1. Background
Cancer is one of the significant causes of mortality behind cardiovascular disease worldwide (1). Neuroblastoma (NB), an aggressive childhood disease of the nervous system, is the second most common type of pediatric tumor with unique features (2). NB is often malignantly and characterized by undifferentiated cells resulting in various clinical presentations of this cancer (3). Since NB is identified in advanced stages, despite intensive therapy, it has survival rates of only 40 - 50% (4). NB treatment includes surgical methods, standard chemotherapy, and chemotherapy. Moreover, the toxic side effects of chemotherapy cause lifelong health issues (4).
In addition to damaging the proliferative cells, resistance to anticancer drugs is the reason for using different agents to control such effects. Natural products have always served as vital resources for cancer therapy, and the application of traditional medicine in the world has gradually increased. It has been known that phytochemicals and phytotherapy may overcome the existing shortcomings of conventional cancer therapies (5).
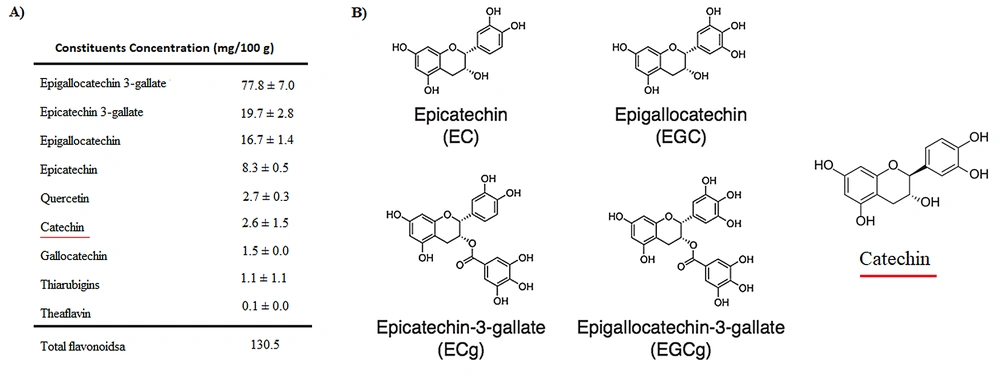
Green tea is one of the most popular drinks in the world. It is derived from the plant Camellia sinensis, with beneficial properties for human health. Green tea has been applied to the prevention of cancer and cardiovascular diseases. It has also contained anti-inflammatory, antiarthritic, antibacterial, antiangiogenic, antioxidative, antiviral, and neuroprotective effects in traditional medicine. The tea contains several bioactive chemicals and is particularly rich in catechins (Figure 1) (6, 7).
Catechins are flavonoids (also known as flavanols), known as most polyphenol compounds in green tea. Catechin varieties in green tea include epicatechin (EC), epicatechin-3-gallate (ECG), epigallocatechin (EGC), and epigallocatechin-3-gallate (EGCG) (8). The anticarcinogenic effect of EGCG was found on the skin and several cancer cells as glioblastoma (9).
Doxorubicin (DOX), known as an anthracycline antibiotic, is the first-line chemotherapeutic for many cancers (10). Damage to non-target tissues, therapeutic dose limitation, and diminishing patients' life quality are the major problems in DOX treatment. Moreover, multidrug resistance (MDR) is another limitation of the long-term use of DOX (11).
2. Objectives
Various efforts have recently been made to ameliorate the side effects of DOX. To reach this goal in the present study, we design a combination of pure natural catechin with DOX to enhance the antitumor efficiency. Therefore, a mixed model containing non-effective concentrations of catechin and DOX was fabricated against the human neuroblastoma cells to evaluate drug synergism on cell apoptosis and cytotoxicity.
3. Methods
3.1. Materials
Human Neuroblastoma cells [BE(2)Cs] were purchased from the National Cell Bank of Iran (NCBI). Fetal bovine serum (FBS), penicillin, streptomycin, and DMEM medium were bought from Gibco (USA). Trypan blue and methyl green dyes were purchased from Merck. Pure green tea catechin, dimethyl sulfoxide (DMSO), propidium iodide (PI), hoechst, and 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT) were obtained from Sigma- Aldrich (UK).
3.2. Cell Culture
The BE(2)C cells were cultured in high glucose DMEM medium supplemented with 10% FBS containing penicillin and streptomycin (100 U/mL and 100 ug/mL) at 37°C and 5% CO2. The HDFNs as normal cells were cultured in RPMI medium containing 10% FBS at the mentioned atmosphere condition.
3.3. MTT Cytotoxic Assay
The cytotoxicity effects of catechin, DOX, and their combination on BE(2)C cells were assessed using the MTT test. Briefly, the cells were seeded in 96-well plates, and after 24h, the culture medium was replaced with a fresh one consisting of different concentrations of samples: (1) DOX: 10, 20, 50, 100, 200, 300, 400, 600 and 800 ng/mL; (2) Catechin: 40, 80, 100, 150, 200, 300, 400, and 500 µg/mL; (3) Catechin and DOX combination.
After 24 and 48h, the cells were incubated with 20μl of 5 mg/mL MTT solution in phosphate-buffered saline (PBS) for 4 hours. The formazan crystals produced from MTT were dissolved in DMSO. Finally, the absorbance was recorded using a multi-well spectrophotometer at 490 nm. IC50 values were calculated by a linear approximation regression of the percentage survival versus the drug concentration:
The combined effect was analyzed with Compusyn software (Published for PC windows in 2005 by ComboSyn, Inc., Paramus, NJ. 07652 USA). According to the quantitative determination, the combination index (CI) was calculated, and the drug combination is considered synergism if CI < 1.
3.4. Morphological Analysis Using Giemsa Staining
The morphological changes of BE(2)C cells were assessed with Giemsa staining. The treated cells were fixed with cold methanol for 10 min, washed with PBS, and then stained with Giemsa solution (100 uL/1mL) for 5 min. Finally, the cells were washed and dried, and their morphological changes were observed and photographed under an inverted light microscope.
3.5. Nuclear Morphology Changes
Morphological changes of the cells were sassed using Hoechst staining which was used to evaluate apoptosis-associated morphological changes. The BE(2)Cs have seeded on glass microscopic slides at a density of 5 × 104 cells and incubated overnight. Then the cells were incubated in various concentrations of the drugs. Eventually, the cells were washed, fixed with methanol, and stained with Hoechst-33342 to diagnose chromatin changes.
3.6. Cell Cycle Analysis
For cell cycle analysis, both the suspended and attached cells were harvested and washed with PBS. Then, the cells were fixed in cold 70% ethanol and washed at least once with cold PBS. The cells suspend in 500 μL PI solution (RNase A, PI, PBS) and incubate at 37°C for 30 minutes. DNA content was analyzed with a flow cytometer using FLOWJO® software (Becton, Dickinson, and Company; 2021, USA).
3.7. Wound Healing/Cell Migration Assay
Cell motility was evaluated by wound healing assay. The cells were grown to form a monolayer. Then, the cell surface was manually scratched with a sterile pipet tip. Finally, the cells were PBS-washed and treated with various concentrations of the drugs. The wound closure and cell migration were monitored by photography at 0, 24, 48, and 72h.
3.8. Clonogenic Assay
The colony-forming activity of BE(2)C cells was also evaluated. The cells were seeded on 12-well plates (800 cells/well). Subsequently, the cells were treated with drugs and cultured for 8 - 10 days. After this period, the cells were fixed and stained with Giemsa at room temperature, images were captured, and the colonies were analyzed.
3.9. Statistical Analysis
All experiments were done at least three times, and the data were shown as mean ± standard error of the mean (SEM). The results were analyzed by one-way ANOVA followed by Tukey's post hoc test. P ≤ 0.05 was considered significant.
4. Results
4.1. Inhibitory Effects of Catechin, DOX, and Their Combination on BE(2)C Cells
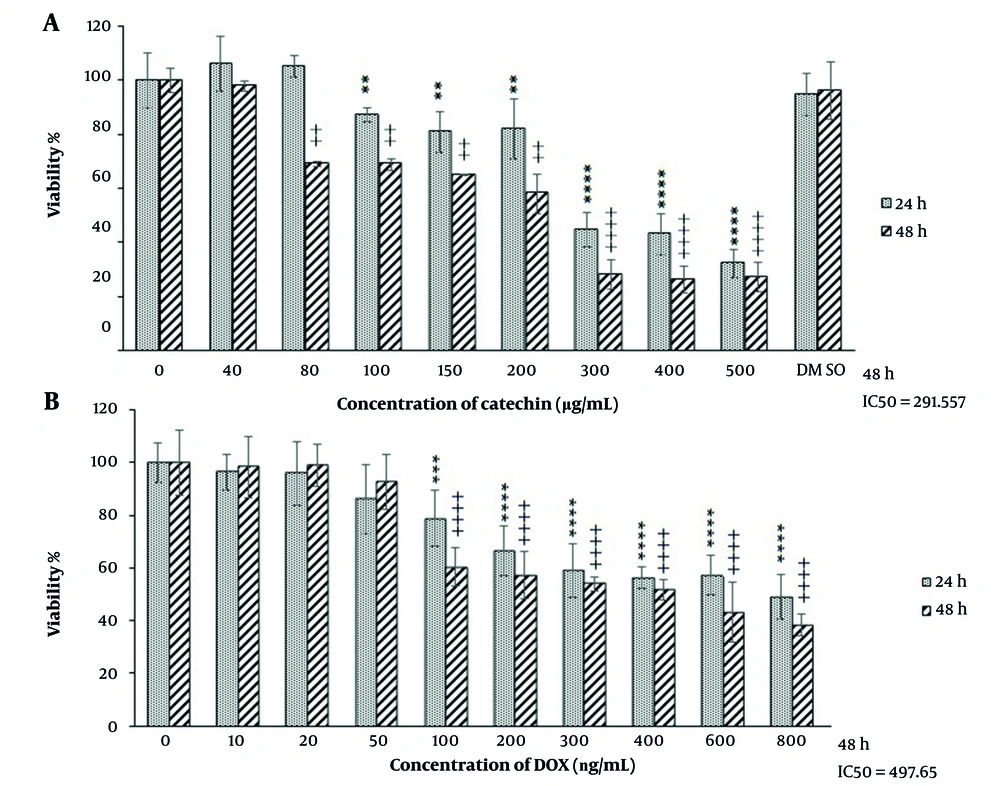
The antiproliferative effect of catechin and doxorubicin was investigated by MTT assay. As shown in Figure 1, high concentrations of catechin inhibited BE(2)C cell growth after 24 h, while the lower concentrations of catechin inhibited cell proliferation in a dose-dependent manner with IC50s of 261.5 µg/mL. Following a 48h incubation period, DOX caused cell death at 497.6 ng/mL concentration (Figure 2).
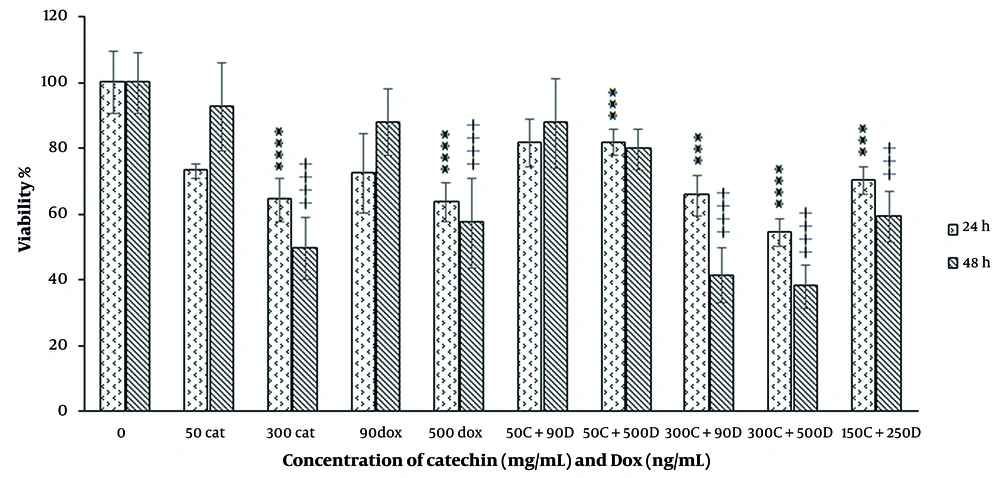
In the combination mode of catechin and DOX, the combination of ineffective doses did not show a significant cytotoxic effect during 48 h treatment. However, the combination of IC50 dose of catechin (300 µg/mL) with an ineffective dose of DOX significantly inhibited the growth of the cell. In this situation, the combination indices calculated by CompuSyn software were lower than 1, which confirmed the synergism between these compounds (Figure 3). In another combination experiment, we used ½ IC50 doses of catechin and DOX, which caused growth inhibition significantly with CI ≤ 1 means synergism during 48 h incubation.
4.2. Morphological Evaluation of Apoptosis
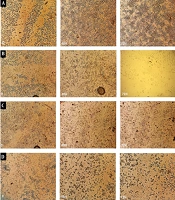
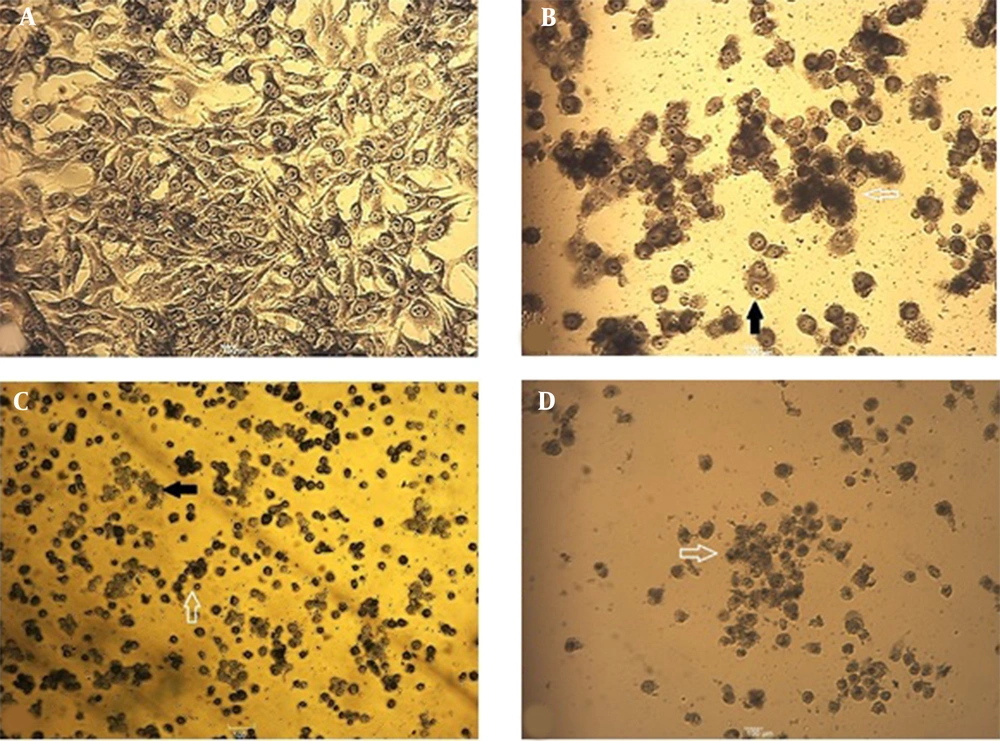
Following the evaluation of catechin and DOX cytotoxicity on the cells, morphological changes of treated cells were analyzed. As shown in Figure 4, following 48 h, cytoplasmic shrinkage (as the first event in cell death via apoptosis) was detected. Indeed, cell adhesion reduction and buoyancy were observed in more than IC50 doses of DOX (500 ng/mL). The control samples contained visible nucleoli under the light microscope, yet not in the nuclei of the treated cells. The treated cells lost their normal orphology and also their nuclei uniformity.
Morphological changes of BE(2)C cells stained with Giemsa after 48 h. The cell volume decreased, and chromatin condensation and cytoplasmic shrinkage were observed. A, Control cells with intact cytoplasm and nuclear; B, The cells were treated with IC50 concentration (300 µg/mL) of catechin; C, The cells were treated with IC50 concentration (500 ng/mL) of DOX; D, The cells were treated with the combination of ½ DOX and ½ catechin (25X). Solid arrows show attached cells, and hollow arrows indicate floating and round cells.
4.3. Chromatin Changes
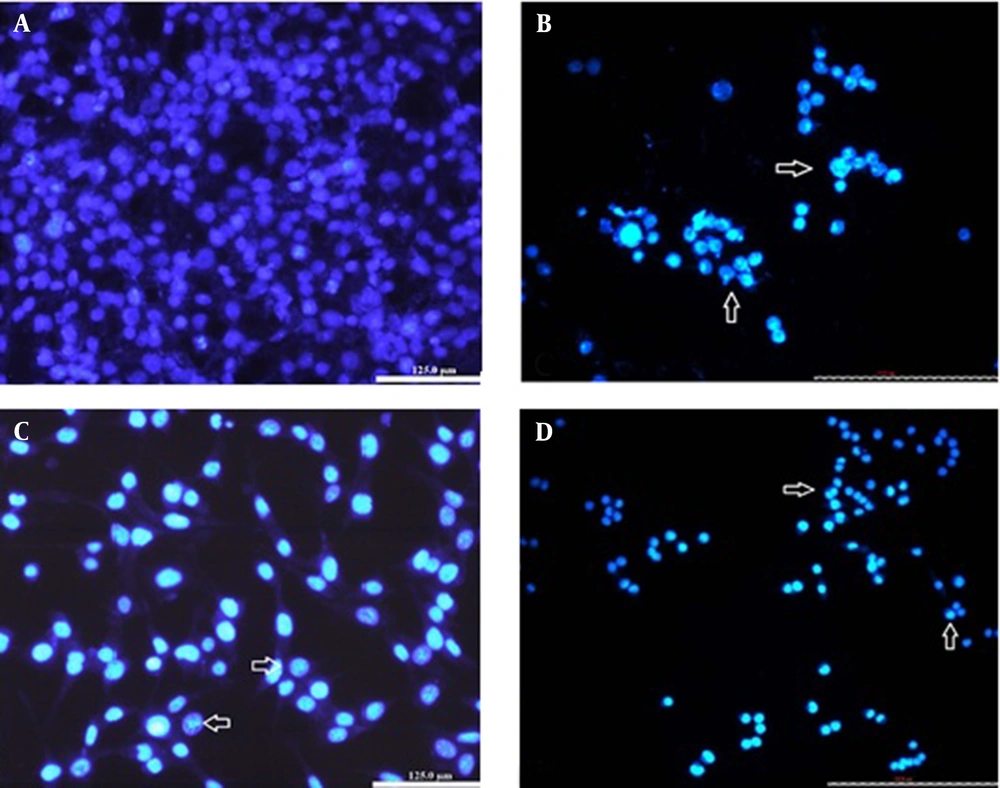
Besides cytoplasmic shrinkage, nuclear morphological changes of apoptosis were assessed with fluorescent die Hoechst-33342 staining. After 48h, the control cells appeared to be intact with shape containing nuclei that were stained with a less bright blue fluorescence (Figure 5). In the cells treated with IC50 of catechin and DOX, cell decrement accrued, and the cells showed apoptotic nuclei with condensed or fragmented chromatin (uniformly fluorescent). In the drug combination samples (1/2 IC50 doses), cell apoptotic bodies were also detected (Figure 5). These nuclear alterations might prove that the drugs induce apoptotic cell death in BE(2)C cancer cells.
The BE(2)C cells with Hoechst staining after 48h. A, Control cells with round nuclei stained evenly; B, The cells were treated with IC50 concentration (300µg/ mL) of catechin; C, The cells were treated with IC50 concentration (500ng/ mL) of DOX; D, The cells were treated with the combination of ½ DOX and ½ catechin (25X). The nuclei are generally fragmented, condensed, and stained intensely. Nuclear morphological changes were shown with arrows.
4.4. Cell Cycle Analysis
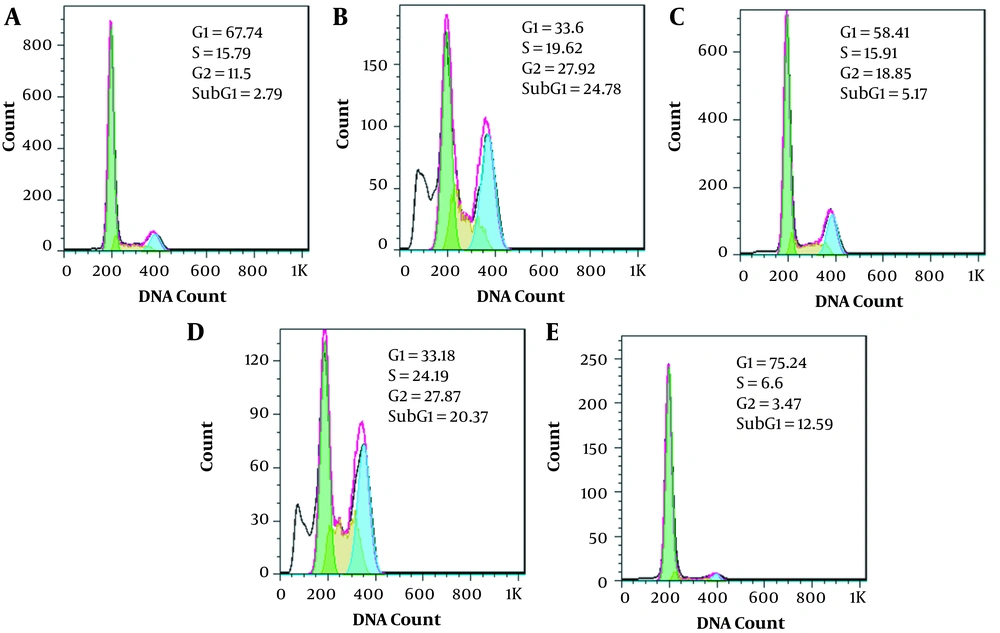
In cancer cells, control of the cell cycle is evaluated to be an effective strategy for tumor growth control (12). It is known that a large number of 180 bp DNA fragments accumulated in the cell at the end of the apoptotic cascade, though a hypodiploid or 'sub-G1' peak in a DNA histogram would be detected (13). The cell cycle analysis demonstrated that the sub-G1 apoptotic fraction was significantly increased in catechin IC50 treatment (Figure 6). The results also indicated a G/S cell cycle arrest in the presence of catechin. The same results were not observed in DOX-treated samples, in which the effective dose (500 ng/mL) caused G2/S cell cycle arrest. An envisaged combination of the effective doses increased the number of sub-G1 cells and cell cycle arrest in the G2/S phase. The interesting point was detected in the combination of ½ IC50 doses of the drugs in which a typical sub-G1 fraction, representing the apoptotic cell population, appeared (Figure 6).
The effect of catechin, DOX, and their combination on the BE(2)Cs cell cycle. A, Control; B, 300 µg/mL of catechin; C, 500 ng/mL of DOX; D, Combination of 300 µg/mL + 500 ng/mL; E, Combination of 150 µg/mL + 250 ng/mL; F, Distribution of cells in the various phases of the cell cycle. Each figure shows one of the three experiments.
4.5. Cell Migration Assays
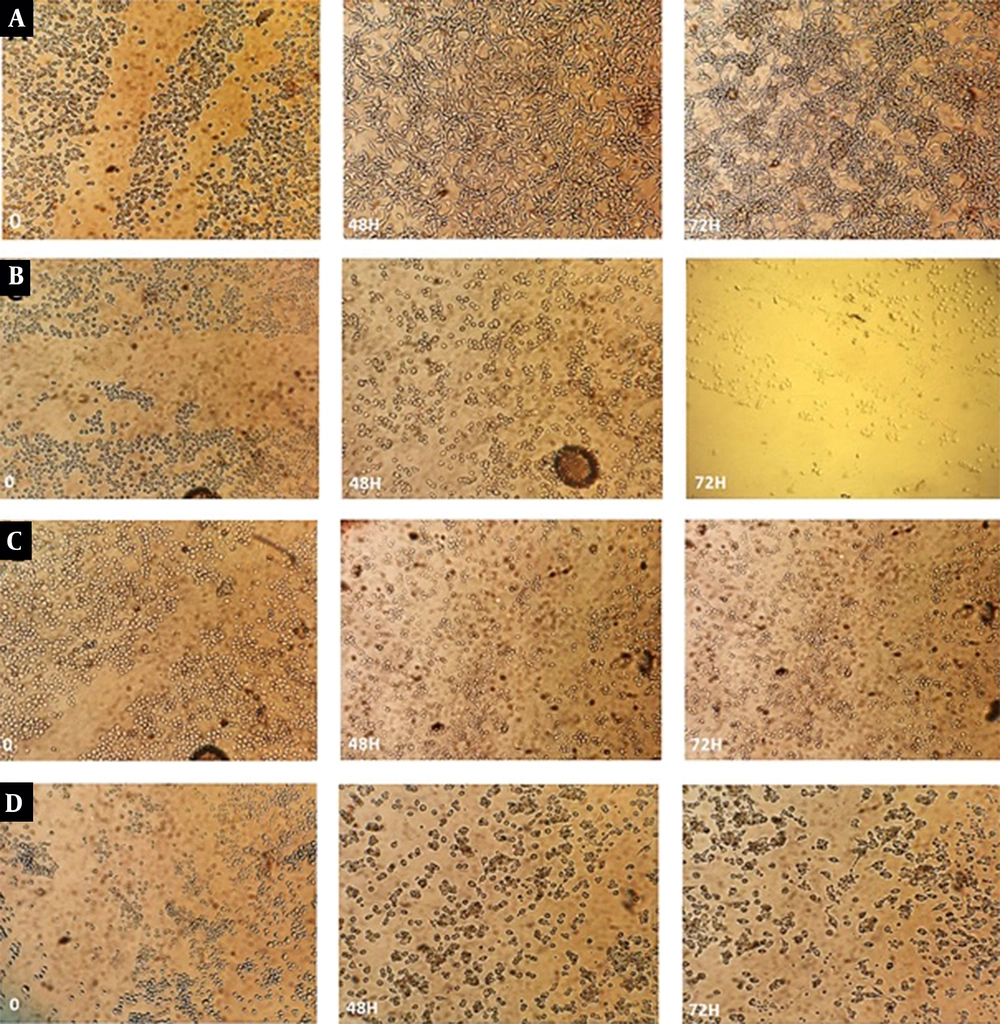
Cell migration and invasion are the key events of tumor metastasis (14). We further confirmed the inhibitory effects of the drugs on cell growth by the migration assay. Compared with the control, catechin and DOX treatments caused inhibition of cell migration in BE(2)C cells (Figure 7). In addition to the combination of effective doses, cell migration through the scratches was restrained significantly in the combination of ½ IC50 doses.
The effect of catechin, DOX, and their combination on cell migration. BE(2)C cells were grown to confluence, wounded (t = 0 h), and then treated with different concentrations of the drugs. Cells were observed under a light microscope and imaged following 72 h. A, Control; B, 300 µg/mL of catechin; C, 500 ng/mL of DOX; D, Combination of 300 µg/mL + 500 ng/mL; E, Combination of 150 µg/mL + 250 ng/mL.
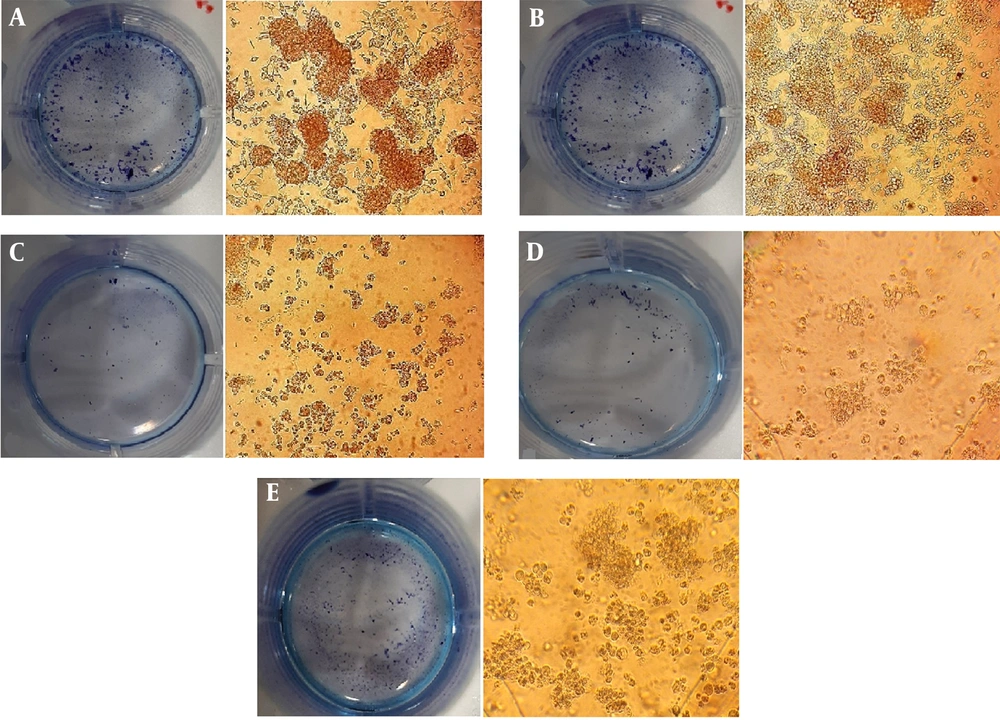
4.6. Colony Formation
The clonogenic assay is an in vitro cell survival assay based on the ability of a single cell to grow into a colony. As shown in Figure 8, following catechin, DOX, and their synergistic combination treatments, the size of the single colonies and the number of colonies obviously reduced after 10 days. Since the drug administration, the reduction of colony numbers revealed cell proliferation inhibition, leading to the anti-tumor activity of cells.
5. Discussion
Polyphenol compounds such as catechins contain powerful protective effects in cancer chemoprevention (15, 16). In cancer treatment, catechin synergistic effects in combination with conventional anticancer drugs are a great deal of attention as they could act as P-glycoprotein (P-gp) modulators and are key benefits in overcoming MDR. This study investigated the protective effects of pure catechin on DOX-induced toxicity in neuroblastoma cancer cells. Due to the MDR, DOX is a significant problem in Neuroblastoma (2, 17). To achieve success in Neuroblastoma restrain, we employed a combination mode of catechin and DOX to inhibit cancer cells more effectively and synergistically.
First of all, the results showed that catechin decreased the BE(2)Cs proliferation significantly in a dose-dependent manner during 48h. It was shown that tea catechins suppressed proliferation and induced apoptosis in different cancer cells such as breast, melanoma, and cervix in vitro (18, 19). In the case of DOX, the present data recorded an IC50 within 500 ng/mL after 48 h, which was correlated with previous reports about DOX-caused dose-dependent cytotoxicity in cancer cells and also Neuroblastoma resistance against DOX (20). For drug combination studies, the cells were exposed to different combination modes of DOX and catechin for 48 h. The results showed that a simultaneous combination of catechin and DOX's effective doses had more significant effects on BE(2)C cells which were not unexpected. Indeed, ineffective concentrations of the drugs (1/2 IC50) showed higher toxicity than the single doses. It was previously shown that several natural products, including flavonoids, could improve the therapeutic index of DOX (21). In BE(2)C neuroblastoma cells, studies determined that the combination of 6-OH-11-O-hydroxyphenanthrene and epigallocatechin-3-gallate caused cell growth limitation synergistically (22). It was also shown that the combination of dextran-catechin and DOX has significant synergistic effects on DOX activity in BE(2)C cells via reducing cell viability (23).
The same subtractive effects were not seen in the normal human dermal fibroblasts (NHDFs), indicating these agents and their combinations were not deleterious to noncancerous cells (data are not shown). It can be concluded that pure catechin as a natural substance was selectively toxic to BE(2)C cells.
Morphological alterations in the treated cells included floating and shrinkage of the cell membrane, nuclei fragmentation, and being intensely stained with Hoechst due to the DNA condensation that was in line with the studies showing the apoptotic and antiproliferative effects of catechin in cancer cells (24). As cell proliferation and apoptosis are linked with cell cycle regulators, the DNA content of the treated cells was analyzed. The effective dose of catechin increased the number of cells in the sub-G1phase with a G2/S arrest which may be related to the time of DNA repairing. Flavonoids can block the cell cycle division at the G2/S (25); therefore, the present data are in line with the effect of flavonoids in the accumulation of neuroblastoma cells in the G2/M phase (26). In DOX treatments, the cell cycle stopped at the G2/M phase, which could correlate with previous data about DOX downregulating cdk1 activity through the G2/S phase (27). In the combination mode, the ½ IC50 combinations were accompanied by a significant increase in cellular accumulation in sub-G1; which could indicate the apoptosis induction in these cells. It may be deduced that catechin and its combination with DOX suppress neuroblastoma cells' proliferation through cell cycle arrest and apoptosis.
Cell migration and invasion as crucial features of metastatic cells were investigated to get a further perception. Studies have shown that EGCG suppresses migration, invasion, and metastasis of subcutaneous carcinoma, breast carcinoma, hepatocyte carcinoma cells, and bladder cancer cells (28, 29). In our study, owing to the treatment of catechin, DOX, and their combination, the BE(2)Cs migration was inhibited significantly. It was also revealed that most halters occurred in the combination of the ½ IC50 doses. The same data were obtained in the clonogenic capacity of the cell's exposure to the catechin, DOX, and their combination, though the number and density of the colonies decreased significantly. These findings agree with the results that catechin compounds inhibited colony growth and cell invasion of cancer cells (30). For as much as inhibition of tumor cell migration and invasion can be important targets of anticancer drugs, it appeared that the mentioned drug administration affected both the rate of colony formation and the ability of cells to migrate and confirmed the inhibition effect of catechin and DOX combination in neuroblastoma cells.
Several studies have stated that catechin compounds and their combinations could induce apoptosis in various cancers (31). In this study, it can be concluded that pure catechin-sensitized neuroblastoma cells targeted more cellular pathways leading to cell death through its combination with DOX. Therefore, lower doses of a chemical drug, DOX, synergically had more efficiency to inhibit neuroblastoma cell proliferation which might be due to the apoptosis induction.