1. Context
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes coronavirus disease 2019 (COVID-19), can infect people from mild to severe. It has blowout quickly over the globe in the months following the pandemic’s detection in December 2019 in China. Minor cases are characterized by fever, drowsiness, and a hacking cough, while severe cases show pneumonia, respiratory failure, and kidney failure (1). In addition, due to lymphopenia and punctate lung inflammation with high concentrations of proliferative inflammatory mediators, the infection can lead to acute respiratory distress syndrome (ARDS) due to the release of cytokines, multiorgan dysfunction, and development of senile septicemia, eventually leading to death (2).
Significant facts related to hereditary pathobiology, having a reactive viral stage, and a retrograde host reaction phase are associated with overall obesity and death in victims of COVID-19 (3). Elevated plasma concentrations of proinflammatory cytokines (IL-6, IL-10, TNF-a, G-CSF, MCP1, and MIP1α), lymphocytopenia, decreased IFN-g protection of CD4+ T cells, and decreased numbers of CD4+ and CD8+ T cells in the marginal blood of previously SARS-CoV-2-contaminated people constitute the riskier and potentially life-threatening events associated with severe COVID-19. Although SARS-CoV-2 is gradually increasing in infection rate, researchers have not yet proposed a particular drug, vaccination program for cancer-causing viruses, or other approved therapeutics to fight SARS-CoV-2, resulting in considerable morbidity and mortality (4, 5).
1.1. New Variants of Severe Acute Respiratory Syndrome Coronavirus 2
Severe acute respiratory syndrome coronavirus 2 variants have emerged in different regions of the world during the ongoing COVID-19 pandemic, including the United Kingdom (UK), South Africa (SA), Brazil, India, and the United States. The spike (S) protein, which is a crucial glycoprotein for infection, immune response, and antiviral therapy against SARS-CoV-2, has been found to have multiple mutations in this new variant (6). Hence, a pressing need remains for safe, potent, and affordable means of preventing and treating SARS-CoV-2 infection (7).
Viruses evolve by mutation, and a new form emerges that does not pose a severe problem. Specific mutations or mixtures of mutations, however, can have selective benefits for the virus, for example, increased transmissibility leading to increased receptor binding and the ability of the host’s immune response to change the surface structures recognized by antibodies (8). Earlier studies by Liu et al. regarding the D614G variant have shown that while the 614G variant provides selective gain, the increased cellular infectivity means no detectable effect on the severity or outcome of infection (9).
The world followed with interest a growing concern in mid-December 2020 as scientists in the UK described the newly discovered coronavirus variant as more transmissible than the viruses already circulating (10). Although many vaccines, such as mRNA vaccines, inactivated vaccines, and adenovirus vaccines, have been licensed in several countries, the danger of SARS-CoV-2 variants persists. To date, Alpha (B.1.1.7), Beta (B.1.351, B.1.351.2, B.1.351.3), Delta (B.1.617.2, AY.1, AY.2, AY. 3), Gamma (P.1, P.1.1, P.1.2), and Iota (B.1.526) circulating in the USA, Kappa (B.1.617.1) in India, Lambda (C.37) in Peru, and Mu (B.1.621) in Colombia are considered the variants of concern and interest (11).
1.2. Host Proteins
A basic approach to treating previral infections is disrupting interactions between hosts and viruses. The functional roles of some host proteins are crucial; for example, when the virus attaches to the host cell membrane and activates the spike protein, the virus can enter the host cell (12).
Angiotensin-converting enzyme 2 (ACE2) in humans can detect the same receptors as SARS-CoV-2 and SARS-CoV, according to ongoing investigations. In addition to proprotein convertase furin, which promotes cell fusion, the serine protease transmembrane serine protease 2 (TMPRSS2) is also a key target enzyme in SARS-CoV-2. This is because TMPRSS2 cleaves away ACE2, increasing viral entry (13). Cathepsin L, like TMPRSS2, also slashes the S-protein, although in the postreceptor binding just before the virus enters the cell. Eventually, another membrane-bound host protease, furin, will also split the coronaviruses-2 S-protein at the furin cleavage site, which is unique to SARS-CoV-2 and not found in any other corona viruses. Dietary cysteine-like protease (3CLpro) protein is a well-known major protease in coronaviruses and is one of the most promising antiviral drug targets against coronaviruses (14).
Recently, researchers’ attention has been drawn to miRNA-based therapies, with some beginning precise in silico studies to link the SARS-CoV-2 genome to the human miRNome to better understand infectious miRNAs’ contribution to SARS-CoV-2 infections (15). One study, drawing on computational studies, suggests that miR-27b plays a critical regulatory role in SARS-CoV-2 infection, showing an important correlation with ACE2. Finding new compounds that block both viral and host proteins linked to SARS-CoV-2 suggests that miR-27b could be part of a drug combination to treat SARS-CoV-2 (16).
1.3. Preventative and Therapeutic Approaches
Even though licensed regular drugs are already used routinely in clinical settings, there is still a pressing need for both specific countermeasures and vaccines. Bioengineered and vectored antibodies, small molecule drugs, and cytokine- and nucleic acid-based therapies that target viral gene expression are all promising treatments for coronavirus infections (17).
Plasma therapy for the treatment of COVID-19 has recently shown unpromising results because of unwanted direct or indirect side effects, all these artificial substances are generally ineffective when used. However, investigating the effects of drug product recombination experimentally can be costly and time-consuming, whereas evaluating them mathematically can provide testable hypotheses for systematic drug product decompression (18).
Complementary medicines involving herbal medicines have been used extensively worldwide since the COVID-19 breakout. The use of herbal medicines with specific active ingredients possessing antimicrobial, antiviral, anti-inflammatory, and immunostimulant effects, like Echinacea, quinine, and curcumin, has become a new trend in the community. Such plant agents are believed to be capable of modulating the immunologic response and are therefore thought to be advantageous in curing or preventing COVID-19 (19).
The WHO states that approximately 80% of the world’s population relies upon healing plants for their medicinal needs. A considerable number of antiviral agents extracted from many herbal species have been used in many studies. In addition, several complex herbal medicines are in clinical research to treat coronavirus-related symptoms (20).
Against this background, clarifying the true potency and safety profile of herbal pharmaceuticals is needed to scientifically support future decisions on the benefits and hazards of their application. Therefore, all current scientific literature on the biological activities of herbal agents has been brought together in a unified framework to support collaborative research for the design of innovative pharmaceuticals/molecular therapeutics to combat current pandemics and beyond (21).
1.4. Natural Products Inhibiting Severe Acute Respiratory Syndrome Coronavirus 2
There is increasing attention to natural products due to their broad therapeutic spectrum and potent antiviral, immune-modulatory, inhibiting inflammatory, and antioxidant properties (22). The present study, hence, aimed to investigate natural products having the potential to both modulate the host immune system and block viral entry into host cells by disrupting engagement with a cellular receptor for the development of a comprehensive and efficient-based roadmap for the treatment of coronaviruses and other virus infections in the coming years. Potential binding inhibitors developed using natural compounds represent a safe and powerful treatment option for coronavirus. Consequently, these natural medicines may be used to treat various signs and symptoms of SARS-CoV-2 infection and other coronaviruses as alternative therapy when no specific antiviral medicine is currently available. Furthermore, natural products are essential in infection prevention, particularly in high-risk patients suffering from coronavirus transmission. In the following, the current research results on compounds against SARS-CoV-2 are discussed, and their specific molecular targets and possible mechanisms of action are summarized (Table 1).
| Name of Plants | Mechanism of Action | Chemical Class | Type of Compounds | Type of Study | Source |
|---|---|---|---|---|---|
| Polyphenols | Inhibiting SARS-COV-2 Mpro and RNA replicase | Phenolics, flavonoids, lignans, hydroxycinnamic acid, stilbenes, and hydroxy acids | Flavonoids, phenolic acid, polyphenolic amides, and other polyphenols | Silico analyses | Fruits, vegetables, herbs, spices, tea, dark chocolate, and wine |
| Naringenin | Cytokine production, reducing viral replication | Antiviral and anti-inflammatory properties | Flavonoids found in citrus fruits | Silico analyses | Citrus fruits like grapefruits, sour orange, tart cherries, tomatoes, Greek oregano |
| Hesperidin | Binding ability to the SARS-CoV-2 3CLpro | Flavonoid | Citrus flavonoids | Silico analyses | Citrus fruit |
| Silymarin | Anti-inflammatory, antioxidant, antiplatelet and antiviral, ability to bind to transmembrane protease serine 2 | Flavonoid called taxifolin | Polyphenolic flavonolignans | Silico analyses | Silybum marianum (milk thistle) plant |
| Resveratrol | Proinflammatory cytokines | Flavonoids | Natural polyphenolic | Silico analyses | Grapes, wine, grape juice, peanuts, cocoa, and berries of Vaccinium species, including blueberries, bilberries, and cranberries |
| Fenoterol | SARS-CoV-2 RNA replicase inhibitors | Polyphenolic | Secondary amino compound, a secondary alcohol and a member of resorcinols | Silico analyses | Synthesis |
| Quercetin | Interaction between the SARS-CoV spike protein and ACE2 and inhibiting viral protease and helicase activity | Flavonoids | Flavonoids | Silico analyses | Fruits and vegetables |
| Eriodictyol | Inhibiting ACE2 | Flavonoid | Flavonoid | Silico analyses | Citrus fruits such as lemons, orange, and grapes |
| Taxifolin | Inhibiting the major protease of SARS-CoV-2 | Flavonoid | Flavonoid | Silico analyses | Onion, milk thistle, French maritime pine bark and Douglas fir bark |
| Alkaloids | Inhibiting protease enzyme, RNA and proteins synthesis | PSM | Nitrogenous compounds of low molecular weight | Silico analyses | Bacteria, fungi, plants, and animals |
| Terpenoids | Antiviral activity against the Mpro and PLpro enzymes of SARS-CoV-2 | Isoprenoids | Isoprene unit | Silico analyses | Marine organisms |
| Glycyrrhizin | Inhibiting viral replication | Glucuronic acid | Triterpene glycoside | Silico analyses | Roots of the liquorice plant |
| Lycorine | Inhibiting viral RdRp activity | Indolizidine alkaloid | Amaryllidaceae alkaloids | Silico analyses | Flowers and bulbs of daffodil, snowdrop (Galanthus) or spider lily (Lycoris) |
Abbreviations: SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; Mpro, main protease; 3CLpro, 3-chymotrypsin-like protease; ACE2, angiotensin-converting enzyme 2; PSM, Plant Secondary Metabolite; PLpro, Papain-like protease 2; RdRp, RNA-dependent RNA polymerase.
1.5. Plants’ Secondary Metabolites Against Severe Acute Respiratory Syndrome Coronavirus 2
Secondary and primary metabolites from plants are considered potential drugs for inhibiting different types of coronaviruses. Their IC50 (the concentration at which there is a 50% loss of enzyme activity), molecular docking, and binding energy are parameters that provide information about the ability of the metabolites to inhibit infectious viruses specifically. Investigators worldwide are trying to find therapeutic agents consisting of plant secondary metabolites (PSMs) against SARS-CoV-2 and new medicinal plant compounds to avert this global strategy crisis (23).
Plant secondary metabolites represent a source of natural antiviral agents that might provide important question since most are safer or cheaper than conventional drugs, although several PSMs are also toxic. Plant pathway metabolites can disrupt enzymatic activity. They participate in the CoV propagation cycle, which includes papain-like proteinase and 3CL protease, stopping the merger of coronavirus S nutrient and host ACE2. They can also inhibit intracellular transduction pathways (24).
2. Experimental Procedure
References for this narrative review were established with four search engines (PubMed, Scopus, Web of Science, and Google Scholar) with listed keywords: Coronavirus disease 2019 pandemic, COVID-19 epidemiology, natural products, herbal remedies, and SARS-CoV-2 variant. The literature was gathered until late August 2021. Results from in silico and in vitro tissue-based and non-surface-based investigations were included. This review includes articles published only in English.
3. Results
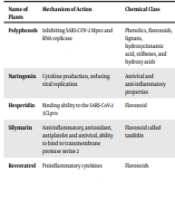
3.1. Role of Polyphenols Against Severe Acute Respiratory Syndrome Coronavirus 2
Various antiviral compounds have been discovered in medicinal plants belonging to different plant families. They include phenols, flavonoids, lignans, hydroxycinnamic acid, stilbenes, and hydroxy acids, all containing several phenolic rings. The benefits of polyphenols include the potential suppression of SARS-CoV-2 spike protein binding to the host cell ACE2 receptors, prevention of viral entry into the host cell, and suppression of viral RNA replication and protein synthesis (23). Molecular model loading studies revealed enhanced binding affinity of polyphenols from Turmeric sp. (curcumin and its derivatives) and Lemon sp. (hesperetin, hesperidin, and tangeretin) for the S-protein compared to the benchmark drug nafamostat. Recently, it has been suggested that ingesting resveratrol, a phenolic compound found at high levels in red grape skin, may influence the severity of SARS-CoV-2 pathogenesis by affecting ACE2 regulation and activity (25). However, the poor solubility and breakdown of most polyphenols in neutral and basic conditions limit their activity (26).
3.1.1. Naringenin
There has been much research on the antiviral and anti-inflammatory properties of naringenin, a flavonoid found in citrus fruits, among many other compounds (27, 28). A study by Alberca et al. provided new experimental evidence that flavanone naringenin, which targets endolysosomal two-pore channels (TPCs), is often added to the list of potential weapons against SARS-CoV-2 inspiration and COVID-19 disease (29). Naringenin’s benefits include reducing or avoiding coronavirus infection (28). In addition, a study conducted by Clementi et al. has shown that it is possible to inhibit the activity of human TPCs by the natural flavonoid compound naringenin, one of the essential flavonoid compounds in people’s nutrition (27). Although the data suggest a novel pharmacological strategy against CoV, naringenin is promising for efficient and safe prophylaxis and therapy (29).
3.1.2. Hesperidin
Citrus flavonoids have been proposed as potential antiviral drugs against coronaviruses and therapeutics to combat inflammatory diseases that may result in symptoms of more severe viral illness. Recent research using molecular model quantification has identified the flavonoid hesperidin, the glycosylated version of hesperidin, and the main form in which this flavonoid is found throughout citrus (30). Nevertheless, at high concentrations of hesperidin, our results showed that in vitro citrus zest has little inhibitory effect against SARS-CoV-2 3CLpro. Furthermore, molecular docking revealed that curcumin, hesperidin, diosmin, apiin, and rutin are potent inhibitors of COVID-19 (31). Hesperidin’s anti-inflammatory properties and ability to bind to the coronavirus spike are its benefits (30). Based on docking, molecular dynamics (MD) simulation behaviors, and known antiviral activities, Kumar et al. showed that hesperidin and conivaptan were recognized as potential SARS-CoV-2 exon inhibitors (32). In conclusion, as a potential COVID-19 treatment, we recommend further exploration of this combination therapy with RNA-dependent RNA polymerase (RdRp) inhibitors and a newly developed exon inhibitor (32).
3.1.3. Silymarin
As an extract fraction, silymarin is derived from the seeds of the traditional thistle plant, a member of the Asteraceae family. Mediterranean regions like Crete, Greece, Iran, and Afghanistan are native to this plant (33). Pharmacologically, silymarin is a complex of seven closely related polyphenolic flavonolignans with a flavonoid called taxifolin. Appreciated for its various pharmacological activities such as anti-inflammatory, antioxidant, antiplatelet, and antiviral with versatile functions regulating immune cytokines, silymarin can bind to TMPRSS2 and the interferon-stimulated gene of the antiviral cytokine for the treatment of coronavirus (34).
Because silymarin has antiplatelet activity and may prevent coagulation, it may be utilized to treat and recover from strokes linked to SARS-CoV-2-induced thrombotic pulmonary embolism (33). A computational study by Speciale et al. showed that silibinin forms a stable complex with the SARS-CoV-2 spike protein RBD, exhibits good negative bonding affinity towards main protease (Mpro), and interacts with many residues at the site of Mpro, supporting its potential to inhibit virus entry and replication (35). Additionally, in silico data suggested that silibinin prevents SARS-CoV-2 from entering and replicating into host cells (35).
The action mechanisms of IL-6-targeting monoclonal antibodies and pan-JAK1/2 inhibitors are predicted to be phenotypically integrated by silibinin, which directly modulates downstream STAT3 activity within the unsuccessful cycle of SARS-CoV-2-injured lung tissue (36). Therefore, silibinin could act as an immunotherapeutic agent to relieve the cytokine storm and T-cell lymphopenia within the clinical scenario of a subset of intense COVID-19 sufferers who completely meet ARDS criteria (35).
Considering silibinin’s anti-inflammatory and anticoagulant traits at the endothelium and its proven cap potential right here to interact with key goal proteins of SARS-CoV-2, silibinin can be a sturdy candidate for the remedy of coronavirus from a multitargeted perspective (37).
3.2. Role of Flavonoids in Combating Coronavirus Disease 2019 Pandemic
Citrus fruits are abundant in flavonoids, which have been shown to have antiviral effects. In some studies, the significance of flavonoids as antiviral agents against other respiratory diseases, including SARS-CoV-2, has even been discussed. The binding of flavonoid compounds to these residues could reduce the catalytic activities of Mpro, ultimately leading to a decrease in viral replication (38). In addition, flavonoids showed evidence of a significant capacity to reduce COVID-19 exacerbation in obesity by promoting fat metabolism. In addition, flavonoids have an excessive protection profile, suitable bioavailability, and no enormous side effects. For instance, flavonoid-wealthy plants are extensively allotted worldwide and may offer suitable safety against coronavirus (39).
Flavonoid-like compounds like apigenin and quercetin exhibited activity directed toward SARS-CoV. Also, SARS-CoV-2 Mpro activity can be blocked by flavonoid substances in vitro and in silico (20). According to Alzaabi et al., the effects strongly advocate the promising multi-goal activity of flavonoids, mainly quercetin and luteolin, against SARS-CoV-2, promoting their use in the present and possibly future epidemics (19). Gentile et al. tested a marine natural product (MNP) library in search of the latest SARS-CoV-2 Mpro inhibitors (21). Phlorotannins, flavonoids, and pseudopeptides are only a few of the chemical families that have been shown to inhibit SARS-CoV-2 Mpro, as was the case with SARS-CoV-1 Mpro. Future tests of the ligands identified in this study’s in vitro activity assays will provide crucial information on novel scaffolds for optimization (21).
Flavonoid glycosides such as rutin and nicotiorin from the plant Dysphania ambrosioides, in addition to their glucuronide and sulfate derivatives, have emerged as potent inhibitors of SARS-CoV-2 Mpro and RdRp through a molecular docking approach (40). The demonstration of the best docking score, the site of the ligands on the inhibitory site, the interplay profile with the catalytic residues, and the suited ADMET parameters of the QSAR version advise that chrysin-7-O-glucuronide can be powerful against SARS-CoV-2 Mpro. In addition, in vitro and in vivo research themselves to the conventional preventive use of Oroxylum indicum and could offer precious statistics on new scaffolds for optimization (41).
3.2.1. Resveratrol
Resveratrol is a natural polyphenolic compound formulated with the aid of numerous plants in reaction to physiological pressure or bacterial and fungal infections (42). The expression of ACE2, regulation of the renin-angiotensin system (RAS), activation of the immune system, and suppression of the release of proinflammatory cytokines are just a few of the significant pathways shown to be attenuated by resveratrol in the pathogenesis of SARS-CoV-2 (43). A study by Ramdani and Bachari highlighted the importance of resveratrol as a potential therapeutic agent in SARS-CoV-2 infections (43). The RAS and the production of ACE2 are two of the critical pathways connected to the pathogenesis of SARS-CoV-2 that have been demonstrated to be attenuated by resveratrol; the stimulation of the immune system and the downregulation of the release of proinflammatory cytokines are the others (43).
According to those reports, it has been recommended that resveratrol is a capable healing agent for the treatment of SARS-CoV-2 (44). Moreover, Pasquereau et al. demonstrated the antiviral impact of resveratrol on human coronavirus (HCoV)-229E and SARS-CoV-2 replication in vitro (45). The SARS-CoV-2 inhibition experiments additionally displayed the antiviral impact of resveratrol in vitro. Finally, they recommended using resveratrol in a scientific setting, with the aid of using itself or as part of a routine against coronavirus (45).
3.2.2. Fenoterol
Using computational approaches, the polyphenolic 2-adrenergic receptor agonist fenoterol, the naturally occurring flavone baicalin from Scutellaria baicalensis, and several xanthones from Swerti apseudochinensis were identified as potent SARS-CoV-2 RNA replicase inhibitors (46). In other in silico studies, epigalloca gallate, myricetin, quercetagetin, and numerous polyphenols have been suggested to have excessive binding affinity for the RdRp of SARS-CoV and SARS-CoV-2 (47).
3.2.3. Quercetin
Quercetin is one of the most important natural flavonoids found in most fruits and vegetables and a few plant leaves. On the viral side, this flavonoid is proposed as a leading candidate because it inhibits the interaction between the SARS-CoV spike protein and ACE2 and inhibits viral protease and helicase activity. At the same time as in the host cell direction, it inhibits ACE activity and increases intracellular zinc (48). In the early stages of SARS-CoV-2 viral infection, quercetin may prevent the onset and progression of the disease (49). Alzaabi et al. showed that quercetin and luteolin might have potential multitarget actions against SARS-CoV-2, which supports their use in current outbreaks and future outbreaks (19).
Moreover, modern molecular docking assays and in silico screening of plant-derived drugs have proven that quercetin is certainly considered one among numerous capable inhibitors of 3CLpro of SARS-CoV-2 (50). Quercetin is currently undergoing research and clinical trials to determine whether it is potentially effective as a drug against SARS-CoV-2. These clinical trials investigate quercetin’s antioxidant and antiviral properties and the possibility of developing drugs without side effects (38).
In addition, a proof of concept was provided by a study by Kushwaha et al., showing the docking of key SARS-CoV-2 proteins to quercetin (51). Their findings revealed that quercetin is a capable drug molecule. More studies are urgently needed in vitro and in vivo to permit using quercetin, primarily based on total drugs, as early as possible (51). Directing quercetin to the active site of RdRp ASP761 might be a possible healing choice to inhibit SARS-CoV-2 replication. Similarly, Aftabet utilized this computational technique and anticipated that ASP761 strongly binds the antiviral drug galidesivir, indicating that quercetin has an ability much like galidesivir (52).
3.2.4. Eriodictyol
A flavonoid belonging to a subclass of flavanones, eriodictyol, is widely distributed among citrus fruits, vegetables, and plants of medicinal importance, resulting in no reported toxicity or adverse reactions. Eriodictyol, an avanone occurring in Eriodictyon californicum, exhibited one of the highest affinities for ACE2 out of all other contenders (53). Quite recently, a docking study by Deshpande et al. reported that eriodictyol exhibits good binding energies and antiviral effects through its cellular multi-targeting capabilities directed against several proteins of SARS-CoV-2, in particular ACE2 (54). In addition, eriodictyol binds strongly to virus-replicating proteins such as the SARS-CoV-2 helicase compared to many already known drugs, which may be helpful in RNA recognition. DNA duplex is a unique characteristic of viruses and the active sites of the SARS-CoV-2 spiked glycoprotein C chain of 10 amino acids. For this reason, eriodictyol has emerged as a novel multitarget molecule against SARS-CoV-2. This indicates that eriodictyol could influence the virus’s life cycle by interacting with several proteins (54).
3.2.5. Taxifolin
Molecular docking was used to evaluate a group of 44 citrus flavonoids against the SARS- CoV-2 highly conserved Mpro. Of the 44 citrus flavonoids, five of them showed a lower affinity for Mpro than the co-crystalline ligand. The lowest reported IC50 value for taxifolin was among these. In order to battle the current pandemic, the current work reveals that taxifolin may also be a possible inhibitor against the primary protease of SARS-CoV-2. This possibility may be further explored through in vitro and in vivo investigations (55).
3.3. Role of Alkaloids Against Severe Acute Respiratory Syndrome Coronavirus 2
Alkaloids fall within the PSM group and may be primary, with at least one nitrogen atom in their structure. Based only on the heterocyclic ring, they are categorized into several classes, including tropanes, pyrrolidines, isoquinoline purines, imidazoles, quinolizidines, indoles, piperidines, and pyrrolizidines. They are made by animals, fungi, and terrestrial plants. Emetine, ipecac, macetaxime, tylophorine, and 7-methoxy-cryptopleurine are only a few of the alkaloids whose anti-SARS action has been demonstrated by suppressing the creation of proteins, RNA, and the protease enzyme (56).
Through blocking pathogenesis-associated targets of the Coronaviridae family, which are necessary for the virus life cycle, alkaloids have demonstrated potential anti-SARS-CoV activity (57). 10-hydroxy usambarensines, an alkaloid discovered in the roots of Strychnos usambarensis, and cryptoquindoline and cryptospirolepine, both found in Cryptolepis sanguinolenta, were found to have an abnormal amount of binding affinity for the SARS-CoV-2 Mpro (58).
Chloroquine, an alkaloid derivative, has been proven powerful against anti-SARS-CoV-2. Consequently, a few PSMs in the shape of alkaloids may offer opportunity drug goals for COVID-19 (59). This examination confirmed that 10-hydroxyusambarensine had the most powerful interactions with 3CLpro of SARS-CoV-2, while cryptospirolepine confirmed the best binding affinity and selectivity for 3CLpro of SARS-CoV and middle east respiratory syndrome-coronavirus (MERS-CoV). Furthermore, some other in silico studies discovered that the alkaloids of Cryptolepis sanguinolenta, particularly cryptomisrine, cryptospirolepine, cryptoquindoline, and biscryptolepine, exhibited sturdy binding to RdRp, indicating that they are potent RdRp inhibitors (57).
In a molecular dynamics study, anisotine, adhatodine, vasicoline, and vasicine alkaloids from the leaves of J. adhatoda showed potent inhibition of SARS-CoV-2-Mpro, making these drugs suitable candidates for protease inhibition for further in vitro and in vivo validation (44). More recently, an assessment of the antiviral efficacy of herbal alkaloids known as homoharringtonine (HTT) and emetine has yielded promising results against coronaviruses, including SARS-CoV. These alkaloids can be used as antiviral agents against SARS-CoV-2 since they have already been reported to inhibit the replication of SARS-CoV and other viruses across cell lines (60). Ismail et al. demonstrated that of the 71 compounds tested, as many as 23 were chosen for molecular docking owing to their pharmacokinetic and toxic profiles (61). The findings revealed that the three targets of SARS-CoV-2 Mpro, S-protein, and ACE2 had significant binding to norquinadoline A, deoxytryptoquivaline, and deoxynortryptoquivaline (61). Norquinadoline A, deoxytryptoquivaline, and deoxynortryptoquivaline have good pharmacokinetic and safety profiles, indicating that they are potential natural multitarget drugs against COVID-19. Therefore, as their efficacy and mechanisms of action are confirmed, these three alkaloids could be starting points for future drug development (62).
3.4. Role of Terpenoids Against Severe Acute Respiratory Syndrome Coronavirus 2
Terpenoids or isoprenoids are described as various and, therefore, the largest group of natural products originating from isoprene moieties, which can function as a source for brand spanking new medicines or prototypes for developing potent pharmacotherapeutic compounds (63). Terpenoids have numerous medicinal qualities, such as antiviral, antibacterial, and antioxidant activity. These natural products have antiviral activity against the Mpro and papain-like protease 2 (PLpro) enzymes of SARS-CoV-2. Computational programs concerning a complete of molecular dynamics simulations resulted withinside the identity from the version of promising Terpenes for inhibition of the proteases of the SARS-CoV-2 virus (64). The methyl tanshinonate, sugiol, and α-cadinol are all expected to be exceptional applicants to inhibit Mpro. At the same time, 8-β-hydroxyabieta-9,13-dien-12-one, dehydroabietate 7-one, and tanshinone I are better positioned for PLpro inhibition of SARS-CoV-2 (65). In addition, evidence confirmed that the terpene ginkgolide A strongly inhibited the SARS-CoV-2 protease enzyme (66).
3.5. Role of Glycyrrhizin Against Severe Acute Respiratory Syndrome Coronavirus 2
A vital constituent of liquorice root, glycyrrhizin (or glycyrrhizic acid), comes from Glycyrrhiza glabra L. and Glycyrrhiza uralensis Fish. ex DC (67). Additionally, glycyrrhizin was shown to possess antiviral activity against HIV-1, SARS-CoV, and respiratory syncytial virus in vitro. Subsequent studies performed on clinical isolates of coronavirus demonstrated glycyrrhizin’s efficacy in inhibiting viral replication and, therefore, the penetration and adsorption of the virus into the host cell at non-cytotoxic levels (68).
Emerging research based on an incorporated computational primarily based method and pharmacological issues offers similar proof to support an adjuvant position for glycyrrhizin in coronavirus treatment (69). Bailly and Vergoten thoughtfully examined in a timely review the prospective improvement of glycyrrhizin analogs, not only as antiviral drugs, but also as an adjuvant remedy for their protector impact on susceptible organs in patients with SARS-CoV-2 infection (67). Given this history, it is time to find a way to get this proven secondary phytochemical that protects against SARS-CoV-2-3CL approved as a medicine so that it can be used in the future.
According to the authors, there may be an ability for glycyrrhizin to bind to ACE2. This result shows that for the remedy of COVID-19, glycyrrhizin can be a promising drug. Meanwhile, previous study determined that glycyrrhizin is an effective and non-toxic broad-spectrum anti-coronavirus molecule in vitro, especially against SARS-CoV-2 (70). Based on Sinha et al., glyasperine A and glycyrrhizic acid can be considered the greatest molecules from liquorice that can be beneficial against COVID-19 (71).
3.6. Role of Lycorine Against Severe Acute Respiratory Syndrome Coronavirus 2
The binding position of lycorine overlaps with the nucleoside rings of remdesivir at an equivalent pocket region of the catalytic site of the SARS-CoV-2 RdRp protein (72). Lycorine’s anti-SARS-CoV-2 activity was attributed to the modulation of host factors during a recent study by Zhang et al. (73). Even so, it could be intriguing to consider this chemical as a potential future contender for preventative intervention against 3CL.
As a bioactive constituent of the plant family Amaryllidaceae, lycorine is determined in Hymenocallis littoralis (Jacq.) Salisb., Lycoris radiate (L’Hér.) Herb. and Narcissus pseudonarcissus.cv. Dutch Master, which are traditionally used for wound recuperation in many nations and the remedy for most cancers and some infectious diseases (74). Lycorin was reported by Jin et al. to be a potent, non-nucleoside, direct-performing antiviral agent against newly rising variants of coronavirus, performing via inhibiting viral RdRp activity; consequently, lycorin can also additionally be a candidate drug against COVID-19 (72).
4. Conclusions and Future Perspective
Infection with COVID-19 is a significant life-threatening disease. As known, no antiviral medicines on the market play an important role in curing COVID-19 patients. Natural products and PSMs have been used for many years to treat viral infections and boost the host’s immune response. Since the pandemic outbreak, there has been increasing scientific evidence that natural products can help alleviate the symptoms of COVID-19. Hence, researchers are trying hard to find highly potent antiviral compounds targeting COVID-19. Our study has discovered several secondary plant metabolites that interfere with critical components of coronavirus pathogenesis and reproduction to have antiviral efficacy against coronaviruses. According to the present review, some PSMs are promising anti-COVID-19 agents, which can block essential proteins, e.g., PLpro, Mpro, ACE2, and RdRp. Among them, green tea beverage (GTB) or its principal ingredient, epigallocatechin gallate (EGCG), bis-benzylisoquinoline alkaloids, and neferine as coronavirus entry inhibitors are highly effective in inhibiting infection of the new variants. More studies are still needed to prevent or evaluate COVID-19 virus infection with appropriate herbal metabolites or natural medicines. However, medicinal plants only work best in combination with modern medical treatment, assistive devices (such as ventilators), and intensive care. Research must continue quickly to find effective PSM compounds to treat the infection.
4.1. Limitations
One of the limitations of our research was the uncertainty of the effect of herbal medicines, the long duration of the drug’s effect on the treatment of the coronavirus, and the lack of sufficient information about it.