1. Background
Osteoarthritis (OA) is a prevalent and debilitating rheumatic disease, posing clinical challenges for effective management. Discovering new strategies to address pain and restore joint function is crucial (1). Studies have examined the role of nutraceuticals in managing OA and demonstrated the promising performance of herbal medicines (2, 3). For instance, Curcuma longa, known for its anti-inflammatory properties, has been recognized as a therapeutic herbal option (4). Herbal preparations are believed to offer a safe and effective alternative with fewer adverse effects than nonsteroidal anti-inflammatory drugs and other pharmacological approaches (5).
Pistacia vera L., a member of the Anacardiaceae family, has a history of use in traditional medicine due to its pharmacologically active components. Various studies have demonstrated the potential beneficial effects of P. vera derivatives on numerous diseases and experimental models (6). Research has explored both the topical and systemic effects of P. vera. For example, Taghipour et al. demonstrated the protective effects of P. vera topical administration on second-degree burn wounds in rats (7). Mohammadi-Nasab et al. showed the neuroprotective effects of P. vera on anxiety and depression in rats with polycystic ovary syndrome (8).
Furthermore, P. vera contains anti-inflammatory compounds, making it a promising candidate for the treatment of inflammatory diseases. Research has focused on exploring the anti-inflammatory properties of P. vera (9). Orhan et al. demonstrated a moderate anti-inflammatory effect in a mouse model of carrageenan-induced hind paw edema (10). Additionally, reports indicate the analgesic properties of P. vera (11).
2. Objectives
In conclusion, this study aims to assess the potential benefits of P. vera seed oil ointment in patients with OA, aiming to identify a safe and effective herbal preparation for managing this complex disease.
3. Methods
3.1. Study Protocols
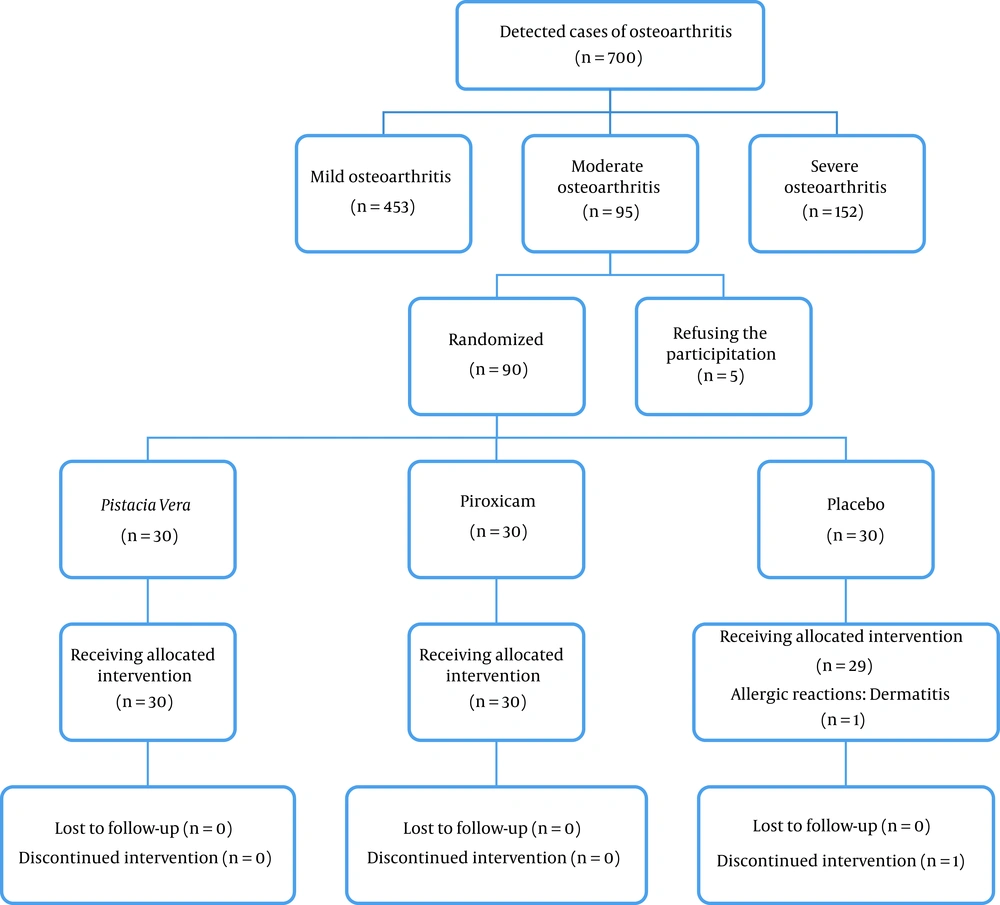
This randomized, double-blind, controlled clinical trial was conducted between March and August 2019 at Rafsanjan Rheumatology Clinic. A total of 89 participants diagnosed with OA were divided into three groups. Figure 1 provides a schematic view of the study design and the steps involved. The study spanned a duration of 3 months and consisted of three parallel intervention arms: Trial medication (P. vera), piroxicam (a topical nonsteroidal anti-inflammatory medication), and placebo (ointment base).
3.2. Inclusion Criteria
The inclusion criteria were as follows: (1) Age between 45 - 70 years; (2) diagnosis of knee OA based on the criteria established by the American College of Rheumatology and confirmed by a rheumatologist (12, 13); and (3) moderate knee pain within 24 hours as assessed by the visual analog scale (VAS: 4 to 7 cm) (14).
3.3. Exclusion Criteria
Patients with the following co-morbidities were excluded from the study: inflammatory diseases, malignancy or serious illness, symptoms or history of liver or kidney failure, recent consumption of oral corticosteroids within the past four weeks or corticosteroid injections within the past six months, continuous use of medicinal herbs, sensitivity to pistachios, presence of fever, dissatisfaction with participation, failure to adhere to study protocols, observation of side effects related to pistachio oil, regular daily use of analgesics, advanced stage of the disease, use of other topical medications at the site of pistachio oil application, oral use of analgesics and other effective compounds for OA treatment up to 10 days prior to the study, skin disease or infection, or wound at the site of pistachio ointment application, and pregnancy (15). Patients who met the inclusion and exclusion criteria were eligible for participation in the trials.
3.4. Study Design and Interventions (Randomization and Blinding)
The patients were randomly assigned to three groups using a computer-generated random number table:
Group A: Piroxicam gel; group B: P. vera oil topical ointment; group C: Placebo (non-medicated ointment base). The treatment protocol spanned three months, with each preparation applied topically twice a day. The duration of the drug massage was 3 - 5 minutes. All patients were advised throughout the study to adhere to health recommendations and make lifestyle modifications.
To ensure double-blinding of the study, the medications were pre-filled into identical tubes by a person other than the researcher and labeled as A, B, and C. Evaluation of signs and symptoms by a rheumatologist was conducted following the protocol, and the rheumatologist remained unaware of which medication was assigned to each patient. Additionally, patients were unaware of which medication tube was prescribed to them.
Follow-up was conducted through clinic visits and weekly phone calls to ensure monitoring of potential side effects and adherence to the study protocol. During the phone calls, the progress of treatment was assessed, along with the occurrence of any additional diseases or drug usage. Complete individual adherence was defined as the consumption of more than 90% of the tube contents, which was determined by measuring the ointment content within the first month. Furthermore, the data analyst was unaware of the specific drug used by each patient.
3.5. Outcome Measurements
Patient pain levels were assessed at baseline, one month into the study, and at the study’s conclusion using a visual analogue scale (VAS), which is a 10 cm ruler. Pain intensity was categorized as 0 - 3 for mild pain, 4 - 7 for moderate pain, and 8 - 10 for severe pain. Patients were asked to indicate their pain intensity on the ruler at baseline and after taking the medication at the end of the third month. Patient performance was evaluated using the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) questionnaire. This validated tool consists of 24 items divided into three sections: five questions for pain intensity, 17 for physical activity, and two for joint stiffness. Each item was scored on a scale of 0 to 4. Participants completed a demographic questionnaire capturing age, gender, marital status, level of education, and employment status. Weight was measured using a digital scale with 0.1 kg accuracy, while height was assessed using a height gauge with 0.5 cm accuracy. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared (weight (kg)/height (m2)) (14-17).
3.6. Pistacia vera Oil Extraction and Ointment Preparation
The study utilized the P. vera fruit of the Akbari species (genetic code: M30). The pistachio was obtained from an herbal market in Rafsanjan, Iran. The cold press method, a simple and effective technique that maintains oil properties and yields high-quality oil, was employed to extract the oil. A pistachio ointment with a concentration of 10% was prepared by incorporating the oil into an inert base (FARABI Co., Tehran, Iran) (7).
3.7. Placebo and Positive Control Preparation
Identical tubes containing a pharmaceutical-grade ointment base (FARABI Co., Tehran, Iran) were prepared alongside the pistachio ointment to maintain blinding. A 0.5% piroxicam gel (Najo) was also used as the positive control.
3.8. Ethical Considerations
This study was approved by the Ethics Committee for Research at Rafsanjan University of Medical Sciences (IR.RUMS.REC.1397.175). Signed informed consent was obtained from all participants. The trial was registered in the Iranian Registry of Clinical Trials database (IRCT20180811040759N1). Patient information was kept confidential.
3.9. Sample Size
The sample size was determined based on previous studies (18), considering a 95% confidence interval and 80% power. The calculation resulted in an estimated sample size of 30 participants in each trial group. The formula used for this section was as follows, with values σ1 = 11, σ2 = 14, and Δ = 11.
3.10. Statistical Analysis
Data analysis was performed using SPSS software (version 20). Quantitative data are presented as mean ± standard deviation and range of changes, while qualitative data are reported as number (percentage). The intention-to-treat (ITT) approach was employed for data analysis. One-way ANOVA was utilized to compare the means of quantitative variables among the investigated groups (Placebo, Piroxicam, and P. vera). The chi-square test or Fisher’s exact test was used to compare the frequency distribution of qualitative variables among the three studied groups. Two-way repeated measures ANOVA was employed to compare the average of the dependent variables (VAS and WOMAC) over the study period (baseline, one month, and three months after intervention). This analysis assessed the effects of group, time, and the interaction between group and time. The homogeneity of covariance matrices in the investigated groups was evaluated using Box’s M test, and no violation was found (P < 0.05). Levene’s test was conducted to assess the homogeneity of variance among the groups, and no violation of this assumption was observed (P < 0.05). The normal distribution of quantitative variables was assessed using the Kolmogorov-Smirnov test and by calculating skewness and kurtosis indices (within the range of -2 to +2). There was no violation of the normality assumption (P < 0.05). A significance level of 0.05 was considered.
4. Results
4.1. Characteristics of Patients
The present clinical trial involved 89 patients with moderate osteoarthritis, divided into three groups. Of these, 17 (19%) were men, and 72 (81%) were women, with a mean age of 57 ± 7.6 years. The average daily activity of the participants was 4 ± 1.63 hours. The patients were categorized as follows: 58 (65%) were housewives, 13 (14%) were employees, 8 (9%) were retired, and 10 (11%) fell into other categories.
Initially, demographic characteristics were assessed within each group. Subsequently, the impact of pistachio ointment on pain severity was evaluated in the patients, followed by a comparison of the average pain severity between groups.
The mean age in the placebo group was 57.41 ± 6.34 years; in the piroxicam group was 57.30 ± 7.84 years; and in the P. vera oil group was 57.80 ± 8.69 years. No statistically significant differences were found in age, gender, BMI, and physical activity among the three groups (P > 0.05, Table 1).
| Different Characteristics in Groups | Piroxicam (n = 30) | Pistacia vera (n = 30) | Placebo (n = 29) | P-Value |
|---|---|---|---|---|
| Age b | 57.30 ± 7.84 | 57.80 ± 8.69 | 57.41 ± 6.34 | 0.966 |
| Gender c | 0.594 | |||
| Male | 4 (13.3) | 7 (23.3) | 6 (20.7) | |
| Female | 26 (86.7) | 23 (76.7) | 23 (79.3) | |
| BMI b | 29.27 ± 3.44 | 27.17 ± 3.93 | 28.07 ± 4 | 0.606 |
| Physical Activity (h) b | 4.80 ± 1.64 | 4.70 ± 1.57 | 4.51 ± 1.72 | 0.801 |
| Job c | 0.759 | |||
| Housewife | 21 (70) | 16 (53.3) | 21 (72.4) | |
| Employee | 4 (13.3) | 5 (16.7) | 4 (13.8) | |
| Retired | 2 (6.7) | 4 (13.3) | 2 (6.9) | |
| Other jobs | 3 (10) | 5 (16.7) | 2 (6.9) |
Baseline Characteristics of Patients in Three Groups a
4.2. The Effect of Treatment with Pistacia vera Ointment in OA
Two-way repeated measures ANOVA was employed to assess changes in the investigated indices among patients in the three groups and at different time points. The results are presented in Table 2. Due to a significant Mauchly’s test of sphericity, the results of the non-parametric Greenhouse-Geisser test were reported.
| Variables | Placebo (n = 29) | Piroxicam (n = 30) | Pistacia vera (n = 30) | Time/Group | Time | Between Groups |
|---|---|---|---|---|---|---|
| VAS/Greenhouse-Geisser | ||||||
| Before treatment | 5.72 ± 0.88 | 5.56 ± 0.97 | 5.90 ± 0.92 | |||
| One month after the intervention | 5.27 ± 0.84 | 4.05 ± 1.45 | 3.60 ± 1.21 | |||
| Three months after the intervention | 5.00 ± 0.92 | 3.86 ± 1.38 | 3.36 ± 1.06 | |||
| F | 31.25 | 269.02 | 8.76 | |||
| df | 2 | 2 | 4 | |||
| Mean square | 10.47 | 90.18 | 26.95 | |||
| Partial eta squared | 0.421 | 0.758 | 0.169 | |||
| P b | < 0.001 | < 0.001 | < 0.001 | |||
| WOMAC (pain)/Greenhouse-Geisser | ||||||
| Before treatment | 11.41 ± 1.93 | 10.36 ± 2.23 | 10.86 ± 1.90 | |||
| One month after the intervention | 11.00 ± 1.73 | 7.03 ± 1.71 | 4.86 ± 1.75 | |||
| Three months after the intervention | 11.00 ± 1.77 | 6.93 ± 1.76 | 4.73 ± 1.68 | |||
| F | 110.28 | 269.02 | 8.76 | |||
| df | 2 | 2 | 4 | |||
| Mean square | 137.94 | 90.18 | 26.95 | |||
| Partial eta squared | 0.719 | 0.758 | 0.169 | |||
| P b | < 0.001 | < 0.001 | < 0.001 | |||
| WOMAC (stiffness)/Greenhouse-Geisser | ||||||
| Before treatment | 3.24 ± 1.02 | 2.76 ± 1.52 | 3.06 ± 1.46 | |||
| One month after the intervention | 3.03 ± 1.34 | 2.00 ± 1.17 | 1.33 ± .92 | |||
| Three months after the intervention | 3.00 ± 1.19 | 1.90 ± 1.12 | 1.23 ± .85 | |||
| F | 13.64 | 58.93 | 10.05 | |||
| df | 2 | 2 | 4 | |||
| Mean square | 9.38 | 40.52 | 34.50 | |||
| Partial eta squared | 0.241 | 0.407 | 0.190 | |||
| P b | < 0.001 | < 0.001 | < 0.001 | |||
| WOMAC (activity)/Greenhouse-Geisser | ||||||
| Before treatment | 35.65 ± 6.91 | 34.43 ± 7.36 | 35.46 ± 7.18 | |||
| One month after the intervention | 35.48 ± 7.08 | 24.50 ± 5.56 | 16.20 ± 4.72 | |||
| Three months after the intervention | 35.34 ± 7.11 | 24.16 ± 5.77 | 16.20 ± 4.72 | |||
| F | 117.20 | 380.54 | 35.12 | |||
| df | 2 | 2 | 4 | |||
| Mean square | 1757.97 | 5708.11 | 3711.36 | |||
| Partial eta squared | 0.732 | 0.816 | 0.450 | |||
| P b | < 0.001 | < 0.001 | < 0.001 | |||
| WOMAC (total)/Greenhouse-Geisser | ||||||
| Before treatment | 50.31 ± 9.09 | 47.76 ± 10.39 | 49.40 ± 9.31 | |||
| One month after the intervention | 49.55 ± 8.78 | 22.50 ± 6.60 | 22.50 ± 6.60 | |||
| Three months after the intervention | 49.34 ± 8.73 | 33.00 ± 7.89 | 22.20 ± 6.65 | |||
| F | 139.52 | 500.81 | 40.270 | |||
| df | 2 | 2 | 4 | |||
| Mean square | 2538.24 | 9111.13 | 7629.70 | |||
| Partial eta squared | 0.764 | 0.853 | 0.484 | |||
| P b | < 0.001 | < 0.001 | < 0.001 |
Mean and Standard Deviation of the Research Variables by Treatment Group During the Study Period and the Intra-group, Inter-group, and Interaction Effects a
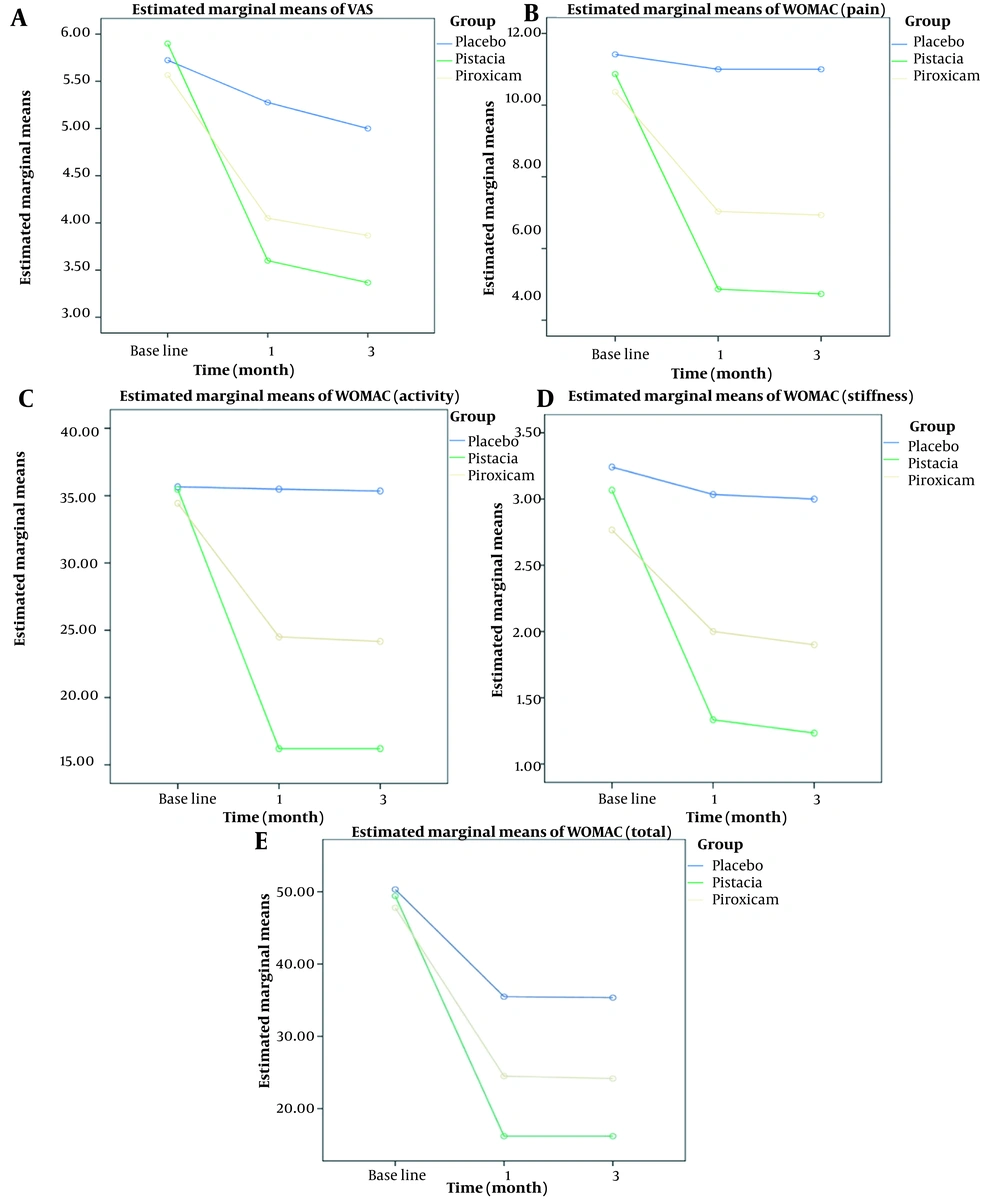
Comparing VAS scores among the piroxicam, pistachio, and placebo groups at different time points (baseline, one month, and three months after intervention) revealed significant effects for group, time, and group-time interaction. The slope of VAS score changes in the placebo group did not show significance throughout the study. Both the piroxicam and pistachio groups exhibited a similar reduction in VAS scores over time. The greatest reduction in VAS scores for both piroxicam and pistachio groups was observed during the initial period of the study (one month after intervention). No further reduction was observed after three months of intervention (Figure 2A). According to Tukey’s post-hoc test with Bonferroni correction (P < 0.025), the mean difference in VAS scores was significantly higher in the piroxicam and pistachio groups compared to the placebo group (P = 0.006, P < 0.001). However, there was no significant difference between the piroxicam and pistachio groups (P = 0.712).
Comparing WOMAC/PAIN scores among the piroxicam, pistachio, and placebo groups at baseline, one month, and three months after intervention revealed significant effects for group, time, and group-time interaction. The slope of WOMAC/PAIN score changes in the placebo group did not show significance during the study. Both the piroxicam and pistachio groups exhibited a similar reduction in WOMAC/PAIN scores over time. The most significant reduction in WOMAC/PAIN scores for piroxicam and pistachio was observed during the initial period of the study (one month after intervention). No significant reduction was observed after three months of intervention compared to the first period (Figure 2B). According to Tukey’s post-hoc test with Bonferroni correction (P < 0.025), the mean difference in WOMAC/PAIN scores was higher in both the piroxicam and pistachio groups compared to the placebo group (P < 0.001, P < 0.001). Additionally, the pistachio group showed a significantly greater difference than the piroxicam group (P = 0.012).
Comparing WOMAC/STIFFNESS scores among the piroxicam, pistachio, and placebo groups at baseline, one month, and three months after intervention revealed significant effects for group, time, and group-time interaction. The slope of the WOMAC/STIFFNESS score change in the placebo group did not show significance during the study. Both the piroxicam and pistachio groups exhibited a similar reduction in WOMAC/STIFFNESS scores over time. The most significant reduction in WOMAC/STIFFNESS scores for piroxicam and pistachio was observed during the initial period of the study (one month after intervention) (Figure 2C). According to Tukey’s post-hoc test with Bonferroni correction (P < 0.025), the mean difference in WOMAC/STIFFNESS scores was higher in both the piroxicam and pistachio groups compared to the placebo group (P < 0.001, P = 0.007). However, there was no significant difference between the piroxicam and pistachio groups (P = 0.429).
The comparison of WOMAC/ACTIVITY scores before treatment, one and three months after intervention in the piroxicam, pistachio, and placebo groups showed significant effects of group, time, and group, time interaction. The slope of the WOMAC/ACTIVITY score change in the placebo group was not significant. The diminution of the WOMAC/ACTIVITY score showed similar slopes in the piroxicam and pistachio groups. The greatest impact of piroxicam and pistachio on WOMAC/ACTIVITY reduction was observed in the first month after intervention, with no further significant reduction at three months (Figure 2D). According to Tukey’s post-hoc test, the mean difference in WOMAC/ACTIVITY scores between piroxicam and pistachio groups was greater than the placebo group (P < 0.001, P = 0.001) after Bonferroni correction (P < 0.025). Furthermore, these differences in the pistachio group were significantly greater than in the piroxicam group (P = 0.004).
The WOMAC/TOTAL scores were compared among the piroxicam, pistachio, and placebo groups before treatment and at one and three months after the intervention. Significant effects were observed for group, time, and the interaction between group and time. The placebo group did not significantly change WOMAC/TOTAL scores throughout the study. The WOMAC/TOTAL score reduction rate was similar in the piroxicam and pistachio groups. The most pronounced reduction in WOMAC/TOTAL scores for both piroxicam and pistachio groups occurred one month after intervention, with no further significant reduction at three months (Figure 2E). According to Tukey’s post-hoc test, the mean WOMAC/TOTAL score was significantly higher in the piroxicam and pistachio groups compared to the placebo group (P < 0.001, P = 0.001) after Bonferroni correction (P < 0.025). Additionally, the pistachio group showed a significantly greater reduction than the piroxicam group (P = 0.005).
4.3. Adverse Effect Observation
No side effects were observed in the participants who received the topical formulation of P. vera in this study. However, one participant in the placebo group experienced skin allergic reactions.
5. Discussion
Our findings indicate that using pistachio seed oil as a topical ointment can effectively reduce pain levels according to the VAS scale. Furthermore, long-term use of pistachio ointment decreased pain and stiffness scores based on the WOMAC assessment. Patients treated with pistachio or piroxicam ointment demonstrated improved function, activity, and overall WOMAC scores. Interestingly, pistachio outperformed piroxicam in certain WOMAC parameters such as pain, activity, and total score. The beneficial effects of pistachio were observed after one month of treatment and remained consistent over three months, with no significant difference between these two points in time. Notably, most participants in the study were women, supporting the observation that OA predominantly affects women rather than men (19).
Osteoarthritis, a degenerative joint disease, is recognized as a primary cause of pain and disability among the elderly (20). Inflammation and the involvement of proinflammatory mediators such as nitric oxide, prostaglandin E2, cytokines, neuropeptides, and proteolytic enzymes contribute to the pathogenesis of OA (21, 22). The management of OA includes three main approaches: Pharmacologic, non-pharmacologic, and surgical interventions (23). The pharmacologic approach typically involves using acetaminophen, topical or systemic nonsteroidal anti-inflammatory drugs (NSAIDs), and corticosteroids (23). However, chronic administration of pharmacologic treatments is associated with various adverse effects. Nonsteroidal anti-inflammatory drugs, for instance, may lead to gastrointestinal irritation, cardiovascular events, renal failure, and hypertension (24).
The use of herbal medicines and remedies has been regarded as safe and effective, with a history of administration for various inflammatory conditions (5). Studies have demonstrated the potential of curcumin in alleviating symptoms of OA by exerting anti-inflammatory, anti-oxidative stress, and anti-catabolic effects, inhibiting inflammatory pathways, COX-2, and matrix metalloprotease-9 (25, 26). Another study by Abbasifard et al. found that topical application of chickpea oil provided pain relief, reduced motion stiffness, and improved activity in patients with knee OA (15). Evidence suggests that other herbal preparations, such as capsaicin and Symphytum officinale, may also have protective effects in treating OA (27, 28). In our study, we aimed to investigate the potential benefits of using a topical ointment containing P. vera seed oil for knee OA, exploring the potential of this herbal approach in targeting this progressive joint disease.
Pistacia vera, a plant belonging to the Anacardiaceae family, possesses various pharmacologic functions. Documented evidence highlights its antibacterial, anti-hyperlipidemia, anti-inflammatory, antioxidant, and analgesic properties. Pistachio extract has been shown to exhibit nephroprotective, hepatoprotective, and neuroprotective effects through its components, such as polyphenols, tocopherols, phytosterols, monoterpenes, flavonoids, gallic acid, carotenoids (29-31). Orhan et al. conducted a study demonstrating the significant anti-inflammatory and antinociceptive activity of P. vera oleoresin in an animal model (10). In a study by Hosseinzadeh et al., it was demonstrated that P. vera extract exhibits dose-dependent central and peripheral analgesic activities, with the involvement of the opioid system in its central effects (11). The extract also displayed anti-inflammatory effects against acute and chronic inflammation (11). Our study’s findings are consistent with other research indicating that topical application of pistachio oil preparation reduces pain in patients, as assessed by the VAS scale or WOMAC index. This supports the beneficial role of P. vera as an herbal strategy for osteoarthritis, as demonstrated in Abbasifard et al.’s study on the effects of chickpea oil in OA patients (15). These findings provide support for the effectiveness of herbal medicine in managing chronic OA. Furthermore, our study observed that patients did not report any notable side effects during the course of the study. This is a significant advantage compared to other medications and highlights the potential of herbal alternatives as a suitable option for individuals with OA.
In this study, the use of pistachio oil formulated as an ointment demonstrated improvements in patient function, physical activity, and stiffness. These findings support the effectiveness of herbal remedies for OA, as observed in the study by Abbasifard et al. (15) and the study by Haroyan et al. on the safety and efficacy of curcumin in OA (32). Furthermore, Abbasifard et al. conducted a randomized placebo-controlled clinical trial investigating the potential protective effects of P. vera pericarp on knee OA (33). Their study revealed that the formulation of P. vera pericarp had pain-relieving properties and improved stiffness and physical performance with no detectable adverse effects (33). The study above, in alignment with our investigation, provides further support for the use of P. vera as a beneficial and safe supplement in managing OA and its associated pain and complications.
The limitations of this study include the penetration and absorption of the active components of the oil into the skin, the standardization process of the topical formulation, and the assessment of the mechanism of action. These parameters require further clarification in future studies.
5.1. Conclusions
Our findings indicate that chronic use of pistachio ointment in patients with knee OA reduces pain, improves motion stiffness, and enhances activity levels compared to placebo. Furthermore, the pistachio seed oil formulation exhibited even more potent effects than topical piroxicam in certain parameters. Notably, no side effects were observed with the chronic administration of pistachio in our study. Further clinical trials are recommended to investigate the exact mechanism of action, as well as the safety and efficacy of this herbal intervention, in a more significant number of patients.