1. Context
Cardiometabolic syndrome is defined as a set of interrelated metabolic risk factors for atherosclerotic cardiovascular disease closely associated with insulin resistance (1). Emerging evidence suggests that the overall prevalence of the cardiometabolic syndrome is increasing globally (2). Given that cardiometabolic syndrome is a pre-condition of critical health problems (1), it is vital to explore the effective and efficient strategies for preventing and managing the determinants of the disease, including the introduction of functional foods in contemporary diets. There is currently a growing awareness on the role of phytochemicals that can potentially address the upstream causes of cardiometabolic syndrome (3).
Cruciferous vegetables are rich sources of essential bioactive components in diets (4) and are believed to possess health-promotional properties (4, 5). Broccoli sprout, identified as the young and newly-grown plants of broccoli (3- to 4-day-old sprouted broccoli seeds) (5), is a multifaceted supplement among cruciferous vegetables. The anti-inflammatory, anti-hypertensive, and cholesterol-lowering effects of broccoli sprout on animal models are well-documented (6, 7). Nevertheless, existing research on endothelial function (8), lipid profile (9, 10), and glycemic status (11) of human subjects following short-term or regular intake of broccoli sprout are yet controversial.
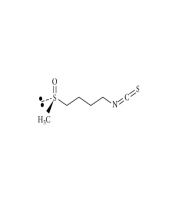
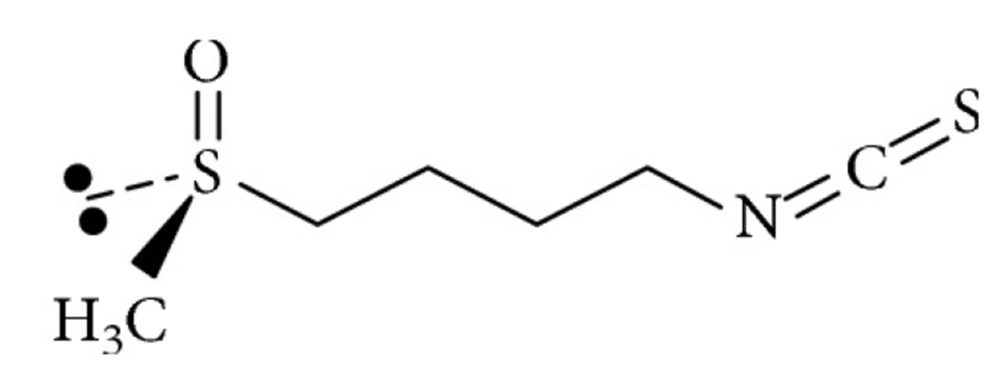
Evidence from experimental studies attributes the health benefits of broccoli sprout to the constituent non-nutritive phytochemicals (5, 12), particularly the glucosinolates (13). Sprouted broccoli seeds contain the highest glucosinolate levels within three to seven days of growth compared to other cruciferous vegetables (3). Glucosinolates interact with the plant myrosinase enzymes (14) or gut bacteria and hydrolyze to biologically active isothiocyanates (15). The most well-known glucosinolates is sulforaphane (1-isothiocyanate-4-methylsulfonyl butane), which is the most potent phase-2 enzyme inducer within food sources and an endogenous antioxidative defense (5, 9, 16). The structure and lipophilicity of glucosinolate confer certain advantages to sulforaphane compared to other phytochemicals, such as a higher bioavailability, which can facilitate its absorption through the membrane of the gut and target cells (3). In fact, the absolute bioavailability of sulforaphane is about 80%, compared to 1 - 8% in other polyphenols with higher molecular weights (3). Figure 1 represents the molecular structure of sulforaphane (4-methylsulfinylbutyl isothiocyanate).
Molecular structure of sulforaphane (4-methylsulfinylbutyl isothiocyanate), adapted from Houghton (3)
2. Objectives
To the best of our knowledge, few studies have investigated broccoli sprout consumption in association with cardiometabolic outcomes. Despite the novelty of this field, no systematic reviews and meta-analyses have reported conclusive results on this topic. Here, we aimed to systematically review studies investigating broccoli sprout supplementation and cardiometabolic health and provide a meta-analysis of the outcomes. The findings from this study can unveil new pharma-nutrition paradigms of medicinal foods in cardiometabolic health.
3. Methods
3.1. Search Strategy and Study Eligibility
This study complies with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) system (17). Eligible publications were extracted from PubMed and Scopus in June 2022. The literature search sought all English articles that investigated the cardiometabolic effects of broccoli sprouts. The original search algorithm used Boolean terms and the following keywords: “broccoli sprout”, “cruciferous vegetable”, cardiometabolic, “metabolic syndrome”, diabetes, glucose, insulin, cholesterol, lipid, lipoprotein, triglyceride, hyperlipidemia, “oxidative stress”, inflammation, obesity, “blood pressure”, “systolic blood pressure”, “diastolic blood pressure”, hypertension, and hepatic. The reference lists of relevant publications and review papers were manually checked for additional studies. The method brought no ethical issues as the required data were extracted from published literature.
3.2. Inclusion and Exclusion Criteria
Initially, the title and abstract of all papers were scanned, and non-English, animal-based, and in vitro studies were excluded. Studies investigating broccoli sprout supplementation associated with the lipid profile, glycemic status, hypertension, inflammation, oxidative stress, and hepatic biomarkers underwent full-text screening. Review studies and chapter books were excluded. To assess the overall eligibility and increase the accuracy, two authors (Z. H. and Z. B.) independently reviewed the included records in detail and retrieved data from potentially relevant articles. The Jadad scale (18) was used to evaluate the quality of articles and provide information on the randomization method, allocation concealment, blinding of outcome assessment, and completeness of the follow-up.
3.3. Data Extraction
The following data were extracted from all included studies for quantitative analyses: Author’s name, publication year, study setting, type and duration of the intervention and placebo, participants’ characteristics (gender, age, health status), cardiometabolic measurements (systolic blood pressure (SBP), diastolic blood pressure (DBP), total cholesterol (TC), triglyceride (TG), high-density lipoprotein (HDL), low-density lipoprotein (LDL), LDL: HDL, TC: HDL, C-reactive protein (CRP), α -tumor necrosis factor (TNF-α), interleukin 6 (IL-6), fasting plasma glucose (FPG), HOMA- IR, HbA1c), and liver enzymes (alanine transaminase (ALT), aspartate transaminase (AST), gamma-glutamyl transpeptidase (γ-GTP)). The quantitative information was collected in mean values. Multiple time intervals and/or different interventional procedures within a single study were treated as distinct subgroups. The primary outcome of this study was to assess the beneficial effects of broccoli sprouts on cardiometabolic factors. As the reporting frequency of the mentioned cardiometabolic variables varied among the eligible studies, we specifically focused on the lipid-related parameters and blood pressure indices with higher frequencies.
3.4. Statistical Methods
This systematic review included 10 trials and studied 16 cardiometabolic variables (summarized in Table 1). Studies with three and more subgroups were included in the analysis. The data were quantitatively assessed based on the weighted mean difference (WMD) and 95% confidence intervals (CIs) before and after the intervention and between the intervention and control groups. A meta-analysis of the absolute mean difference of cardiometabolic variables relative to the baseline values was also carried out in the broccoli sprout-supplemented group. Furthermore, the I2 static and Cochrane’s Q statistic for specific categories were used to assess the statistical heterogeneity (low = 25%, moderate = 50%, and high = 75%) (15).
| Author | Study Design | Study Population | Study Duration (Weeks) | Type and Dosage of Intervention | Sulforaphane Dosage (μmol/g) | Findings | Adverse Effects | |
|---|---|---|---|---|---|---|---|---|
| 1 | Murashima et al. (19) | Clinical phase 1 study | 12 | 1 | Fresh BS; 100 g/day | NG | Males: ↓ TC, TG, ↑ HDL; females: ↓ TC, LDL | NG |
| 2 | Axelsson et al. (20) | Randomized controlled trial | 97 | 12 | Dried powder broccoli sprouts; administered as aqueous extract; NG | 3 | Obese and non-obese well-regulated diabetics: ↑ FPG, HbA1c, IR, TG; non-obese dysregulated: ↓ FPG, IR, ↑ HbA1c, TG; obese dysregulated: ↓ HbA1c | Loose stools, flatulence, nausea, headache |
| 3 | Mirmiran et al. (21) | Randomized controlled trial | 77 | 1/2 vs. 1.5 | 20 mg omeprazole + 500 mg clarithromycin + 1000 mg amoxicillin vs. STT + 6 g/d BSP | 22.5 | Intervention 1: ↑ TC, TG, TG: HDL, HDL intervention 2: ↓ SBP, DBP, ↑ TC | Diarrhea and nausea |
| 4 | Mirmiran et al. (22) | Randomized controlled trial | 63 | 4 | BSP 10 and 5 g/day | 22.5 | ↓ FBS, hs-CRP, IL-6, TNF-α | NG |
| 5 | Bahadoran et al. (23) | Randomized controlled trial | 63 | 4 | BSP 10 and 5 g/day | 22.5 | ↓ FBS, serum insulin concentration, IR index | NG |
| 6 | Bahadoran et al. (10) | Randomized controlled trial | 63 | 4 | BSP 10 and 5 g/day | 22.5 | ↓ FBS, TC, TG, LDL, OX-LDL: LDL, AIP, TC: HDL and HDL: LDL | NG |
| 7 | Bahadoran et al. (11) | Randomized controlled trial | 63 | 4 | BSP 10 and 5 g/day | 22.5 | ↓ FBS, MDA, OX-LDL, TOS, OSI | NG |
| 8 | Kikuchi et al. (24) | Randomized controlled trial | 52 | 8 | BS extract powder; NG | 23 | ALT, ↑ TG ↓, ALT, γ-GTP, Alb, ALP, | NG |
| 9 | Christiansen et al. (8) | Randomized clinical trial | 40 | 4 | Dried BS 10 g/day | 48.5 a | ↓ SBP, TC, HDL; ↑ LDL, FMD, DBP | Unappealing taste |
| 10 | Lopez- Chillon et al. (25) | Randomized clinical trial | 40 | 10 | Raw, fresh BS 30 g/day | 65.47 a | ↓ IL- 6, CRP | NG |
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; BS, broccoli sprout; BSP, broccoli sprout powder; CRP, C-reactive protein; DBP, diastolic blood pressure; FPG, fasting plasma glucose; γ-GTP, gamma-glutamyl transpeptidase; HDL, high-density lipoprotein; IL-6, interleukin 6; LDL, low-density lipoprotein; LDL/HDL, low-density lipoprotein to high-density lipoprotein ratio; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; TC/ HDL, total cholesterol to high-density lipoprotein ratio
a Glucosinolate content
| Variable | Subgroups | WMD (95% CI) | P-value | I2 (%) |
|---|---|---|---|---|
| SBP (mmHg) | 4 | -10.9 (-17.0, -4.86) | 0.001 | 0 |
| DBP (mmHg) | 4 | -6.95 (-10.6, -3.28) | 0.001 | 0 |
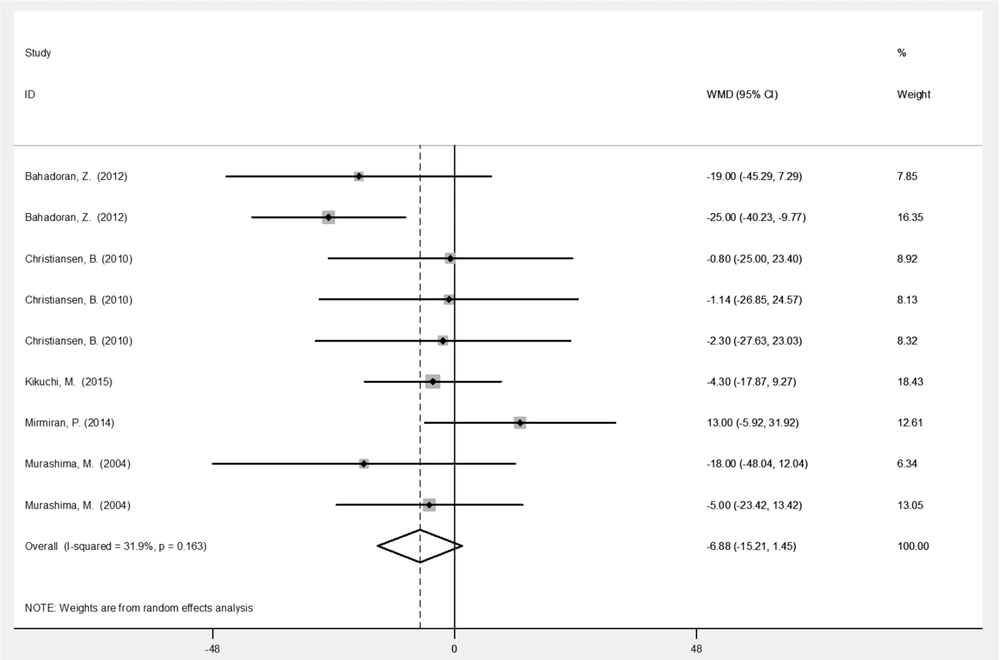
| TC (mg/dL) | 9 | - 6.89 (-15.2, 1.45) | 0.105 | 31.9 |
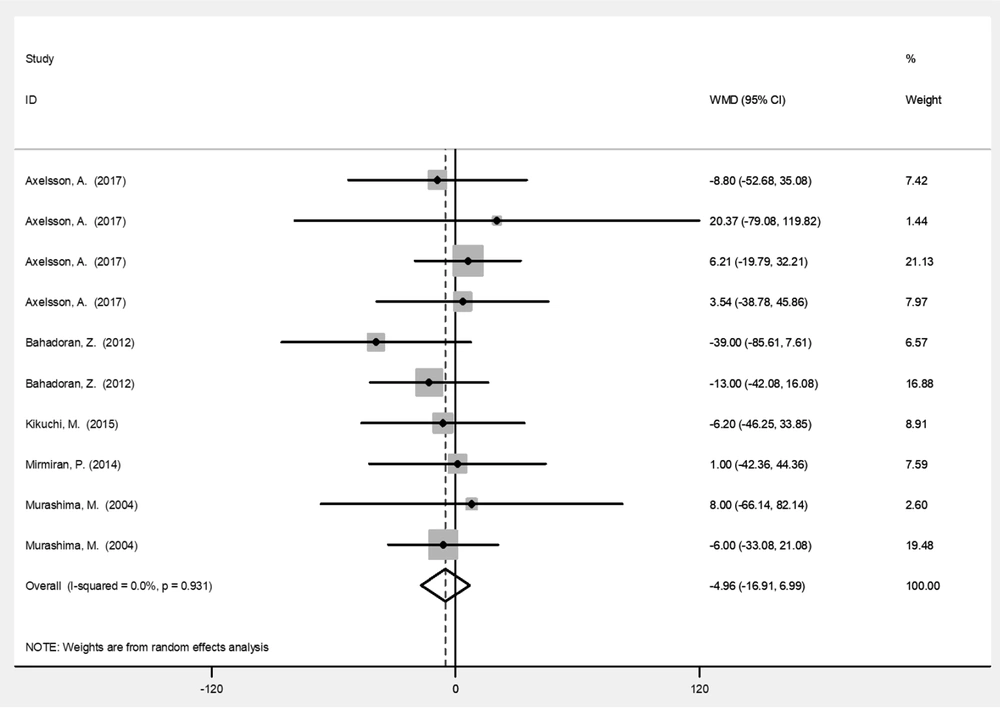
| TG (mg/dL) | 10 | -4.96 (-16.9, 6.99) | 0.416 | 0 |
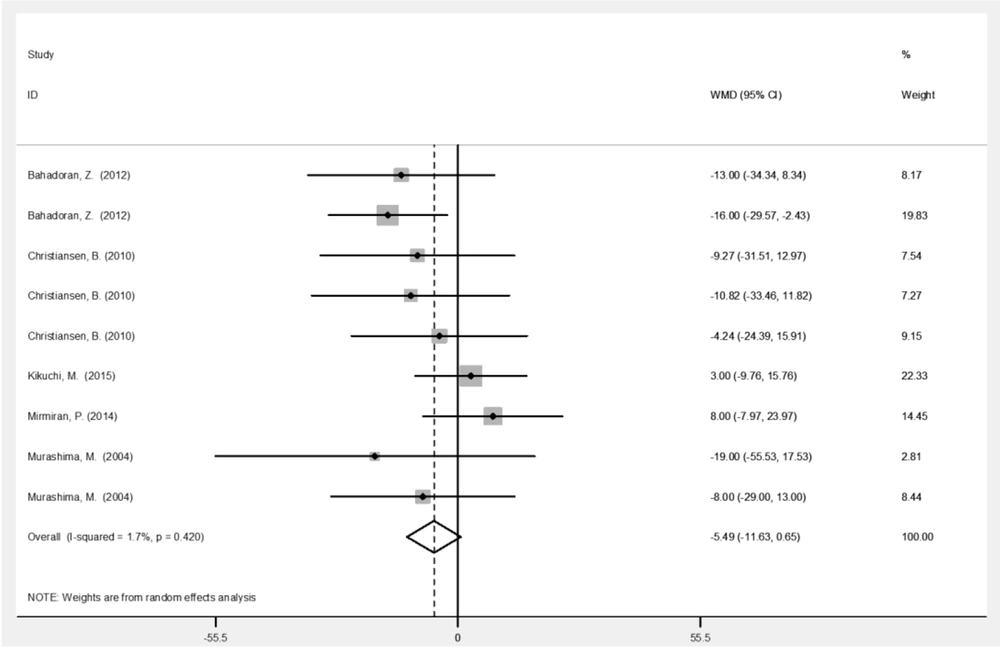
| LDL (mg/dL) | 9 | -5.49 (-11.6, 0.65) | 0.080 | 1.7 |
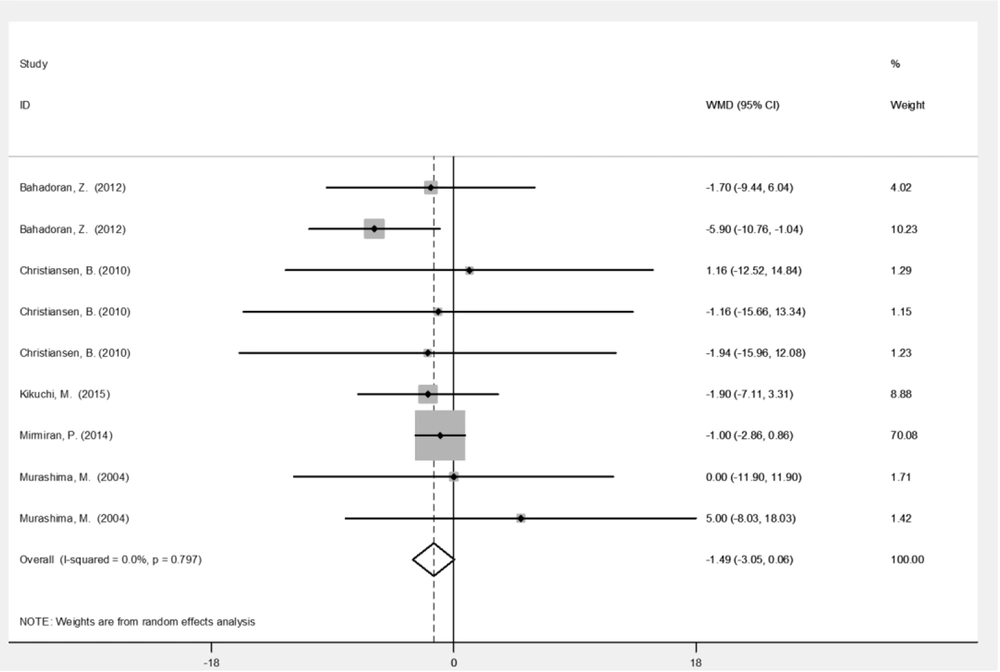
| HDL (mg/dL) | 9 | -1.49 (-3.05, 0.06) | 0.060 | 0 |
| LDL/HDL (mg/dL) | 3 | -0.05 (-0.31, 0.22) | 0.729 | 0 |
| TC/HDL (mg/dL) | 3 | -0.08 (-0.43, 0.27) | 0.668 | 0 |
| CRP (ng/mL) | 5 | -0.28 (-0.75, 0.2) | 0.258 | 29.2 |
| FPG (mg/dL) | 7 | 0.5 (-4.29, 5.29) | 0.839 | 0 |
| HOMA-IR | 6 | 0.16 (-0.32, 0.64) | 0.521 | 25.5 |
| HbA1c (%) | 5 | 0.02 (-0.23, 0.27) | 0.877 | 0 |
| IL-6 (pg/ml) | 3 | -1.09 (-3.07, 0.9) | 0.283 | 93.4 |
| ALT (IU/L) | 3 | -0.95 (-5.49, 3.59) | 0.681 | 0 |
| AST (IU/L) | 3 | -0.05 (-2.21, 2.12) | 0.965 | 0 |
| γ-GTP (IU/L) | 3 | 5.26 (-7.88, 18.41) | 0.433 | 79.2 |
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; BS, broccoli sprout; BSP, broccoli sprout powder; CRP, C-reactive protein; DBP, diastolic blood pressure; FPG, fasting plasma glucose; γ-GTP, gamma-glutamyl transpeptidase; HDL, high-density lipoprotein; IL-6, interleukin 6; LDL, low-density lipoprotein; LDL/HDL, low-density lipoprotein to high-density lipoprotein ratio; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; TC/ HDL, total cholesterol to high-density lipoprotein ratio; WMD, weighted mean difference
Fixed and random-effect models calculated the pooled mean difference of cardiometabolic variables in response to broccoli sprout supplementation. However, due to the significant heterogeneity between the studies, findings from the random-effect model were only reported. Funnel plots and Egger’s regression test asymmetry were used to assess the potential publication bias. All analyses were performed by Stata version 11 (StataCorp), and the tests were 2- tailed with P < 0.05 considered statistically significant.
4. Results
4.1. Studies’ Characteristics
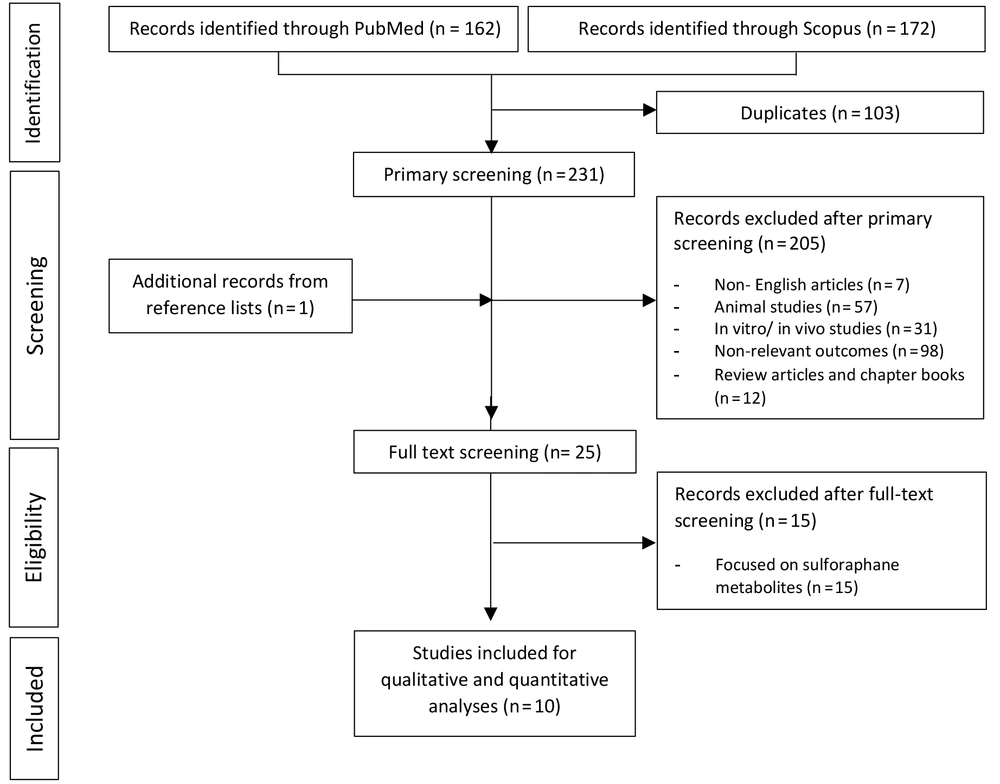
Overall, 334 potentially relevant articles were extracted from the literature, and 231 studies proceeded through the primary screening. Quality assessment of the studies reduced this number, and ultimately 25 articles underwent full-text screening. Of these, 10 articles (8, 10, 11, 19-25) met the inclusion criteria and were deemed eligible for the quantitative analysis. Figure 2 illustrates the successive steps involved in the study selection.
In total, 579 participants were assigned into 21 intervention (n = 396) and 15 control (n = 183) groups. Of these, eight controlled trials compared the treatment results between the intervention and control groups and reported the beneficial effects of broccoli sprouts on the incidence of type 2 diabetes, endothelial disorders, oxidative stress, and inflammation. The non-controlled studies were solely included to estimate the pooled effect of intervention before and after broccoli sprout supplementation.
The methodology and quality of the eligible studies were assessed by the Jadad scoring system (18) (Appendix 1). Taken together, four studies were scored below 5 and ranked as low quality (8, 19, 21, 25). The earliest included study was published in 2004 (19), and the most recent was released in 2019 (25). Some articles focused on a single outcome, whereas others tackled multiple outcomes; two articles provided information on blood pressure measurements (8, 21), seven publications reported results for the lipid-related biomarkers (8, 10, 19-21, 23, 24), four studies focused on the glycemic status (11, 21, 24, 25), five papers assessed the indicators of inflammation and oxidative stress (22-25), and two papers provided specific data on the hepatic enzymes (19, 24). Trial durations varied between one and 12 weeks. The major characteristics of the selected studies are highlighted in Table 1. The average age of participants ranged between 46 ± 6 and 58 ± 9 years. One study did not mention the mean age of the recruited population (19). While one study merely included male participants (24), nine articles reported the male-to-female ratio (5, 8, 10, 11, 19, 21-23, 25) and one study did not state the gender status (20). Participants were given the broccoli sprouts in diverse forms, such as powder (5, 10, 11, 21-23), capsules (24), and fresh (19, 25) or dried vegetables (8), and doses mainly were reported per day. For those indicated, the sulforaphane yield of the sprouts ranged between 225 μmol per 10 g to 112 μmol per 5 g. Only two studies included healthy individuals (19, 25), and others recruited participants with diabetes (5, 10, 11, 20-23), hypertension (8), and fatty liver (24).
| Variable | Subgroups | WMD (95% CI) | P-Value | I2 (%) |
|---|---|---|---|---|
| SBP (mmHg) | 4 | -0.21 (-5.21, 4.81) | 0.936 | 0 |
| DBP (mmHg) | 4 | 0.23 (-3.04, 3.51) | 0.888 | 0 |
| FMD (%) | 3 | -0.74 (-2.83, 1.35) | 0.49 | 68.7 |
| TC (mg/dL) | 7 | -2.01 (-9.76, 5.7) | 0.607 | 0 |
| TG (mg/dL) | 8 | -7.25 (-30.9, 16.4) | 0.547 | 57.1 |
| LDL (mg/dL) | 7 | -1.39 (-7.74, 4.96) | 0.667 | 0 |
| HDL (mg/dL) | 7 | 0.46 (-1.66, 2.59) | 0.67 | 18.6 |
| LDL/HDL (mg/dL) | 3 | -0.08 (-0.37, 0.2) | 0.558 | 0 |
| TC/ HDL (mg/dL) | 3 | -0.29 (-0.68, 0.09) | 0.137 | 0 |
| CRP (ng/mL) | 4 | -0.58 (-1.36, 0.2) | 0.15 | 63.2 |
| FPG (mg/dL) | 7 | 0.45 (-4.65, 5.54) | 0.86 | 0 |
| HOMA-IR | 6 | -0.09 (-0.45, 0.27) | 0.601 | 33.2 |
| HbA1c (%) | 5 | -0.006 (-0.12, 0.11) | 0.914 | 38.8 |
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; BS, broccoli sprout; BSP, broccoli sprout powder; CRP, C-reactive protein; DBP, diastolic blood pressure; FPG, fasting plasma glucose; γ-GTP, gamma-glutamyl transpeptidase; HDL, high-density lipoprotein; IL-6, interleukin 6; LDL, low-density lipoprotein; LDL/HDL, low-density lipoprotein to high-density lipoprotein ratio; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; TC/ HDL, total cholesterol to high-density lipoprotein ratio; WMD, weighted Mean Difference
4.2. Meta-analysis
Table 2 represents the analysis results for variables before and after the intervention with broccoli sprouts. Based on the results, broccoli sprout significantly improved SBP (-10.9 mmHg; 95% CI: -17.0, -4.86; P < 0.001) and DBP (-6.95 mmHg; 95% CI: -10.6, -3.28; P < 0.001) levels. Administration of broccoli sprouts reduced the levels of HDL (-1. 49 mg/dL; 95% CI: -3.05, 0.06; P = 0.06) and LDL (-5.49 mg/dL; 95% CI: -11.6, 0.65; P = 0.08) cholesterols marginally significantly compared to the baseline. Figures 3 - 6 illustrate the overall effect of broccoli sprout supplementation on lipid-related parameters compared to the baseline values. Overall, SBP (-10.9 mmHg), DBP (-6.95 mmHg), TC (-6.89 mmHg), LDL (-5.49 mg/dL), and TG (-4.96 mg/dL) had the maximum change post-intervention in the group consuming broccoli sprouts.
Table 3 provides data on the mean differences between the intervention and control groups. No significant differences were observed between the intervention and control groups for the variables. Meanwhile, the final SBP and DBP levels were lower in the broccoli-supplemented groups compared to the control groups. In this regard, TG, TC, and LDL had the most significant between-group mean differences.
5. Discussion
In this meta-analysis, broccoli sprout supplementation was associated with significant reductions in SBP and DBP compared to the baseline. This finding is in agreement with the study of Mirmiran and colleagues, who reported moderate yet significant reductions in SBP (136 to 122 mmHg; P < 0.05) and DBP (89.8 to 80.4 mmHg; P < 0.05) following broccoli sprout administration in H. pylori-infected patients (21). Changes in blood pressure and endothelial function of hypertensive participants were primarily attributed to the age and gender of the population in another publication (8). Few studies have discussed the anti-hypertensive properties of broccoli sprouts in human populations; however, investigations on spontaneously hypertensive stroke-prone rat models confirm the significant improvements in SBP, DBP, and inflammation status following broccoli sprout administration (6). Detected changes were explained by the actions of glucoraphanin and sulforaphane through antioxidative properties (26). It is believed that sulforaphane-induced upregulation of phase II enzymes could decrease the oxidative stress experienced by vascular smooth muscle cells. Also, sulforaphane increases existing contents of glutathione conjugation and subsequently alters activities of glutathione reductase and glutathione peroxidase; these changes are correlated with improved relaxation of the aorta and significantly decrease the blood pressure (27).
This study found clinically significant reductions in HDL and LDL following dietary broccoli sprout intake (Figures 4 and 5), which is partly consistent with the literature (21). In a phase 1 study, 100 g/d of fresh broccoli sprouts improved the lipid metabolism of participants one week after the intervention (9). Nevertheless, a 2014 clinical trial reported no significant changes in the TG and TG/ HDL levels following broccoli sprout administration (21). Similarly, in a four-week clinical trial, hypertensive participants showed no significant changes in LDL, HDL, and TC levels (8). The effect of broccoli sprout on lipid-related parameters was gender-specific in animal studies; the pattern of hypocholesterolemic changes varied between the male and female Syrian hamsters (28), and acute administration of broccoli sprouts’ ethanol extracts only decreased the serum levels of TC, TG, LDL, and TG/ HDL in male rats fed on high-fat diets (7). Reduced levels of fat absorption by the phytonutrient content (29) and the inhibition of lipoprotein lipase activity in the adipose tissue (7) may explain the mechanisms of changes in this context.
The potential health benefits of high sulforaphane-yielding broccoli sprouts are discussed in various clinical trials, animal studies, and in vitro models. Nevertheless, few human trials have focused primarily on cardiometabolic outcomes. Due to the lack of adequate data, as well as high levels of heterogeneity between the studies in terms of the intervention duration and dosage, and the health status of the participants, we could not detect any significant effects on glycemic, oxidative, and inflammation-related biomarkers. Meanwhile, existing literature suggests that adherence to a broccoli sprout-containing diet positively attenuates glycemic profiles and diabetic status. A double-blind clinical trial concluded that 5 g/day and 10 g/day of low sulforaphane-yielding sprout powder (0.4% compared with 1 - 2% in other studies) significantly decreased the blood glucose level (11), which could be related to the anti-inflammatory properties of broccoli sprouts. The inhibitory effects of sulforaphane on the NF-κB pathway (30) and the potential role of sulforaphane in the activation of peroxisome proliferator-activated receptors (PPARs) are among the other possible mechanisms (31).
Anti-inflammatory properties of broccoli sprouts are key in the potential effects on the cardiometabolic health. Accordingly, Mirmiran and colleagues confirmed significant reductions in hs-CRP, IL-6, and TNF-α concentrations following broccoli sprout supplementation (22). Broccoli sprouts are a potential inhibitor of proinflammatory cytokines production (32). Sulforaphane could also act as an inhibitory factor in the expression of cyclooxygenase-2 and an inducible factor in lipopolysaccharide-stimulated nitric oxide synthase (33), which all in all, limits the lipopolysaccharide-stimulated production of TNF-a and IL-1b in vitro (6). Likewise, the antioxidative features of broccoli sprouts prevent the onset and progression of diverse micro and macro-vascular issues (6, 34) by the potent induction of phase 2 antioxidant enzymes and reduction of oxidative species (35). Acute supplementation with broccoli sprout is linked with a significant reduction in malondialdehyde, oxidized LDL, and oxidative stress index levels and an increase in total antioxidant capacity (23). Also, daily consumption of fresh broccoli sprout is linked with the reduction of urinary 8-isoprostane and increase in the CoQ10H2/coenzyme Q10 ratio level among healthy individuals (19). Therefore, incubating human microvascular endothelial cells with sulforaphane potentially can prevent mitochondrial dysfunction, trigger the formation of reactive oxygen species, and attenuate hyperglycemia-induced activation of hexosamine and protein kinase C (PKC) pathways and protein glycation under hyperglycemic conditions (31).
To the best of our knowledge, this is the first systematic review and meta-analysis that exclusively focused on the cardiometabolic effects of broccoli sprouts. This research can pave the way for further studies of functional foods with an emphasis on cruciferous vegetables. Nevertheless, the high heterogeneity between the studies is among the limitations of this research, which can be explained by the varying doses and forms of broccoli sprout administration, sulforaphane yield of the supplements, and differences in the study populations and participants’ health status. Also, the low number of eligible studies, the lack of adequate data for subgroup analysis, and the broad inclusion criteria for the qualification of the studies may have increased the heterogeneity and challenged the application of final findings to other populations.
6. Conclusions
In conclusion, this systematic review and meta-analysis study supports the positive effect of broccoli sprout supplementation on managing hypertension and, possibly, hyperlipidemia. Further clinical studies with larger sample sizes, longer duration, and supplementation with standardized glucoraphanin and sulforaphane doses are required to confirm the effectiveness of broccoli sprouts in the management and treatment of other cardiometabolic indicators.