1. Background
Pharmaceutical products in all forms are prone to contamination with various microorganisms. Microbial contamination leads to the decomposition of the products or, in the case of pathogens, is considered a threat to consumers' health. Such occurrences may cause severe economic losses to the manufacturer. Therefore, the microbiological safety of the drug has always been the focus of the pharmaceutical industry. To this end, antimicrobial preservatives are included in the formulation of pharmaceutical products to prevent microbial decomposition of the products and increase their lifespan (1). A recent approach for preserving pharmaceutical products is to avoid synthetic preservatives, which can create undesirable reactions in people sensitive to a particular additive or may increase the risk of cancer due to the adverse effects of some preservatives (2).
Benzalkonium chloride (BKC) is an antimicrobial agent commonly used as a preservative in nasal sprays. According to the World Health Organization (WHO) reports, using BKC in the form of inhalation leads to obstructive bronchitis, followed by pneumonia (3). According to the studies conducted on the effects of the drugs containing BKC in mouse nasal mucosa, this preservative caused mucosal swelling, clogged arteries, and increased mononucleosis. Thus, the adverse effects derived from preservatives are not only limited to allergic reactions. Identifying their side effects is very difficult as most of them occur dilatory or in a specific weak way. Therefore, the longtime consumption of preservatives should be avoided; otherwise, an alternative with lower toxicity than common chemical preservatives should be found (4).
Recently, studies on using essential oils from medicinal plants as antimicrobial agents have increased due to their wide range of activities, natural origin, and generally their safe status. Furthermore, it was found that essential oils often have antifungal, anti-parasitic, antibacterial, and antiviral properties (5, 6). The antimicrobial effect of the components of the essential oils is related to the lipophilicity property of hydrocarbons and the hydrophilicity of their main functional groups (7). Eucalyptus is one of the most diverse flowering plants in the world and belongs to the family of Myrtaceae. Eucalyptus is indigenous to Australia and Tasmania and has spread worldwide (8, 9). Eucalyptus species are considered as pharmaceutical plants because of their biological and therapeutic properties; however, Eucalyptus globulus has been introduced as the most important and original species of Eucalyptus in international pharmacopeia (10). Previous antimicrobial studies have confirmed the therapeutic value of E. globulus and have supported its usage in traditional medicine (10-13). In addition, as secondary plant metabolites, E. globulus oils present huge possibilities as natural preservatives in the perfume and food industries.
However, no study has been done on using Eucalyptus essential oil in nasal sprays as an antimicrobial preservative. Thus, fluticasone propionate nasal spray, an inhaled and topical anti-inflammatory medicine, has been considered a pharmaceutical model.
2. Objectives
The E. globulus essential oil was studied alone and in combination with BKC as an antimicrobial preservative to mitigate the harmful effects of chemical preservatives.
3. Methods
3.1. Microorganisms and Media
The microorganisms used in the current research were obtained from the Iranian (Persian) Type Culture Collection, and they are as follows: Staphylococcus aureus (PTCC 1112), Escherichia coli (PTCC 1330), Pseudomonas aeruginosa (PTCC 1074), Candida albicans (PTCC 5027), and Aspergillus brasiliensis (PTCC 5011). Tryptone Soya Agar (TSA; Merck, Darmstadt, Germany) was inoculated with bacteria and was incubated for 24 h at 37°C. Candida albicans and A. brasiliensis were grown on Sabouraud Dextrose Agar (SDA; Merck, Darmstadt, Germany) at 25°C for 48 h and 5 d, respectively. To harvest A. brasiliensis culture, a sterile saline test solution containing 0.05% (v/v) of tween 80 was applied.
3.2. Essential Oil
Commercially available E. globulus essential oil (EGO) (Zardband pharmaceutical company, Tehran, Iran) was utilized in this experiment.
3.3. Manufacturing of Nasal Spray
The 4500 mL fluticasone propionate nasal spray was prepared with raw materials mentioned in Table 1, according to the Iran Avandfar company's instruction. The challenge test was conducted in six 750 mL preservation conditions: spray preserved with EGO (0.9% (v/v)), spray preserved with EGO (0.675% (v/v)) and BKC (0.005% (v/v)), spray preserved with EGO (0.45% (v/v)) and BKC (0.01 % (v/v)), spray preserved with EGO (0.225% (v/v)) and BKC (0.015% (v/v)), spray preserved with BKC (0.02 % v/v) (Carloerba, Italy) (commercially available sample), and spray without any preservative (negative control).
| Suspension | Proportion (%) |
|---|---|
| Aqueous phase | |
| Fluticasone propionate (FARMA BIOS, Germany) | 0.05 |
| Vivapur (JRS FARMA, Germany) | 1.2 |
| Tween 80 (Merck, Darmstadt, Germany) | 0.02 |
| Dextrose (Scharlau, Spain) | 5 |
| H2O | Up to 100 |
| pH | 6.0 |
3.4. Antimicrobial Activity of EGO
The antimicrobial activity of EGO was evaluated by broth micro-dilution method according to the Clinical and Laboratory Standards Institute (CLSI; 2016) procedure (14). Initially, 100 µL of Mueller Hinton Broth (MHB) medium was transferred to the wells of a microtiter plate, and then EGO concentrations ranging from 17.5 - 0.034 mg/mL were prepared in each row. Ethanol was used to disperse EGO. Each test microorganisms with a standard concentration of 0.5 McFarland was inoculated into each well to reach the final concentration of 105 CFU/mL or 105 spores/mL. MHB without EGO and wells containing ethanol and tween 80 were considered as control. Microtiter plates were incubated for 24 - 72 h at the appropriate temperature for each test microorganism, as mentioned above. The lowest concentration that prevented microbial growth was considered minimum inhibitory concentration (MIC). The experiment was performed in triplicate for all microorganisms.
3.5. Stability Evaluation of Nasal Spray Formulation
3.5.1. Physical Analysis
The color and the texture of sprays were checked by transferring 10 mL of each preservation condition (stored at 40°C and RH: NMT 25 %) into transparent containers. These tests were performed along with pH measurement at the manufacturing time of nasal spray (at zero time) and after that for three and six months (15).
3.5.2. Microbial Control
The microbial load of nasal spray base (without preservative) and nasal sprays of the above-mentioned preservation conditions were evaluated following the American Pharmacopoeia (USP 41) (15). Briefly, the serial dilutions of the product were prepared, and each dilution was transferred into TSA and SDA by conventional plate count, including pour plate and surface spread plate methods for bacteria and mycetes, respectively. TSA plates were incubated at 30 - 35°C for three days, and the SDA plates were incubated at 20 - 25°C for five days. The bacterial load of < 102 CFU/mL and the fungal load of < 10 CFU/mL are acceptable according to the limitations recommended by American Pharmacopoeia.
3.6. Evaluation of Preservative Effectiveness
3.6.1. Challenge Test
The antimicrobial preservative efficacy test was performed following the US Pharmacopoeia (USP 40, 2017), using standard microbial strains (15). Each preservative formulation (samples of 50 mL) was transferred into sterile containers and individually inoculated with each microbial test suspension to reach the final density of 105 - 106 CFU/mL or 105 - 106 spores/mL. The trial was set up in triplicate, and the inoculated samples were incubated at 25 or 37 °C according to the type of test microorganisms. Over time intervals of 0, 1, 7, 14, 21, and 28 days, the samples were taken and neutralized using Casein-peptone lecithin polysorbate broth (Merck, Darmstadt, Germany). Then, microbial colonies on TSA and SDA were counted after 18 - 24 h and 2-5 days of incubation at 37 and 25°C for bacteria and mycetes, respectively. The average log10 colony-forming units per milliliter (CFU/mL) were used to quantify the results. As per USP 40 (2017), the antimicrobial effectiveness of a preservative in a nasal spray is confirmed if a 2 log reduction is observed in bacterial count per milliliter on day 14 of the challenge compared to the initial count with no subsequent increase of cell number on day 14 to day 28. Concerning yeast and mycetes, the number of CFU per milliliter at 14 and 28 days of challenge should not be exceeded the initial count.
3.7. Statistical Analysis
All outputs were represented by their mean value and standard deviation (mean ± SD). Statistical analysis was done using SPSS 21.0 software (SPSS, Chicago, IL). The repeated measure and one-way analysis of variance (ANOVA) were used to analyze the results. Besides, for multiple comparisons among preservation conditions means, Tukey’s tests were applied. All experiments were carried out in triplicate, and the statistical significance level of P < 0.05 was considered.
4. Results
4.1. Antimicrobial Activity of EGO In Vitro
According to the broth microdilution method results, the MIC value of EGO for S. aureus, E. coli, P. aeruginosa, and A. brasiliensis was equal to 9 mg/mL and for C. albicans was 4.4 mg/mL.
4.2. Stability Study of Nasal Spray Formulation
Evaluation of the color and texture of nasal spray base and preserved nasal sprays did not show any visual appearance at the time of preparation, and after three and six months of storage at 40°C, RH: NMT 25 %, and their colors were recorded as white. On day zero and the third and sixth months of the stability study, pH values for all nasal sprays obtained were 6, 5.3, and 6.3, respectively, not statistically different (P < 0.05). Moreover, current research preservation conditions' microbial control of nasal sprays indicated that the total viable count of aerobic mesophilic microorganisms on zero-day and following three and six months, compared to the nasal spray base, was less than 10 CFU/mL. Moreover, P. aeruginosa, S. aureus, and C. albicans have not been detected in the formulations (Table 2).
| Products (Fluticasone Propionate Nasal Spray) | Microbial Count (CFU/mL) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zero Time | 3 Months | 6 Months | ||||||||||
| TAMC | TYMC | Staphylococcus aureus | Pseudomonas aeruginosa | TAMC | TYMC | S. aureus | P. aeruginosa | TAMC | TYMC | S. aureus | P. aeruginosa | |
| Preserved with BKC (0.02 %) | < 10 | < 10 | 0 | 0 | < 10 | < 10 | 0 | 0 | < 10 | < 10 | 0 | 0 |
| Preserved with combination of BKC/GEO a | < 10 | < 10 | 0 | 0 | <10 | < 10 | 0 | 0 | < 10 | < 10 | 0 | 0 |
| Preserved with GEO (0.9 %) | < 10 | < 10 | 0 | 0 | 10 | < 10 | 0 | 0 | < 10 | < 10 | 0 | 0 |
| Preservative free (nasal spray base) | 4 × 10 | < 10 | 0 | 0 | 20 × 102 | 10 | 0 | 0 | 2.8 × 103 | 6 × 10 | 0 | 0 |
Abbreviations: TAMC, total aerobic mesophilic count; TYMC, total yeast/mold count (total mycete count).
a All three studied combination concentrations of BKC/EGO.
4.3. Preservative Effectiveness Testing
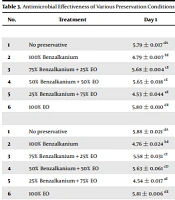
The results of the microbial challenge tests are illustrated in Table 3 (A-E). The primary S. aureus, E. coli, and P. aeruginosa counts at day zero were about 5.86, 5.85, and 5.89 log CFU/mL, respectively, for all preserved and unpreserved formulations. Based on the findings of the bacterial challenge test, in the preservative-free nasal spray, an incremental trend in the bacterial number was observed up to day 14; however, there was no statistical difference (P > 0.05), and this trend was maintained until the end of the study. In nasal spray preserved with a commonly used synthetic preservative, BKC (100%), the initial counts of S. aureus, E. coli, and P. aeruginosa reached 2.64, 2.72, and 2.84 log CFU/mL, respectively, within 14 days of challenge, and this considerable decrease continued up to day 28. By introducing EGO as a sole preservative (100%) in the current formulation, S. aureus and E. coli primitive counts attained 2.70 log CFU/mL and 2.74 log CFU/mL, respectively, after 14 days of storage, while P. aeruginosa level reached 4.80 log CFU/mL, which was not compliant with the criteria of the USP 40 (2017). Coexistence of BKC / EGO at the ratios of 75%: 25% and 50%:50% in nasal spray decreased the levels of S. aureus to 2.52 and 2.58 log CFU/mL, E. coli to 3.58 and 3.57 log CFU/mL, and P. aeruginosa to 4.82 and 4.73 log CFU/mL, respectively, by day 14. These ratios were insufficient for P. aeruginosa, although the subsequent reduction occurred after this period. Adding both BKC and EGO at a ratio of 25%:75% in nasal spray formulation represented the most prominent reduction in bacterial levels compared with the other preservation conditions so that the primary counts of S. aureus, E. coli, and P. aeruginosa reached 2.49, 2.40, and 2.41 log CFU/mL on day 14 and 1.33, 1.44, and 1.67 log CFU/mL by day 28, respectively.
Concerning the fungal challenge test findings, the primitive number of C. albicans (5.86 log CFU/mL) and A. brasiliensis (5.90 log CFU/mL) in preservative-free nasal spray increased over the 28 days of storage but were not significantly different (P > 0.05) and therefore, did not meet the USP40 (2017) requirements. In contrast, in BKC preserved spray, the initial numbers of C. albicans and A. brasiliensis reached 2.71 and 3.68 log CFU/mL after 14 days of challenge test and further decreased to 1.69 and 1.60 CFU/mL, respectively, on the 28th day. In the case of nasal spray preserved with EGO alone, the mean values of 3.64 log CFU/mL for C. albicans and 3.65 log CFU/mL for A. brasiliensis were recorded on day 14, and these values steadily decreased until the end of the study. The mean values of mycetes, 14 days after exposure to 75:25 and 50:50 ratios of BKC/EGO combination in nasal spray were determined as follows; 2.55 and 2.70 log CFU/mL for C. albicans and 2.58 and 2.65 log CFU/mL for A. brasiliensis and they continuously decreased by day 28. Nasal spray incorporated with a 25:75 combination of BKC and EGO represented the values of 2.38 log CFU/mL and 2.56 log CFU/mL on the 14th day and 1.36 and 1.38 log CFU/mL on the 28th day for C. albicans and A. brasiliensis, respectively. Comparing the preservation conditions mentioned above, the spray preserved with a 25:75 combination of BKC and EGO and the spray preserved with BKC alone revealed the highest preserving efficacy against the studied mycetes.
| No. | Treatment | Day 1 | Day 7 | Day 14 | Day 21 | Day 28 |
|---|---|---|---|---|---|---|
| (A) Staphylococcus aureus | ||||||
| 1 | No preservative | 5.79 ± 0.017 dA | 5.84 ± 0.008 eB | 5.86 ± 0.007 d B | 5.86 ± 0.007 dB | 5.86 ± 0.015 cB |
| 2 | 100% Benzalkanium | 4.79 ± 0.007 bE | 3.54 ± 0.020 dD | 2.64 ± 0.011 bcC | 1.83 ± 0.018 bB | 1.50 ± 0.132 aA |
| 3 | 75% Benzalkanium + 25% EO | 5.68 ± 0.004 cE | 3.58 ± 0.028 dD | 2.52 ± 0.066 abC | 1.91 ± 0.022 bB | 1.50 ± 0.132 aA |
| 4 | 50% Benzalkanium + 50% EO | 5.65 ± 0.018 cE | 2.75 ± 0.010 bD | 2.58 ± 0.032 abcC | 1.85 ± 0.043 bB | 1.63 ± 0.042 aA |
| 5 | 25% Benzalkanium + 75% EO | 4.53 ± 0.044 aE | 2.67 ± 0.014 aD | 2.49 ± 0.009 aC | 1.61 ± 0.022 aB | 1.33 ± 0.042 aA |
| 6 | 100% EO | 5.80 ± 0.010 dE | 2.85 ± 0.005 cD | 2.70 ± 0.006 cC | 2.15 ± 0.014 cB | 2.01 ± 0.001 bA |
| (B) Escherichia coli | ||||||
| 1 | No preservative | 5.88 ± 0.021 dA | 5.90 ± 0.003 eA | 5.91 ± 0.004 dA | 5.91 ± 0.017 eA | 5.90 ± 0.013 fA |
| 2 | 100% Benzalkanium | 4.76 ± 0.024 bE | 3.52 ± 0.002 cD | 2.72 ± 0.047 bC | 1.89 ± 0.015 bB | 1.66 ± 0.001 bA |
| 3 | 75% Benzalkanium + 25% EO | 5.58 ± 0.031 cE | 3.75 ± 0.003 dD | 3.58 ± 0.031 cC | 2.40 ± 0.020 dB | 2.22 ± 0.012 eA |
| 4 | 50% Benzalkanium + 50% EO | 5.63 ± 0.061 cD | 3.75 ± 0.001 dC | 3.57 ± 0.010 cB | 1.89 ± 0.015 bA | 1.80 ± 0.014 cA |
| 5 | 25% Benzalkanium + 75% EO | 4.54 ± 0.017 aE | 2.65 ± 0.002 aD | 2.40 ± 0.012 aC | 1.68 ± 0.025 aB | 1.44 ± 0.043 aA |
| 6 | 100% EO | 5.81 ± 0.006 dE | 2.84 ± 0.007 bD | 2.74 ± 0.001 bC | 2.28 ± 0.011 cB | 2.10 ± 0.024 dA |
| (C) Pseudomonas aeruginosa | ||||||
| 1 | No preservative | 5.88 ± 0.006 eA | 5.91 ± 0.001 eB | 5.96 ± 0.005 eB | 5.93 ± 0.003 eB | 5.92 ± 0.001 cB |
| 2 | 100% Benzalkanium | 4.83 ± 0.003 bE | 3.66 ± 0.019 bD | 2.84 ± 0.026 bC | 1.91 ± 0.011 bB | 1.64 ± 0.020 aA |
| 3 | 75% Benzalkanium + 25% EO | 5.72 ± 0.019 cE | 4.87 ± 0.009 dD | 4.82 ± 0.012 dC | 3.86 ± 0.011 dB | 2.84 ± 0.008 bA |
| 4 | 50% Benzalkanium + 50% EO | 5.70 ± 0.018 cD | 4.76 ± 0.005 cC | 4.73 ± 0.003 cC | 3.77 ± 0.005 cB | 2.75 ± 0.005 bA |
| 5 | 25% Benzalkanium + 75% EO | 4.56 ± 0.016 aE | 2.66 ± 0.008 aD | 2.41 ± 0.016 aC | 1.82 ± 0.032 aB | 1.67 ± 0.064 aA |
| 6 | 100% EO | 5.80 ± 0.001 dD | 4.85 ± 0.017 dC | 4.80 ± 0.001 dC | 3.76 ± 0.003 cB | 2.77 ± 0.020 bA |
| (D) Candida albicans | ||||||
| 1 | No preservative | 5.86 ± 0.030 cA | 5.89 ± 0.001 eAB | 5.91 ± 0.008 dAB | 5.92 ± 0.001 cB | 5.91 ± 0.008 dAB |
| 2 | 100% Benzalkanium | 4.60 ± 0.002 aE | 3.42 ± 0.008 cD | 2.71 ± 0.026 bC | 1.79 ± 0.029 abB | 1.60 ± 0.001 bA |
| 3 | 75% Benzalkanium + 25% EO | 5.63 ± 0.005 bE | 2.70 ± 0.005 aD | 2.55 ± 0.005 bC | 1.91 ± 0.011 bB | 1.78 ± 0.014 cA |
| 4 | 50% Benzalkanium + 50% EO | 5.70 ± 0.016 bE | 2.81 ± 0.001 bD | 2.70 ± 0.014 bC | 1.73 ± 0.016 aB | 1.61 ± 0.022 bA |
| 5 | 25% Benzalkanium + 75% EO | 4.54 ± 0.032 aE | 2.66 ± 0.017 aD | 2.38 ± 0.094 aC | 1.67 ± 0.064 aB | 1.36 ± 0.001 aA |
| 6 | 100% EO | 5.81 ± 0.019 cD | 3.71 ± 0.025 dC | 3.64 ± 0.027 cC | 1.87 ± 0.041 bB | 1.73 ± 0.016 cA |
| (E) Aspergillus brasiliensis | ||||||
| 1 | No preservative | 5.88 ± 0.003 dA | 5.91 ± 0.006 eB | 5.91 ± 0.002 dB | 5.92 ± 0.005 bB | 5.92 ± 0.006 dB |
| 2 | 100% Benzalkanium | 4.61 ± 0.012 aE | 3.48 ± 0.027 cD | 2.68 ± 0.017 bC | 1.86 ± 0.025 aB | 1.69 ± 0.043 bcA |
| 3 | 75% Benzalkanium + 25% EO | 5.67 ± 0.006 bE | 2.75 ± 0.009 abD | 2.58 ± 0.016 aC | 1.87 ± 0.012 aB | 1.78 ± 0.014 cA |
| 4 | 50% Benzalkanium + 50% EO | 5.69 ± 0.001 bE | 2.79 ± 0.006 bD | 2.64 ± 0.007 bC | 1.77 ± 0.036 aB | 1.60 ± 0.001 bA |
| 5 | 25% Benzalkanium + 75% EO | 4.57 ± 0.028 aE | 2.70 ± 0.005 aD | 2.56 ± 0.014 aC | 1.79 ± 0.029 aB | 1.38 ± 0.037 aA |
| 6 | 100% EO | 5.82 ± 0.005 cD | 3.74 ± 0.003 dC | 3.65 ± 0.004 cC | 1.82 ± 0.060 aB | 1.68 ± 0.025 bA |
1The lower cases indicate the statistical differences on different days (columns), and capital letters show the statistical differences between treatments (rows).
5. Discussion
The long-term use of artificial preservatives leads to harmful effects, such as hypersensitivity, asthma, and even cancer. Many customers have become increasingly worried about the safety of synthetic preservatives. The rising demand for more natural and preservative-free pharmaceutical products reinforces the idea of replacing synthetic preservatives with natural antimicrobials like essential oils (EOs) (16). In the current study, the commercial EGO was assumed to have adequate antimicrobial activity against investigated microorganisms (S. aureus, E. coli, P. aeruginosa, A. braziliansis, and C. albicans). Furthermore, researchers worldwide have well reported the strong antibacterial and antifungal potency of EGO and have recommended it as an alternative antimicrobial agent in the food and pharmaceutical industries (10, 11, 17). The impressive antimicrobial potential of EGO has been attributed to the existence of monoterpenes, oxygenated monoterpenes (notably 1, 8-cineole), and their synergistic effect (10, 18). The conclusions of previous studies on the application of essential oil like EGO as a potential natural preservative for pharmaceutical products (1, 11, 18) and present antimicrobial assay findings persuaded us to launch a study to investigate the antimicrobial effectiveness of EGO in the fluticasone nasal spray individually and in combination with BKC. Moreover, stability testing was conducted to assess whether the products (in their final container) retained the desired physical, chemical, and microbiological properties when stored under appropriate conditions. Based on the outcomes, all nasal sprays of studied preservation conditions compared to nasal spray base were able to maintain the intended quality over a defined period. It is more likely due to the presence of antimicrobials in the formulation preparation (19).
In the microbial challenge tests, it must be ensured that the used microorganisms have been challenged against preservative agents for 28 days. Therefore, a preservative-free nasal spray was applied to demonstrate the viability of the inoculated microorganisms and their growth ability during the experiment period (Table 3). The analysis of the challenge test for bacteria represented a reduction of three logarithmic cycles in the growth of S. aureus and E. coli on day seven and persistent reduction until the 28th challenge day for the nasal sprays preserved with EGO (0.9%) and BKC/EGO combinations at the concentrations of 0.01%/0.45% and 0.005%/0.675% (v/v). The preserved nasal sprays with BKC (0.02%) and 0.015%/0.225% concentration of BKC/EGO combination showed a similar decline (two logarithmic cycles reduction) in these bacterial strains, with no significant difference (P > 0.05). Generally, all preservation conditions showed a full preservative effect against S. aureus and E. coli based on USP criteria. In contrast, the microbial challenge test for P. aeruginosa, an opportunistic pathogen inherently resistant to the wide range of antimicrobials, did not present a satisfactory result when EGO was applied as the sole preservative in a nasal spray formulation. This might be due to the impermeability of the outer membrane of P. aeruginosa to hydrophobic antimicrobials (20). The improved antimicrobial activity of BKC/EGO mixtures against P. aeruginosa in nasal spray formulation supported that BKC, a positively charged amphiphilic derivative of ammonium compounds, enables EGO to penetrate the inner part of this bacterium through destabilizing pathogen surface (21). Based on the results (Table 3 [C]) in formulation preserved with EGO (0.675%) and low concentration of BKC (0.005%) simultaneously, the population of P. aeruginosa declined as much as that mentioned for S. aureus and E. coli. This outcome is in line with another study which revealed that the use of Calamintha officinalis essential oil as the only preservative in the cetomacrogol cream could not be effective against P. aeruginosa and its combination with EDTA as a metal chelator considerably reduced the development of P. aeruginosa through impairing its membrane permeability (21). Preservation challenge test for mycetes revealed a three-log reduction in C. albicans and A. brasiliensis population for nasal sprays preserved with a combination of BKC / EGO concentrations (0.015%/0.225%, 0.01%/0.45%, and 0.005%/0.675%) within seven days. It permanently continued until the end of the study. As for the preserved formulations with BKC (0.02%) and EGO (0.9%) individually, a two-log reduction was observed for both C. albicans and A. brasiliensis. Although all preserved conditions met the USP (40) criteria, the BKC/EGO combination at a concentration of 0.005%/0.675% was the most satisfactory preservative compared to BKC alone. More importantly, this combined preservative system dramatically reduced the amount of both antimicrobials in nasal spray preparation, particularly the synthetic one, from 0.02% (v/v) (suggested by the manufacturer) to 0.005% (v/v). Similar findings have been reported by other researchers who used EO in combination with synthetic preservatives, chelators, or emulsifiers in cosmetic products, including the combined use of tea tree oil and ethanol addition (22), C. officinalis EO and EDTA (as a metal chelator) (20), Laurus nobilis, E. globulus, and Salvia officinalis EOs with methyl p-hydroxybenzoate (16) and lavender, tea tree, and lemon oils with 1, 3‐dimethylol‐5, 5‐dimethylhydantoin, and 3‐iodo‐2‐propynyl butyl (19). Furthermore, such combined preservative systems diminish the pungent odor of EO and increase consumer satisfaction with cosmetic and pharmaceutical products (1).
5.1. Conclusions
The development of natural preservatives provides a way to replace or decrease the level of synthetic preservatives commonly utilized in the pharmaceutical industry. In addition, these agents have less toxic effects and represent a possible natural and safer alternative to synthetic preservatives. Our research demonstrated that E. globulus essential oil in combination with benzalkonium chloride at concentrations of 0.675% and 0.005% (v/v), respectively, effectively decreased the growth of reference microorganisms in comparison with that of benzalkonium chloride in fluticasone propionate nasal spray formulation and fulfilled the USP criteria for the antimicrobial effectiveness test. Therefore, it can be recommended as an effective candidate for natural pharmaceutical preservatives in nasal spray and inhalation solutions after complementary studies.