1. Background
Dental caries is a breakdown of teeth as a result of activities of certain bacteria (1). Although the human oral flora is extremely complex and diverse, only a few specific species of bacteria including Streptococcus mutans and Lactobacillus species are believed as the main causative agents of tooth caries (2). The acid generation and acid lenience of above mention microorganisms also serve as virulence agents, given that the final products of acids generated over the metabolism of fermentable sugars and the capability of microorganisms to endure acidic conditions in dental plaque are vital for the progression of tooth caries (3). Moreover, the development of biofilm formation associated with S. mutans is a chief parameter accountable for the appearance of a variety of maladies, comprising of tooth cavities. Hence, a rational approach for hindering dental caries would be controlling both the growth of S. mutans and biofilm formation.
The use of medicinal plant in traditional folk medicines goes back many years. It revealed a promise for a reliable supply of constituents for improvement of novel antimicrobial, antiviral and anticancer drugs (4). In the dentistry field, numerous researches were carried out to explain the feasibility of applying remedial herbs as remediation of oral diseases including dental cavities. However, the use of microalgae-based medicine for preventive effects on dental caries has not been well evaluated.
Microalgae are microscopic algae, naturally found in marine and freshwater systems living in both the sediment and column water. Microalgae are a supply of natural products and have been recently considered for biotechnological applications. The diversity of microalgae provides a potentially rich supply for a variety of chemical compounds with implementation in pharmaceutical, cosmetic, nutritional, and medicinal industries. The marine extracts of microalgae are a rich supply of vitamins, proteins, and minerals. In this regard, 2 species of microalgae named Chlorella vulgaris and Dunaliella salina have attracted much interest in the field of nutritional and medicinal industries (5). D. salina is halophile green unicellular, photosynthetic and motile flagellate microalgae morphologically that can be found in salty lakes and sea salt fields (6, 7). This microorganism can tolerate different concentrations of sodium chloride, ranging from 0.2% to approximately 35% (8).
D. salina can produce large amounts of carotenoids with antioxidant properties, that have wide applications for use in cosmetics, nutritional supplements, aquaculture feeds, food, colouring, and dietary supplements (9, 10).
C. vulgaris, is a member of green algae that has been considered as a reliable supply of foodstuff worldwide (11). This microalgae provides an excellent supply for proteins, lipids, carotenoids, minerals, vitamins, and pigments (12). Furthermore, the extracts of Chlorella sp. have antibacterial activities against some bacterial strains like Bacillus subtilis, Pseudomonas aeruginosa, and Escherichia coli (9, 13, 14). The antibacterial activity of C. vulgaris was attributed to the existence of cyclic peptides, terpenoids, alkaloids, steroids, and tannins (12).
The specific nature of the antibacterial and antiadherent traits of D. salina and C. vulgaris to control dental caries is not well known and is now currently under evaluation. In this regard, most of the studies on these microalgae are predominantly focused on their nutritional and medicinal traits. For the first time in the present study, the antimicrobial, anti-biofilm properties, and inhibitory effects of D. salina and C. vulgaris extracts were evaluated by several antimicrobial methods against S. mutans as the most important agents in dental caries. Moreover, evaluation of these 2 extracts toxicity was also studied on Artemia urmiana.
2. Methods
2.1. Microorganisms and Culture Conditions
The microalgae C. vulgaris was isolated from lake and rice paddy fields of Kamfiruz, in Fars province, Iran and D. salina was isolated from Maharlu Lake, Shiraz, Iran. Individual cells were morphologically studied under a light microscope (labo America, lnc-USA) and confirmed by sequencing 18s rRNA gene using the PCR method (15, 16).
The single colonies of each C. vulgaris and D. salina were maintained on the BG-11 and Johnson medium, respectively. Microalgae growth was monitored at 25 ± 2ºC, under low illumination (37 µmol m-2 s-1) and unlimited aerated condition during 17 and 21 - 31 days for C. vulgaris and D. salina, respectively (17). After colonization, pure cultures were prepared by subculturing on the agar plate and then transferring to broth mediums.
S. mutans PTCC 1683 was purchased from the Iranian Biological Resource Centre in Iran in the form of lyophilized tube. This bacterium was cultivated in brain heart infusion (BHI) broth including 5% sucrose, for 24 h at 37°C under microaerophilic conditions.
2.2. Microalgae Growth Factors
The optical density and cell number was quantified during the cultivation period in the Erlenmeyer flasks. For cell enumerating, 20µL of microalgae suspension was picked via sampling tube and then directly counted using Neubauer hemocytometer (ISOTHERM) under light microscope (TCM400-LABOMED). Optical density was measured by a UV/Visible spectrophotometer at 670 nm (U-0080D-TOYKYO, JAPAN).
2.3. Microalgae Materials and Extraction
The body mass of 14 days of growth of both microalgae were collected by centrifuge (at 6000 rpm, 10 min). The supernatant of each microalgae was discarded and then the wet biomass was frozen for 12h at -70°C and freeze-dried (GLD-136C-JAPAN) at -70°C under a vacuum.
The dried products were collected and stored in 50 ml falcon tubes. For preparing micro algal extract, 5g of freeze-dried microalgae biomass was suspended in chloroform/ methanol/ acetone in ratio of 2.1.1 (500 ml) and soaked for 72h. Then, extraction of each microalgae was prepared by using rotary evaporator (TW-10, JAPAN) at the temperature of 45ºC for 8h.
2.4. Preliminary Phytochemical Analysis
Phytochemical experiments were performed for a variety of components of C. vulgaris and D. salina using the following reagents and chemicals (18, 19). Mayer and Dragendorff’s reagents were used for alkaloids, FeCl3 for tannins, NaCl and HCl for flavonoids, Liebermann-Burchard test for sterol/terpenes, frothing test for saponins, ethyl chloroformate as derivatization reagent for the determination of free fatty acids, and Molisch reagent for carbohydrates. In general, examinations for the absence or presence of substances using these approaches include the addition of the suitable chemical indicator to C. vulgaris and D. salina in a test tube. Finally, each observation was recorded and then analyzed (18, 19).
2.5. Antimicrobial Assays
2.5.1. Disc Diffusion Method
The antimicrobial agar diffusion assay was carried out according to modified Kirby-Bauer disc diffusion method CSLI (2016) (20) against S. mutans PTCC 1683. A total of 30 μl of each extract was spotted on sterile filter paper discs (6 mm in diameter) and left to air-dry; then, it was placed on the surface of agar medium (Mueller-Hinton Agar + 5% blood sheep, pH 7.4 ± 0.2 at 25 °C) (21). The opaqueness of the suspension was adjusted with a spectrophotometer at 530 nm to achieve a final standard concentration of a 0.5 McFarland (1.5 × 108 CFU). The plates were then inoculated by immersing a swab in the inoculums and spread it onto the entire surface of the media and then incubated at 37°C for 24h (21, 22).
After incubation for 24 h, the formation of clear zone of inhibition around a disc was confirmation of antibacterial activity. The zones of inhibition diameters were recorded in millimetres (The Zone of inhibition ≤ 11 was considered as resistant). Each examination was performed in triplicates. Discs containing each extracting solvent were considered as negative control and ampicillin were considered as positive control.
2.5.2. Well Diffusion Method
After culture of bacteria in brain heart infusion (BHI) broth and spreading the suspension on the Muller Hinton agar medium and 5% blood sheep, the agar was punched with a puncher in a 6 mm diameter. A total of 50 μl of each extract was loaded on each well. After 24h of incubation, diameters of the zones of inhibition were measured in millimeters. Each test was prepared in triplicates (21).
2.5.3. Determination of Minimum Inhibitory Concentration (MIC)
Minimal inhibitory concentrations (MIC) of D. salina and C. vulgaris extracts were evaluated on the basis of a micro dilution approach in 96 well microtiter plates. Briefly, S. mutans was cultured at 37ºC for overnight on BHI broth and then adapted to a final density of 1.5 × 108 CFU.
Then, the cell suspension with different concentration of each extract was added to Muller Hinton broth in each well. After 24h of incubation, their optical density was read by the Elisa reader as well as a spectrophotometer and then compared with each other. Medium without the extract was a negative control and ampicillin was the positive control. This experiment was done in triplicate (22).
2.5.4. Determination of Minimum Bactericidal Concentrations (MBC)
Minimum bactericidal concentrations (MBC) of extracts were determined due to NCCLS protocol (20). Samples with the extract concentrations equal and higher than MIC were cultured on BHI agar with 5% blood sheep. After 48h of incubation, the plate with lower concentration of extract showing no bacterial growth was considered as MBC. This experiment was done in triplicate (22).
2.5.5. Anti-Biofilm Formation Test
The commonly used microtitre-plate method, for determining bacterial adhesion to plastic (96-well polystyrene microtitre-plate), based on the methodology, often employed for biofilm formation was used (3). The assays were performed in 96 well polystyrene micro plates (Nunc® MicroWellTM). Each well filled of 50 µL of BHI broth, 2% sucrose, and 50 µL of S. mutans suspension, corresponding to approximately 1.5 × 108 CFU with 50 µL of different concentrations of C. vulgaris and D. salina. As a positive control, 50 µL of ampicillin was loaded in place of the plant extract. Negative control samples were prepared from 50 µL of each of the inoculums and the culture media. The microplates were incubated for 48h under microaerophilic and 5% CO2 conditions. After incubation, supernatants were discarded from the 96-well plate, then, mildly rinsed 4 times with distilled water to eliminate the weakly adhered cells to polystyrene. Subsequently, 200 µL of pure methanol was added to each well until fixation of the adherence cells. After 30 minutes the adhered cells to the walls of the wells were stained with addition of 200µl of crystal violet (0. 0125% w/v) and left for 5 minutes at room temperature. Then, the microplates were washed 3 times with distilled water. Destaining was done by addition of 200 µL glacial acid/ alcohol for 5 min. After solubilisation, a volume of 50 µL from each well was transferred to quartz cuvette, and the absorbance was measured at 512 nm to assess the formation of biofilm in every concentration of extracts (23, 24).
2.6. Toxicity Examination Against the Brine Shrimp
2.6.1. Hatching Shrimp
The cysts of brine shrimp were purchased from fisheries of Urmia city, Iran. Brine shrimp lethality assay was used to investigate the toxicity of 2 kinds of microalgae D. salina and C. vulgaris. Brine shrimps (Artemia urmiana) were hatched using 1g of cysts in a flask with 1L artificial seawater (prepared from various lake salts in 38 g l-1 then 1N NaOH was used for adjusting to pH 8.5) for 48h under constant aeration (25). Finally, the active nauplii were collected with a sampler for the study.
2.6.2. Brine Shrimp Assay
All the toxicity experiments were carried out in 12 well tissue culture plates (BIOFIL). A total of 10 newly hatched (24h old) brine shrimp (Artemia urmiana) nauplii were added into each well pre filled with 0.5 ml microalgae extract with different concentrations and 4.5 ml of salt water (26). The number of inanimate nauplii was recorded at 5 time intervals, 15 min, 2, 8, 16, and 24h after exposure to the microalgae extracts (25).
2.6.3. Analysis
Each experiment was performed as a triplicate and then analyzed using ANOVA and the t-test. The level of significance was P < 0.05 for all statistical tests. Statistical analysis was carried out using the SPSS 16.0 software (SPSS Inc., Chicago, IL, USA).
3. Results
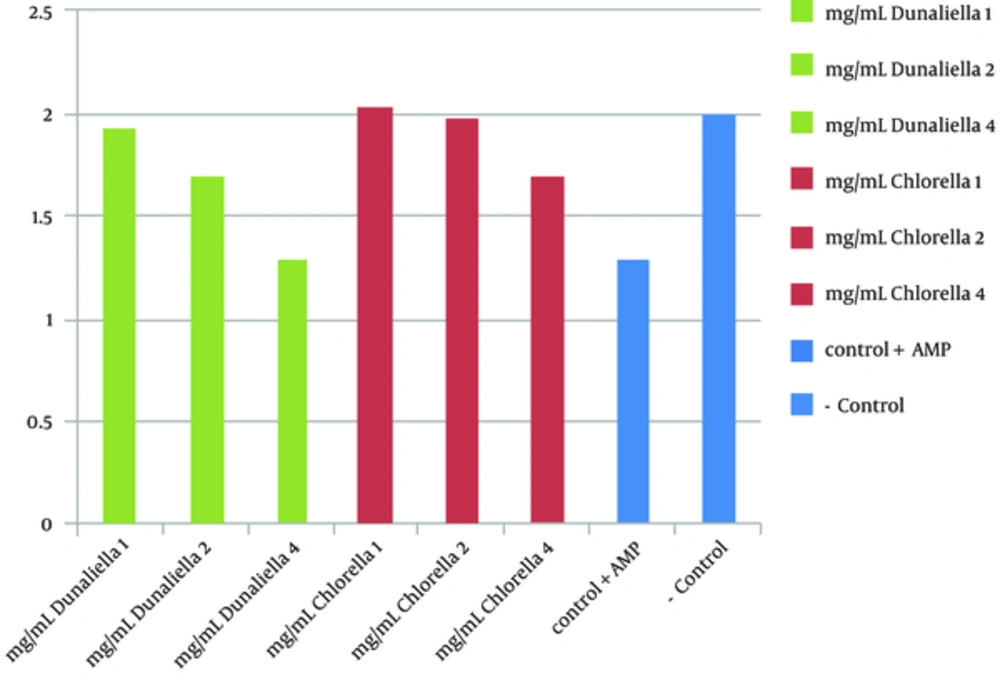
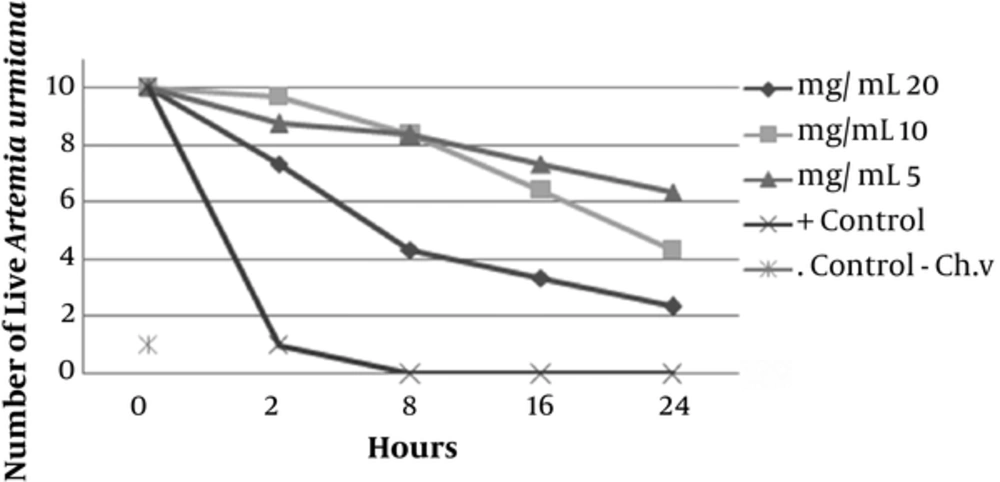
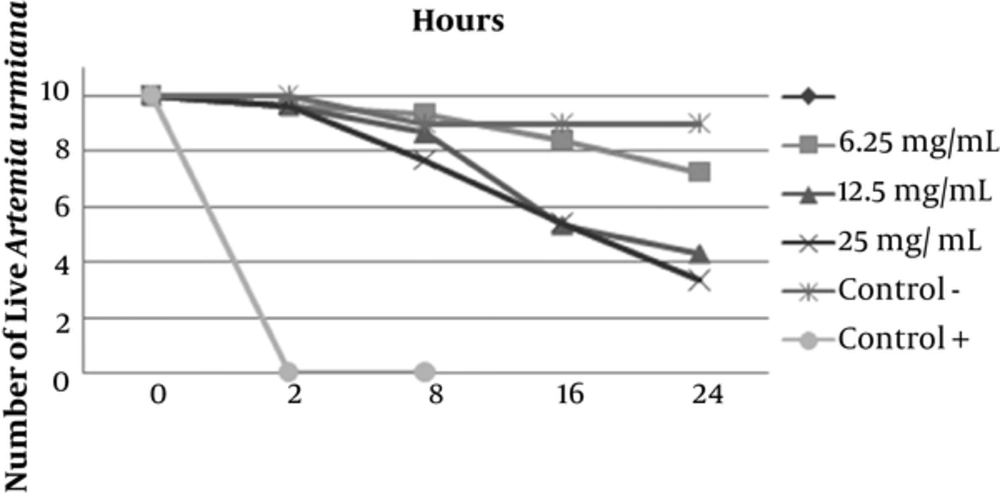
Preliminary phytochemical screening of D. salina and C. vulgaris revealed the presence of sterol/terpenes, indoles, phenols, fatty acids, and carbohydrates. The antimicrobial activity of disc diffusion assay of C. vulgaris extract at concentrations of 0.625, 1.25, and 2.5 mg/disc was examined against S. mutans PTCC 1683. Among them, the largest inhibition zone appeared around the disc loading of 2.5 mg/disc of extract with a diameter of 13.5 ± 0.92mm (P < 0.05). The antimicrobial activity of disc diffusion assay of D. salina extract in concentrations of 1.5, 3 and 6 mg/disk was evaluated while the highest zone of inhibition showed around the disc loading with 6 mg/disc of extract with a diameter of 18.5 ± 0.97 mm (P > 0.05). Ampicillin (10µg Disc-1) was used as positive control, which showed clear zone of inhibition 21 ± 0.98 mm. The antimicrobial activity of well diffusion assay of C. vulgaris in concentrations of 2.5, 5 and 7.5 mg ml-1 was evaluated. The highest zone of inhibition around the well of 7.5 mg ml-1 was reported with the average diameter of 19 ± 0.87 mm. The results of well diffusion assay of D. salina in concentrations of 3.75, 7.5, and 15 mg ml-1 showed the highest antimicrobial activity (25.4 ± 0.97 mm) around the well with 15 mg ml-1 of extraction. Ampicillin, as positive control, showed clear zone of 28 ± 1 mm. The MIC value of C. vulgaris extract in serial dilution concentrations of 0.625 to 20 mg ml-1 showed the inhibitory effect in 5 mg ml-1. On the other hand the MIC value of D. salina extract in serial dilution concentrations between 0.75 to 25mg ml-1 started in 6.25 mg ml-1. MBC value of C. vulgaris and D. salina extracts revealed the desire effect in 20mg ml -1 and 12.5mg ml-1 concentration, respectively. Anti biofilm activity of C. vulgaris extract in concentrations of 1, 2, and 4mg ml-1 showed appropriate results in 4mg ml-1 (no biofilm was formed). The same obtained results for D. salina extract was achieved in 2 mg ml-1 (Figure 1). Brine shrimp test toxicity of 2 microalgae extracts with different concentrations (5, 10, and 20 mg ml-1) for C. vulgaris showed LC50 value of 20 mg ml-1 (Figure 2) and for D. salina (6.25, 12.5 and 25 mg ml-1), the LC50 value of 12.5 mg ml-1 was observed (Figure 3).
4. Discussion
Dental caries is one of the most predominant chronic illnesses in both adults and children, albeit it is mostly avoidable. A broadly adopted treatment to diminish the occurrence of the illness is the implementation of chemoprophylaxis agents. These agents have pulled toward extensive considerations due to its capability to control patient-directed methods for plaque management. However, a number of these agents, such as antibiotics and chlorhexidine, have unfavorable side effects involving tooth coloring and the appearance of bacterial multi-drug resistance. Recently, medicative plants and microalgae have revealed hope as an alternate option to chemoprophylaxis agents for hindering tooth decay. In this regard, we have concentrated on C. vulgaris and D. salina, which has been applied in habitual medication for controlling dental disorders. Although the biomass of C. vulgaris and D. salina have been studied broadly for their biological activity (4, 27, 28), in accordance to the best of our knowledge, this is the 1st study on prohibitive impacts of these microalgae against S. mutans viability and the biofilm formation.
There has been some controversy in regards to classifying a particular bacterial species or a consortium of bacteria is the causative agent for the development of tooth cavities. Due to the fact that various non-mutans bacteria spp. are adequately acid tolerate, acid producer, and many of them were able to generate the amount of acid necessary for the progression of tooth cavities (3, 22, 29), some researchers disagreed that S. mutans spp., except for only some specific cases, are the causative agent of tooth cavities (30, 31). However, this research relied on the assumption that S. mutans spp. are the main tooth-decaying microorganisms, though earlier immunological and epidemiological studies provided enough proof for the relationship of S. mutans spp. with tooth cavities in individuals (24). Among various type of mutans streptococci spp., S. mutans is the prominent mutans streptococci in human type, which is thoroughly related to individual tooth cavities (3). Therefore, S. mutans was selected as the main test bacteria.
Disc and well diffusion method are both verified by CSLI (2016) for evaluation of antibacterial properties of compounds. In this study, both of them were applied for revealing, which one of them show the best results. In this regard, the well diffusion method could mimic a more similar condition to mouth wash solutions rather than the disk diffusion method (20). Generally, antimicrobial activity of D. salina showed better results than C. vulgaris extract. In addition, the results of extracts compare with ampicillin showed approximately equal effects for D. salina and less antimicrobial properties for C. vulgaris. The exact bioactive compounds of C. vulgaris and D. salina that offer the prohibitive effects on S. mutans are unclear. In a study by Mendiola et al., supercritical CO2 extraction of D. salina showed antimicrobial activity against Escherichia coli, Staphylococcus aureus, Candida albicans, and Aspergillus niger (14). According to the initial phytochemical analysis, the activity of C. vulgaris and D. salina might be related to the presence of indoles, tannins, terpenes, acetogenins, phenols, and fatty acids. Some studies reported that microalgae phenols as well as fatty acids and volatile halogenated hydrocarbons possess an extensive variety of pharmacological activity including antimicrobial activity. In this regard, Cowan (32) reported that the phenolic antibacterial activity might be due to the oxidized compounds, which react with sulfhydryl groups or via additional non-specific interactions with the proteins. Moreover, there were some reports that terpenes and alkaloids are active against bacteria (33, 34). In a research by Uma et al., it was reported that flavonoids, tannin, phenolic compounds, terpenoids, and saponins are the most active antibacterial agents in C. vulgaris against Klebsiella pneumonia, Vibrio cholera, and Staphylococcus aureus (36). The exact mechanism accountable for the function of these compounds are not fully understood, however it is hypothesized to encompass the capability of terpenoids to membrane disruption by the lipophilic compounds and intercalate with DNA (33). The possible role of fatty acids in antimicrobial activity might be related to disruption of the electron transport chain and oxidative phosphorylation, destruction of nutrient uptake, auto-oxidation degradation products and production of peroxidation, direct lysis of bacterial cells, or prohibition of enzyme activity (35). However, according to our solvent extracts, it’s suggested that flavonoids, tannins, and terpenoids in C. vulgaris extract and 3, 3, 5-trimethylheptane, n-hexadecane together with polyunsaturated fatty acids and compounds related to carotene metabolism, such as β-ionone and neophytadiene in D. salina extract might be responsible for antimicrobial properties. In contrast, according to obtained shrimp test toxicity, accumulation of secondary metabolites such as flavonids, phenols, saponins, and tannins in both extracts, results in toxicity and mortality of nauplii (36, 37).
The antibiofilm activity of C. vulgaris and D. salina might be attributed to the glucosyltransferases (GTF) suppressive activity of C. vulgaris and D. salina extracts, which prohibits the biosynthesis of water-insoluble glucans. Lauritano et al. showed that some types of microalgae have antibiofilm activity Staphylococcus epidermidis (38). It should be noted that generation of water-insoluble glucans from sucrose enhances attachment to the teeth (39, 40), which helps the making of tooth plaque.
4.1. Conclusions
To sum up, the results of this research revealed that both C. vulgaris and D. salina extracts had antibacterial and anti biofilm formation activity against S. mutans. These inhibitory effects might be due to the existence of biologically active ingredients where their characteristics and chemical structures would enable them to function as an antibacterial substance against some pathogens. The outcomes of this research pave the way for developing novel herbal and safe formulations in mouth hygiene against oral pathogens, especially S. mutans for efficient controlling dental cavities.