1. Background
Severe acute respiratory infection (SARI) induced by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) results in lung failure and requires mechanical ventilation (1-3). The mechanisms by which the virus impacts the alveolar epithelium are unknown (4). When the virus spike protein reaches the lower airways, it links to angiotensin-converting enzyme 2 (ACE2) and utilizes it as a vehicle to reach alveolar cells (5).
Angiotensin-converting enzyme 2 is an enzyme that seems to be inactivated by virus activity and catalyzes the angiotensin II to angiotensin 1-7 conversion (6). However, elevated serum angiotensin II levels have been shown in severe COVID-19, a viral disease affecting the lower respiratory tract that has become a public health emergency and pandemic (7-10). It has been suggested that higher ACE2 expression in adults than in children causes a difference in the prevalence of the disease in this age range (11). Angiotensin II’s physiological intracellular signaling increases reactive oxygen species (ROS) production or the activity of NADPH oxidase (NOX) or mitochondria (12, 13). Initially, these ROSs are used in the signaling mechanism; however, their excessive levels may result in apoptosis or cell necrosis (14).
Since the 1960s, NAC has been utilized clinically as a mucolytic (15). It is also utilized to treat acute liver failure and acetaminophen poisoning (16, 17). Its safety has been well established, and its efficacy in lung disease could extend beyond its mucolytic activity, as it could interfere with the inflammatory response and bronchial tone (18). The NAC could help fill the depleted intracellular reservoirs by supplying cysteine, a necessary precursor to glutathione synthesis (GSH) (19). Thus, NAC administration may restore the reduced primary intracellular antioxidant system and intracellular oxidation signaling by increasing the decreased activity of GSH-GSSG (reduced glutathione-oxidized glutathione) (20).
2. Objectives
Knowing the action mechanism of NAC and the need for effective COVID-19 treatments, especially severe COVID-19, we designed a single-blind randomized clinical trial with a control group to evaluate the effectiveness of NAC in severe COVID-19 patients hospitalized in the ICU based on changes in respiration rate per minute, oxygen saturation (SpO2), lactate dehydrogenase (LDH), C-reactive protein (CRP), blood parameters, lymphocyte count, mortality rate, and ward and ICU stay duration.
3. Methods
The present study is a two-arm parallel single-blinded phase III randomized controlled clinical trial. The study population included all definite COVID-19 patients confirmed by PCR admitted to the ICU. Because of a few earlier studies in the field, at least 40 patients with the same conditions meeting the eligibility criteria were assigned randomly to the intervention (20 patients) and control (20 patients) groups, using online web-based tools and by permuted block randomization method in a ratio of 1: 1.
Inclusion criteria included a definitive COVID-19 infection confirmed by PCR. Tachypnea (respiratory rate more than 30 beats per minute, hypoxemia below or equal to 93%, or partial arterial oxygen pressure ratio less than 300), progressive lymphopenia, pulmonary infiltration (more than 50% involvement of the lung field in 24 to 48 h), and LDH above 345 U/L were the criteria for COVID-19 diagnosis. Eligible cases were admitted to the ICU of Shahid Mohammadi Hospital, Bandar Abbas, Iran.
This study was approved under the ethical code of IR.HUMS.REC.1399.539 and registered in the Iranian Registry of Clinical Trials (IRCT) with the code IRCT20200509047364N3. Patients signed written consent to participate in the research and receive the drug. They were included in the research after explaining drugs’ positive and negative points. Cases with the same condition were randomly grouped into the intervention and control groups. The intervention group received standard drug treatment based on the National Committee of COVID-19 and NAC treatment protocols at 300 mg per kg as a slow single injection intravenously on the first day of ICU stay. These cases were monitored until their discharge or for a maximum of 14 days. The control group was provided with standard drug treatment based on the National Committee’s treatment protocols and a placebo; they were monitored until their discharge or for a maximum of 14 days.
Laboratory tests and patients’ clinical status were assessed on the 14th or the day of patients’ discharge without knowing the grouping of patients. Based on a recent study, the criteria of severity included platelet count greater than 100,000 per microliter, respiration rate less than 30 per minute, LDH less than or equal to 245 U/L, CPR less than +2, oxygen saturation more than 93%, and average lymphocyte count or modified lymphopenia, which were considered as a positive result (21). Mortality, worsening clinical condition, platelets less than 100,000, respiratory rate above or equal to 30 per minute, CRP greater than +2, oxygen saturation below or equal to 93%, LDH more than 245 U/L, and lymphopenia were considered as a negative result. Standard medicine treatment was performed based on the COVID-19 National Committee’s treatment protocols as follows:
(1) Hydroxychloroquine/chloroquine phosphate: Pills of 200 mg hydroxychloroquine sulfate or 250 mg chloroquine phosphate (equivalent to 150 mg base dose) on the first day, two pills every 12 h, then one pill every 12 h for a minimum of one week and maximum of two weeks;
(2) One of the drugs below at the treating physician’s discretion:
A, Kaletra pills (lopinavir/ritonavir) 200/50 mg every 12 h twice a day for at least one week and up to two weeks;
B, Atazanavir/Ritonavir 300/100 mg pills: One daily with food or 400 mg daily atazanavir for a minimum of one week and a maximum of two weeks.
The primary endpoint of this study was the combined endpoint of ICU stay length and the patient’s clinical status. These results were measured at baseline (before intervention), 14 days after the intervention, or on the discharge day. Patients participating in the study were unaware of whether they were assigned to the intervention or control group. Nevertheless, principal investigators, medical staff, data collectors, and those who evaluated the results were aware of patient grouping (22).
The data were imported into SPSS version 25 software and analyzed using descriptive statistics (mean and percentage). The chi-square and Fisher’s exact tests were employed to compare qualitative variables between the NAC and control groups. Based on the results of the Kolmogorov-Smirnov test, Mann-Whitney and independent t-tests were used to assess the quantitative variables in the baseline and final evaluation and their differences between the two groups. A P value below 0.05 was significant.
4. Results
In this study, 40 COVID-19 patients admitted to the ICU were examined. The intervention group received NAC in addition to standard treatments, and the control group received a placebo in addition to standard treatments (Table 1).
| Variables | Study Groups | P-Value | |
|---|---|---|---|
| Control (n = 20) | NAC (n = 20) | ||
| Age | 58.30 ± 15.56 | 58.75 ± 15.91 | 0.928 |
| Sex | 0.525 | ||
| Male | 10 (50) | 12 (60) | |
| Female | 10 (50) | 8 (40) | |
| Weight (kg) | 71.91 ± 7.80 | 70.85 ± 5.04 | 0.614 |
| Height (cm) | 160.45 ± 5.94 | 163.10 ± 5.66 | 0.167 |
| SBP (mmHg) | 124.80 ± 12.37 | 131.50 ± 21.48 | 0.165 |
| DBP (mmHg) | 73.80 ± 12.32 | 75.40 ± 16.15 | 0.727 |
| SpO2 (%) | 92.70 ± 6.31 | 92.75 ± 5.80 | 0.979 |
| RR (/min) | 26.80 ± 8.01 | 23.84 ± 6.41 | 0.087 |
| HR (/min) | 81.60 ± 14.76 | 83.00 ± 17.51 | 0.786 |
| Temperature (°C) | 36.68 ± 0.45 | 36.76 ± 0.62 | 0.659 |
| WBC count (/μL) | 7190.00 ± 3427.43 | 8960.00 ± 5997.49 | 0.259 |
| Hb (g/dL) | 12.36 ± 1.14 | 12.40 ± 2.54 | 0.943 |
| Plt count (/μL) | 205300.00 ± 68807.21 | 203150.00 ± 67490.65 | 0.921 |
| RDW (%) | 14.02 ± 1.66 | 14.45 ± 1.92 | 0.480 |
| Lymphocyte count (/μL) | 604.46 ± 305.79 | 765.87 ± 332.94 | 0.124 |
| Lymphocyte percentage | 10.48 ± 7.65 | 12.15 ± 10.03 | 0.673 |
| Neutrophil count (/μL) | 6629.13 ± 3499.28 | 8189.48 ± 6172.07 | 0.368 |
| Neutrophil percentage | 86.89 ± 7.64 | 84.46 ± 9.57 | 0.488 |
| ESR (mm/h) | 38.93 ± 20.47 | 36.12 ± 21.91 | 0.711 |
| CRP (mg/dL) | 50.52 ± 28.50 | 48.67 ± 33.11 | 0.440 |
| LDH (U/L) | 943.50 ± 431.64 | 1011.37 ± 609.31 | 0.922 |
| D-dimer (ng/mL) | 512.88 ± 1020.77 | 356.54 ± 562.85 | 0.644 |
| Ferritin (μg/L) | 1382.41 ± 861.34 | 1042.15 ± 1003.95 | 0.220 |
| BUN (mg/dL) | 25.59 ± 25.57 | 20.73 ± 9.12 | 0.786 |
| Cr (mg/dL) | 1.18 ± 0.55 | 1.25 ± 0.63 | 0.252 |
| PT (s) | 20.73 ± 39.39 | 11.90 ± 1.27 | 0.735 |
| PTT (s) | 29.70 ± 11.64 | 29.66 ± 7.28 | 0.423 |
| INR | 1.00 ± 0.16 | 0.97 ± 0.11 | 0.480 |
| AST (U/L) | 78.71 ± 77.58 | 49.33 ± 19.22 | 0.437 |
| ALT (U/L) | 49.88 ± 40.21 | 33.22 ± 13.71 | 0.150 |
| ALP (U/L) | 187.63 ± 84.94 | 182.72 ± 77.75 | 0.768 |
| Total bilirubin (mg/dL) | 0.86 ± 0.60 | 1.99 ± 3.79 | 0.225 |
| Direct bilirubin (mg/dL) | 0.32 ± 0.26 | 1.33 ± 3.37 | 0.234 |
| pH | 7.44 ± 0.05 | 7.42 ± 0.06 | 0.322 |
| PCO2 (mmHg) | 39.91 ± 7.94 | 42.93 ± 6.77 | 0.204 |
| HCO3 (mEq/L) | 27.18 ± 6.53 | 27.88 ± 4.55 | 0.583 |
| Calcium (mg/dL) | 8.43 ± 0.51 | 8.49 ± 0.68 | 0.811 |
| Phosphorus (mg/dL) | 2.50 ± 0.72 | 3.11 ± 0.79 | 0.044 |
| Intubation | 0.487 | ||
| No | 20 (100) | 18 (90) | |
| Yes | 0 (0) | 2 (10) | |
| Mechanical ventilation | 0.288 | ||
| No | 13 (65) | 16 (80) | |
| Yes | 7 (35) | 4 (20) | |
| Oxygen with a mask | 1.000 | ||
| No | 20 (100) | 19 (95) | |
| Yes | 0 (0) | 1 (5) | |
| Nasal oxygen | 0.490 | ||
| No | 13 (65) | 15 (75) | |
| Yes | 7 (35) | 5 (25) | |
Comparison of General Characteristics and Other Study Variables in the Baseline Between N-acetylcysteine and Control Groups a
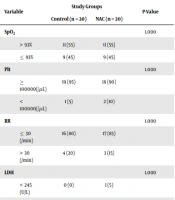
In the final evaluation, the proportion of patients in the control group with LDH less than or equal to 245 U/L was significantly higher than those in the NAC group (25% vs. 0%, P = 0.047). On the other hand, the percentage of patients with SpO2 less than or equal to 93% and lymphocytes less than 1000 per microliter (lymphopenia) was similar in both groups (P = 1,000). Patients with platelets less than 100,000 per mL were more in the NAC group than in the control group (90% vs. 80%, P = 0.661). Patients with RR less than or equal to 30 per minute were also slightly more in the NAC group (90% vs. 75%, P = 0.407) (Tables 2 and 3).
| Variables | Study Groups | P-Value | |
|---|---|---|---|
| Control (n = 20) | NAC (n = 20) | ||
| SpO2(%) | 1.000 | ||
| > 93 | 11 (55) | 11 (55) | |
| ≤ 93 | 9 (45) | 9 (45) | |
| Plt (/μL) | 1.000 | ||
| ≥ 100000 | 19 (95) | 18 (90) | |
| < 100000 | 1 (5) | 2 (10) | |
| RR (/min) | 1.000 | ||
| ≤ 30 | 16 (80) | 17 (85) | |
| > 30 | 4 (20) | 3 (15) | |
| LDH (U/L) | 1.000 | ||
| < 245 | 0 (0) | 1 (5) | |
| > 245 | 20 (100) | 19 (95) | |
| Lymphocyte count (/μL) | 1.000 | ||
| ≥ 1000 | 3 (15) | 4 (20) | |
| < 1000 | 17 (85) | 16 (80) | |
Comparison of the Frequency of Severe Coronavirus Disease 2019 Variables Between N-acetylcysteine and Control Groups a
| Variables | Study Groups | P-Value | |
|---|---|---|---|
| Control (n = 20) | NAC (n = 20) | ||
| SpO2(%) | 1.000 | ||
| > 93 | 10 (50) | 10 (50) | |
| ≤ 93 | 10 (50) | 10 (50) | |
| Plt (/μL) | 0.661 | ||
| ≥ 100000 | 16 (80) | 18 (90) | |
| < 100000 | 4 (20) | 2 (10) | |
| RR (/min) | 0.407 | ||
| ≤ 30 | 15 (75) | 18 (90) | |
| > 30 | 5 (25) | 2 (10) | |
| LDH (U/L) | 0.047 | ||
| < 245 | 5 (25) | 0 (0) | |
| > 245 | 15 (75) | 20 (100) | |
| Lymphocyte count (/μL) | 1.000 | ||
| ≥ 1000 | 3 (15) | 3 (15) | |
| < 1000 | 17 (85) | 17 (85) | |
Comparison of the Frequency of the Variables Between N-acetylcysteine and Control Groups a
Although hospital and ICU stay lengths were shorter in the NAC group than in the controls, the difference between the groups was statistically insignificant (P = 0.598 and P = 0.629, respectively). On the other hand, mortality was higher in the control group (75%) than in the NAC group (50%); however, there was no statistically significant difference between the groups (P = 0.102) (Table 4).
| Variables | Study Groups | P-Value | |
|---|---|---|---|
| Control (n = 20) | NAC (n = 20) | ||
| Duration of hospitalization in the ward (days) | 2.35 ± 2.25 | 1.75 ± 1.52 | 0.598 |
| Duration of hospitalization in the ICU (days) | 13.95 ± 7.10 | 12.90 ± 6.49 | 0.629 |
| Mortality | 0.102 | ||
| No | 5 (25) | 10 (50) | |
| Yes | 15 (75) | 10 (50) | |
Comparison of Ward and Intensive Care Unit Stay Length and Mortality Between N-acetylcysteine and Control Groups a
Diastolic blood pressure decreased significantly in the NAC group compared to the control group. On the other hand, a decrease in hemoglobin (Hb), respiratory rate (RR), systolic blood pressure (SBP), lymphocyte percentage, C-reactive protein (CRP), creatinine (Cr), platelet (Plt) count, red distribution width (RDW), neutrophil percentage, international normalized ratio (INR), partial thromboplastin time (PTT), blood urea nitrogen (BUN), lactate dehydrogenase (LDH), and alkaline phosphatase (ALP) was more in the NAC group than in the control group. However, a decrease in SpO2, body temperature, erythrocyte sedimentation rate (ESR), and pH, and an increase in heart rate (HR), white blood cells (WBC) count, neutrophil count, PCO2, alanine aminotransferase (ALT), aspartate aminotransferase (AST), ferritin, and phosphorus were lower in the NAC group than in the control group. Also, the number of lymphocytes, direct bilirubin, total bilirubin, D-dimer, and HCO3 declined in the NAC group and raised in the control group, while prothrombin time (PT) and calcium increased in the NAC group and decreased in the control group. However, except for changes in diastolic blood pressure, changes in other variables were insignificantly different between the groups. The rate of mechanical intubation and ventilation in the NAC group and the oxygen supplied with mask and nasal oxygen in the control group were lower but did not show a statistically significant difference. It should be noted that in the final evaluation, no patient was positive for troponin (Table 5).
| Variables | Study Groups | P-Value | |
|---|---|---|---|
| Control (n = 20) | NAC (n = 20) | ||
| SBP (mmHg) | -4.85 ± 25.89 | -12.90 ± 23.29 | 0.116 |
| DBP (mmHg) | 2.65 ± 19.51 | -4.45 ± 13.39 | 0.042 |
| SpO2 (%) | -2.55 ± 11.44 | -0.50 ± 7.32 | 0.841 |
| RR (/min) | 1.00 ± 9.85 | -2.63 ± 5.67 | 0.568 |
| HR (/min) | 3.05 ± 20.29 | 2.70 ± 18.59 | 0.607 |
| Temperature (°C) | -0.95 ± 2.34 | -0.26 ± 0.66 | 0.368 |
| WBC count (/μL) | 5536.84 ± 6571.51 | 2855.00 ± 9085.29 | 0.300 |
| Hb (g/dL) | -0.60 ± 1.47 | -0.89 ± 2.52 | 0.881 |
| Plt count (/μL) | 17800.00 ± 95168.88 | 49150.00 ± 99248.37 | 0.314 |
| RDW (%) | 0.64 ± 0.91 | 1.19 ± 2.09 | 0.849 |
| Lymphocyte count (/μL) | 95.47 ± 542.11 | -93.09 ± 329.87 | 0.193 |
| Lymphocyte percentage | -4.27 ± 7.74 | -4.33 ± 6.79 | 0.982 |
| Neutrophil count (/μL) | 5004.74 ± 6623.99 | 2575.54 ± 9275.14 | 0.392 |
| Neutrophil percentage | 3.66 ± 8.02 | 3.77 ± 5.95 | 0.381 |
| ESR (mm/h) | -11.00 ± 18.71 | -9.18 ± 25.19 | 0.552 |
| CRP (mg/dL) | -22.34 ± 35.78 | -34.66 ± 33.64 | 0.302 |
| LDH (U/L) | 365.40 ± 405.34 | 436.32 ± 978.22 | 0.509 |
| D-dimer (ng/mL) | 992.55 ± 1242.08 | -42.75 ± 803.73 | 0.068 |
| Ferritin (μg/L) | 462.49 ± 1827.09 | 330.15 ± 1047.63 | 0.484 |
| BUN (mg/dL) | 6.26 ± 9.93 | 8.08 ± 23.82 | 0.296 |
| Cr (mg/dL) | -0.04 ± 0.33 | -0.14 ± 0.77 | 0.307 |
| PT (s) | -8.51 ± 39.47 | 3.86 ± 12.83 | 0.689 |
| PTT (s) | 2.76 ± 16.47 | 5.52 ± 21.93 | 0.627 |
| INR | 0.12 ± 0.31 | 0.52 ± 1.34 | 0.245 |
| AST (U/L) | 13.50 ± 78.15 | 4.08 ± 29.68 | 0.862 |
| ALT (U/L) | 30.83 ± 90.68 | 8.00 ± 19.12 | 0.749 |
| ALP (U/L) | 60.82 ± 86.24 | 109.00 ± 141.15 | 0.321 |
| Total bilirubin (mg/dL) | 0.46 ± 1.06 | -1.63 ± 4.50 | 0.335 |
| Direct bilirubin (mg/dL) | 0.40 ± 0.70 | -1.60 ± 4.18 | 0.180 |
| pH | -0.03 ± 0.09 | -0.02 ± 0.11 | 0.628 |
| PCO2 (mmHg) | 3.69 ± 12.77 | 2.74 ± 12.07 | 0.809 |
| HCO3 (mEq/L) | 0.65 ± 8.13 | -0.46 ± 5.25 | 0.612 |
| Calcium (mg/dL) | -0.16 ± 0.71 | 0.00 ± 0.87 | 0.749 |
| Phosphorus (mg/dL) | 1.78 ± 0.96 | 1.70 ± 2.78 | 0.443 |
Comparison of Changes in Study Variables from Initial to Final Between N-acetylcysteine and Control Groups a
5. Discussion
So far, various drugs have been the main or adjunctive treatment for COVID-19. N-acetylcysteine has long been used as a mucolytic in lung disease. This drug seems to have a role in restoring the function of the intracellular antioxidant system. This research aimed to determine NAC’s effectiveness in recovering severe COVID-19 patients admitted to the ICU. The current research showed that NAC was related to a significant reduction in respiratory rate, D-dimer, and changes in diastolic blood pressure in severe COVID-19 patients admitted to the ICU. Furthermore, compared to the control group, LDH less than or equal to 245 U/L was significantly lower in the NAC group. No significant difference was observed between the two groups in other laboratory and clinical parameters, either concerning their changes or in the final evaluation. Also, although the length of stay in the ward and ICU was shorter in the NAC group, the difference between the groups was statistically insignificant. On the other hand, mortality was higher in the control group; however, no statistically significant difference was observed between the groups.
de Alencar et al. compared NAC with placebo for COVID-19 treatment in Brazil (23). They also examined patients with severe COVID-19, although the criteria for the intensity of COVID-19 in this study differed from the present study. Their results were similar to ours in terms of primary and secondary outcomes, including the need for mechanical ventilation, duration of mechanical ventilation, ICU hospitalization, ICU hospitalization duration, and mortality (23).
Bhattacharya et al. indicated that the average hospital stay was 12 days in moderate to severe patients receiving standard NAC treatment (24). The hospital discharge rate was higher in these patients. The average duration of oxygen therapy was eight days, and the mortality rate was lower (24). In the current research, the mean duration of ICU hospitalization in the NAC group was 12.90 days, and the ward hospitalization duration in this group was 1.75 days. Similarly, mortality, although insignificantly, was lower, and hospital discharge was higher in the NAC group in the present study. The two studies differ in the type and design of studies and the consideration of moderate and severe patients in the study of Bhattacharya et al. and severe patients (alone) in the current research (24).
In another pilot study by Taher et al. to evaluate intravenous NAC treatment in people with mild to moderate COVID-19 in Iran, NAC was compared with a placebo (25). In this study, patients admitted to the ICU were also studied (25). This study was in line with the present study in terms of outcomes. According to the results, no difference in 28-day mortality was observed between the two groups. Although the clinical condition on day 28 favored better outcomes in the NAC group, the difference between the groups was statistically insignificant. Similar results were obtained regarding the frequency of patients requiring invasive support and methylation, the number of days without the need for a ventilator, and the median hospitalization time in the ICU and hospital (25). However, in the study of Taher et al., patients with moderate or severe ARDS were evaluated, and in addition, the dose and duration of NAC were different from ours (25).
In contrast to the present study, Assimakopoulos et al. showed that the rate of progression to SRF with oral NAC treatment was significantly lower compared to the control group (26). Patients in the NAC group also had much lower mortality at 14 and 28 days than the controls. On the other hand, these researchers showed that NAC improved PaO2/FiO2 and decreased white blood cells, CRP, D-dimer, and LDH (26). Differences between the two studies can be explained by differences in demographic characteristics, the design of the two studies, NAC dose, and the intensity of COVID-19.
In a case report published by Schetting et al., a 59-year-old man, who presented with respiratory symptoms and a chest radiograph showing classic COVID-19 pneumonia, was treated with a nebulized formulation of cyclodextrin (quercetin) at a dose of 20 mg/mL and NAC at a dose of 100 mg/mL three times a day for 14 days (27). This formula significantly and rapidly improved respiratory symptoms (27).
In another case report published by Puyo et al., a COVID-19 patient, age 54 years old, was hospitalized with multiple organ dysfunction and treated with a combination of oral hydroxychloroquine and intravenous NAC (28). This combination therapy resulted in clinically significant improvement and reduction in various inflammatory markers, especially ferritin, CRP, and lactic acid (28).
In a report of several patients from the United States published by Ibrahim et al., the deficiency was treated with hydroxychloroquine in one patient with severe COVID-19 and G6PD; intravenous NAC was also beneficial (29). In addition to inhibiting hemolysis, NAC reduced hepatic enzymes, CRP, and ferritin; the patient was separated from the ventilator and venovenous ECMO and fully recovered. NAC was further prescribed to nine other COVID-19 ventilator-dependent cases who were not G6PD-deficient. In these patients, NAC also led to a clinically significant improvement and reduction in CRP and a decrease in ferritin in nine out of 10 patients (29).
In a meta-analysis by Lu et al. to evaluate clinical trials of NAC efficacy in patients with ARDS, NAC failed to reduce overall mortality compared to the control group (30). However, NAC significantly reduced the length of ICU stay. Thus, NAC could not reduce mortality but helped reduce the length of hospital stay (30).
One of the limitations of this study is that the final evaluation was not homogeneous, so final examinations were performed either at the end of day 14 or at the time of discharge, which could have affected the results. Future research should use a larger sample size to make the findings more generalizable.
5.1. Conclusions
Based on the current study, NAC is associated with a significant decrease in RR, D-dimer, and DBP in patients with severe COVID-19. Also, LDH was significantly lower in the NAC group than in the controls. Further studies with larger sample sizes are required to confirm the current study findings.