Hg-induced disorders in organs. Moreover, some previous studies have reported improved levels of ROS due to HgCl2 exposure. Therefore, ROS attacks almost all the cell components (eg, membrane lipids) and increases the MDA level due to lipid peroxidation (4, 10, 46).
The findings of this study indicated the significant protective effects of GA against HgCl2-induced oxidative stress. It was revealed that GA facilitates the reduction of oxidative damage. In the present study, GA administration resulted in the increased level of GSH in liver tissues and attenuated the decline in GSH content, induced by HgCl2. It should be noted that the obtained data on GA are in line with earlier published reports (39).
GA administration to HgCl2-treated rats significantly increased SOD and CAT activities, which could be attributed to the free radical scavenging and antioxidant properties of GA. These results are compatible with the findings of another study, which showed that GA decreased SOD and CAT activities in sodium fluoride-induced damage in experimental rats (38). Furthermore, our results demonstrated that GA supplementation for HgCl2-treated rats increased the concentration of GPx. An increase in intracellular GPx level, induced by GA, has been reported in earlier research (39).
Simultaneously, oral administration of GA significantly decreased the hepatic MDA level in group 4, compared to the HgCl2 group (P < 0.05). These results on GA may explain protection against pathological changes in the liver of rats, induced by HgCl2. In this regard, similar results have been reported by other researchers, indicating that GA diminishes the formation of MDA (39, 47).
The literature review demonstrated that GA antioxidant properties, which were verified in this study, are attributed to their ability to scavenge ROS (eg, hydroxyl radicals, hydrogen peroxides, and superoxide anions) in the liver of rats (48, 49). The GA molecular structure contained trihydroxyl groups, which validated an earlier report, showing that phenolic hydroxyl groups are very important in producing a strong radical-scavenging effect.
The hydroxyl group in the para form to the carboxylic group can particularly affect the antioxidant activity of GA (50). The results of our study showed that the antioxidant attributes may be accountable for the liver protective effects of GA. This finding is in compliance with earlier surveys, showing that GA protects the cells via reducing the generation of free radicals (39, 47, 50, 51).
The results of this study suggested that oral gallic acid could protect liver tissues against oxidative damage, induced by HgCl2 through modifying antioxidant enzyme activities and nonenzymatic antioxidant levels. GA can reverse HgCl2-induced oxidative stress in liver tissues due to its antioxidant potential and capacity to improve the antioxidant status. In addition, the findings confirmed its effective protective application against pathological changes, induced by HgCl2 in the liver of rats. Based on these findings, it can be concluded that GA is a promising candidate for the management of liver intoxication.