1. Background
Hydatid cyst is well-known as a parasitic disease initiated by Echinococcus granulosus from the category of cestode worms (1). Adult worms are formed by eating cyst-infected viscera and in the intestine of the dogs as the final host. Humans are the intermediate or accidental hosts of this parasite, and they become infected by consuming plants or vegetables contaminated with worm eggs or by contact with an infected dog (2). Sometimes the disease incubation period is very long, and the disease may be diagnosed 20 - 25 years after infection. Symptoms of hydatidosis depend on the cysts’ number, dimension, and site (3). There is still much controversy about treating this disease, and as a general protocol, if the cyst is solitary or confined to one area (one liver lip with one lung lip), the treatment of choice is surgery to eradicate the cysts (4). In cases where surgery is impossible, medical treatments such as albendazole and mebendazole are used (5, 6).
Today, leakage of cysts or leaks of their fillings during surgery is the most critical upset for surgeons (7, 8). The use of scolicidal agents, e.g., hypertonic salt 20% and silver nitrate (Ag-nitrate), has been considered for reducing the risk of protoscoleces spread and relapse of the disease (9), but some complications (fibrosis of biliary ducts and liver cirrhosis) have been reported with these agents (10).
Due to high availability, high efficacy, low cost, and minimum toxicity, herbs and derivatives are generally applied for treating infectious diseases, especially parasitic ones (11). Isoflavones with the chemical formula of C20H18O6 are a subset of flavonoid compounds with pharmacological benefits for human health (12, 13). Formononetin (FMN) (C16H12O4) is a natural isoflavone found in many foods belonging to the Fabaceae family (14). The chemical name of the pheromone is biochanin B, and its IUPAC name is 7-hydroxy-4’-methoxy isoflavone (14). Isoflavonoids are found in large quantities in plants such as soybeans and clover (14). Formononetin has pharmacological benefits, e.g., antimicrobial, antioxidant, anti-hyperlipidemic, anti-diabetic, anti-tumor, neuroprotective, and cardioprotective activity (14, 15).
2. Objectives
Since FMN has shown various biological and therapeutic effects, in this study, we intended to study the in vitro and ex vivo antiparasitic effect of FMN on hydatid cyst protoscoleces.
3. Methods
3.1. Chemicals
Formononetin (purity > 99%) and Eosin powder were procured from Sigma-Aldrich, Germany. Other chemicals were provided in the best grades.
3.2. Preparation of Protoscoleces
Protoscoleces were prepared from the liver of sheep infected with hydatid cysts based on the method described by Cheraghipour et al. (16).
3.3. Viability Assay
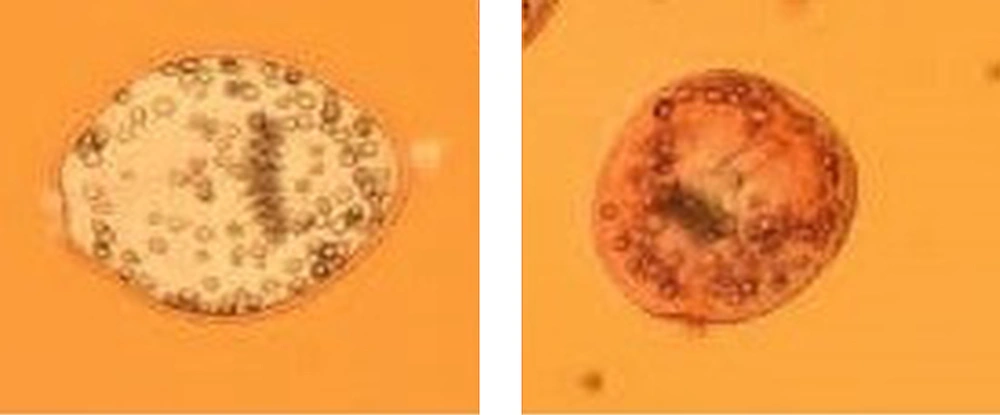
The viability of protoscoleces was evaluated by eosin staining assay according to the method described by Mahmoudvand et al. (17). Living protoscoleces were transparent and colorless, while dead protoscoleces were seen in red.
3.4. In Vitro Scolicidal Activity
In the first step, FMN concentrations of 75, 150, and 300 μg/mL were mixed with protoscoleces (1 × 103/mL), and the viability was recorded after 5 to 60 minutes in 48-chamber plates as triplicate. Consecutively, sterile normal saline + tween 20 and silver nitrate were reported as the negative and positive controls, respectively (18).
3.5. Ex vivo Scolicidal Effects
Nearly 50% of the hydatid cyst contents were drained, and various concentrations of FMN were then separately injected to fill the cyst. Then, the remaining cyst fluid was obtained for 5 - 60 minutes, and the viability of the protoscoleces was assessed by eosin staining assay (19).
3.6. Effect of Caspase-3 Activity in Protoscoleces
The activity of caspase-3 in protoscoleces after incubation with FMN was studied based on a commercial colorimetric protease kit (Sigma, Germany) according to the producer protocols (20).
3.7. Effect on the Plasma Membrane Permeability
Formononetin effect on the permeability of plasma membrane in protoscoleces was studied by the SYTOX stain approach through the Triton commercial kit (Sigma-Aldrich, Germany) after 30, 60, 120, and 240 minutes of protoscoleces treatment with FMN (19).
3.8. Statistical Analysis
Data analysis was done using SPSS version 25.0 software at P < 0.05 as a significance level.
4. Results
4.1. In Vitro Protoscolicidal Effect
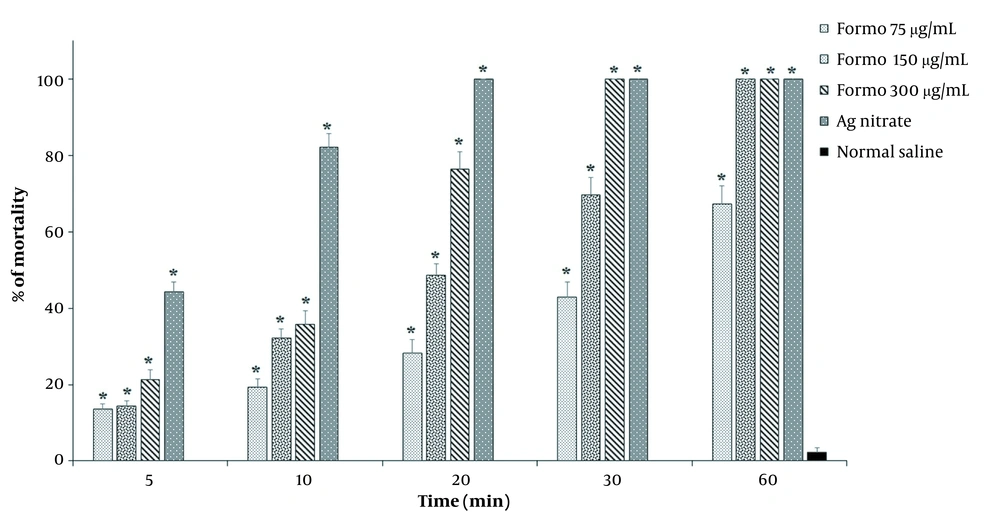
Formononetin at 300 μg/mL concentration absolutely demolished hydatid cyst protoscoleces after 30 minutes of exposure (Figure 1), while at 150 μg/mL after 60 minutes, 100% of protoscoleces were eliminated. Among the studied concentrations, FMN at 75 μg/mL showed the lowest level of protoscolicidal effects, so that after 60 minutes of exposure, 67.3% of protoscoleces were destroyed. The results showed that FMN at different concentrations (75, 150, and 300 μg/mL) meaningfully declined the viability of protoscoleces compared to the control group (Figure 2).
4.2. Ex Vivo Protoscolicidal Effect
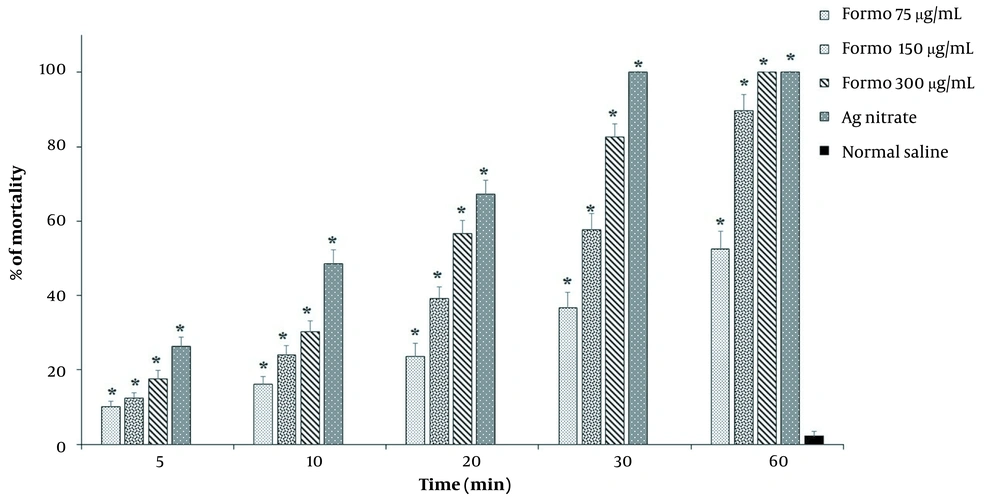
Formononetin showed its antiparasitic effect for longer periods. Based on these results, FMN at 300 μg/mL concentration completely eliminated hydatid cyst protoscoleces after 60 minutes of exposure, while at 150 μg/mL after 60 minutes, 89.6% of protoscoleces were destroyed. Similar to in vitro results, it showed the lowest level of protoscolicidal effects among the studied concentrations, so after 60 minutes of exposure, it destroyed 52.6% of protoscoleces. The results showed that FMN at different concentrations (75, 150, and 300 μg/mL) significantly killed (P < 0.001) protoscoleces compared to the control group (Figure 3).
4.3. Effect on Caspase-3 Activity
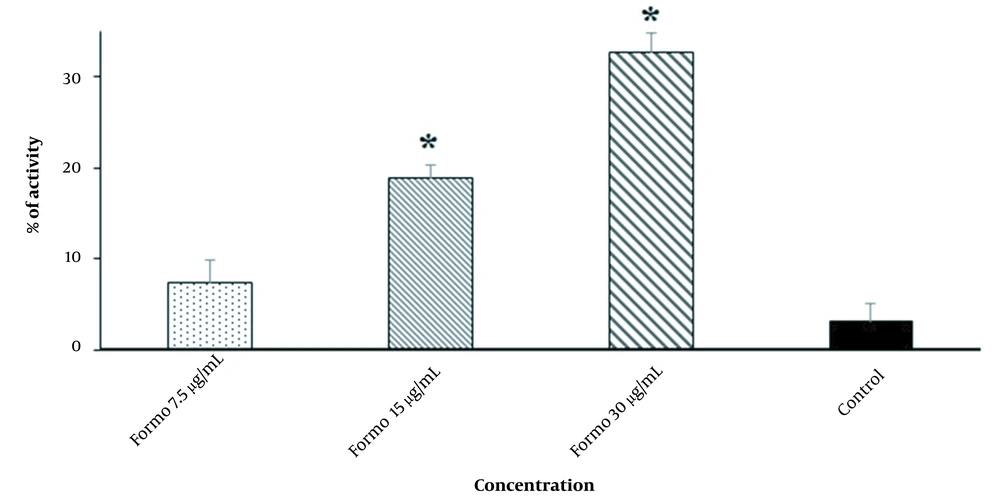
Formononetin at 7.5, 15, and 30 μg/mL triggered the caspase-3 activity by 7.4%, 18.9%, and 32.7%, respectively (Figure 4).
4.4. Effect of Formononetin on Permeability of Plasma Membrane
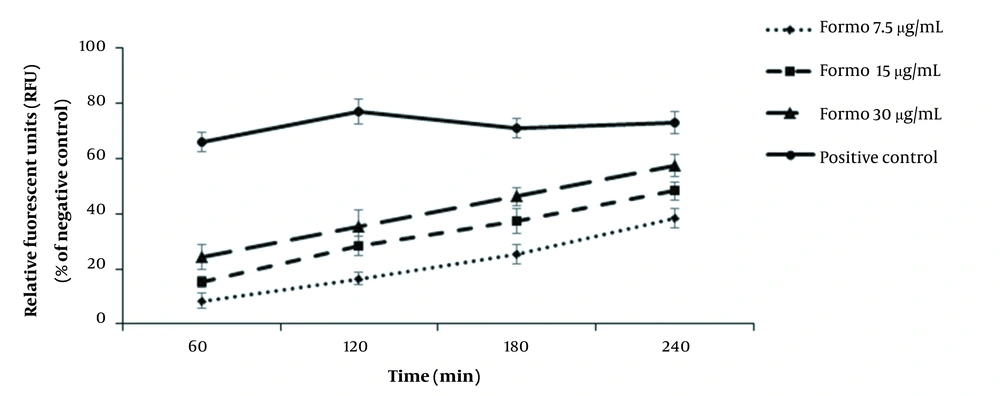
The findings displayed that the plasma membrane permeability of protoscoleces noticeably elevated after FMN treatment dose-dependently (Figure 5).
5. Discussion
Based on the obtained results, FMN, especially at 150 and 300 μg/mL, showed strong protoscolicidal effects, whereas it noticeably (P < 0.001) increased the activity of caspase-3 and permeability of protoscoleces dose-dependently. Hydatid cyst treatment is primarily done by surgery unless there is a reason. In such cases, medications are used, such as benzimidazole, albendazole, and mebendazole (8, 21). Drug treatment can only be effective for small, inactive cysts, while large and active cysts inevitably fall into surgical treatment. Surgery has a risk of cyst rupture and leakage of its internal materials and subsequent infection of adjacent organs (9, 10). Therefore, in the surgical protocol of hydatid cysts, protoscolicidal materials, e.g., silver nitrate, povidone-iodane, and 20% hypertonic salt, are mandatorily used (10). Hence, achieving efficient protoscolicidal agents with minimal side effects is necessary.
We revealed that FMN at 300 μg/mL completely destroyed hydatid cyst protoscoleces after 30 minutes, while at 150 μg/mL, after 60 minutes of contact, 100% of protoscoleces were eliminated. By ex vivo assay, FMN showed its antiparasitic effect for longer periods. Based on these results, FMN at 300 μg/mL completely eliminated hydatid cyst protoscoleces after 60 minutes of exposure, whereas, at a concentration of 150 μg/mL after 60 minutes, 89.6% of protoscoleces were destroyed. A noteworthy point in this study is that ex vivo FMN exhibited its antiparasitic effect over longer periods. This might be because some proteins, substances, and inhibitors are present in the fluid of the hydatid cyst, reducing the drug’s effectiveness.
Yang et al. reported the antimicrobial effects of FMN against Gram-positive and Gram-negative bacteria, e.g., Enterobacter cloacae, Escherichia coli, and Pseudomonas aeruginosa (22). Wang et al. revealed the antiviral effects of FMN on enterovirus-51, whereas this fraction reduced enterovirus-51 RNA and protein synthesis (23).
In another study, Lauwaet et al. showed that isoflavone FMN inhibited the adhesion, motility, and viability of Giardia lamblia trophozoites (24). Mead and McNair showed that among some tested flavonoids and isoflavone compounds (e.g., galangin, luteolin, silibinin, quercetin, genistein, apigenin, and naringenin), quercetin, apigenin, and genistein revealed the highest in vitro antiparasitic effects against Cryptosporidium parvum and Encephalitozoon intestinalis in concentrations ranging from 5.5 to 15 μM (25). Recently, Faixova et al. reported the antiparasitic effects of some isoflavones (genistein, curcumin, silymarin, and quercetin) against some flatworms, e.g., Raillietina spp., Fasciola spp., Schistosoma spp., and Echinococcus spp., through affecting the tegumental construction and disturbance in metabolism by interaction with enzymes or signaling molecules (26).
Stimulation of programmed cell death (apoptosis) as a primary mechanism of the new antimicrobial agents induced by external factors ultimately results in cell death (27). Caspases, mainly caspase-3, are recognized as essential factors of apoptosis (28). We determined that FMN at the doses of 7.5, 15, and 30 μg/mL triggered caspase-3 activity by 7.4%, 18.9%, and 32.7%, respectively, indicating that the induction of apoptosis may be suggested as a protoscolicidal mechanisms of FMN. The effect on plasma membrane permeability of microbial pathogens is another cellular mechanism in killing and eliminating pathogenic microbes (29). In the current study, we exhibited that the level of plasma membrane permeability of protoscoleces was noticeably elevated dose-dependently, although the action mechanism of FMN against protoscoleces has not yet been evaluated. However, previous studies reported that isoflavones have antimicrobial mechanisms mainly against plasma membranes and apoptosis, disrupting the lipid bilayer structure and decreasing the membrane’s electrical potential leading to the outflow of critical materials, e.g., amino acids, proteins, and ions, such as potassium and calcium, causing cell death (10-12, 30-32).
5.1. Conclusions
As a natural product, FMN showed promising effects on the protoscoleces of hydatid cysts, indicating that it can be considered a valuable scolicidal agent. However, more investigations are necessary to evaluate its efficacy in animal models and human subjects.