1. Background
Herbal medicine is an essential part of conventional remedies. The World Health Organization (WHO) delineates conventional remedy as diverse beliefs, practices, techniques, and knowledge that employs minerals, plants, animals, meditation, and spiritual training alone or combined for diagnosis, well-being maintenance, prevention, and treatment of illness (1). Underdeveloped countries frequently use different basils to indulge diseases because it is considered inexpensive and safer than allopaths. According to WHO, 80% of the Asian and African populations consume herbal medicine (2), as they contain phytochemicals, which possess diverse biological significances (3) and could be beneficial in treating different diseases (4). Medicinal plants have the greatest potential for transformation into medical relevance due to essential oils, terpenes, flavonoids, glucosinolates, and alkaloids. Cleome's pharmacological effects are associated with its phytoconstituents, resulting in fever, pain relief, and inflammatory reduction (5).
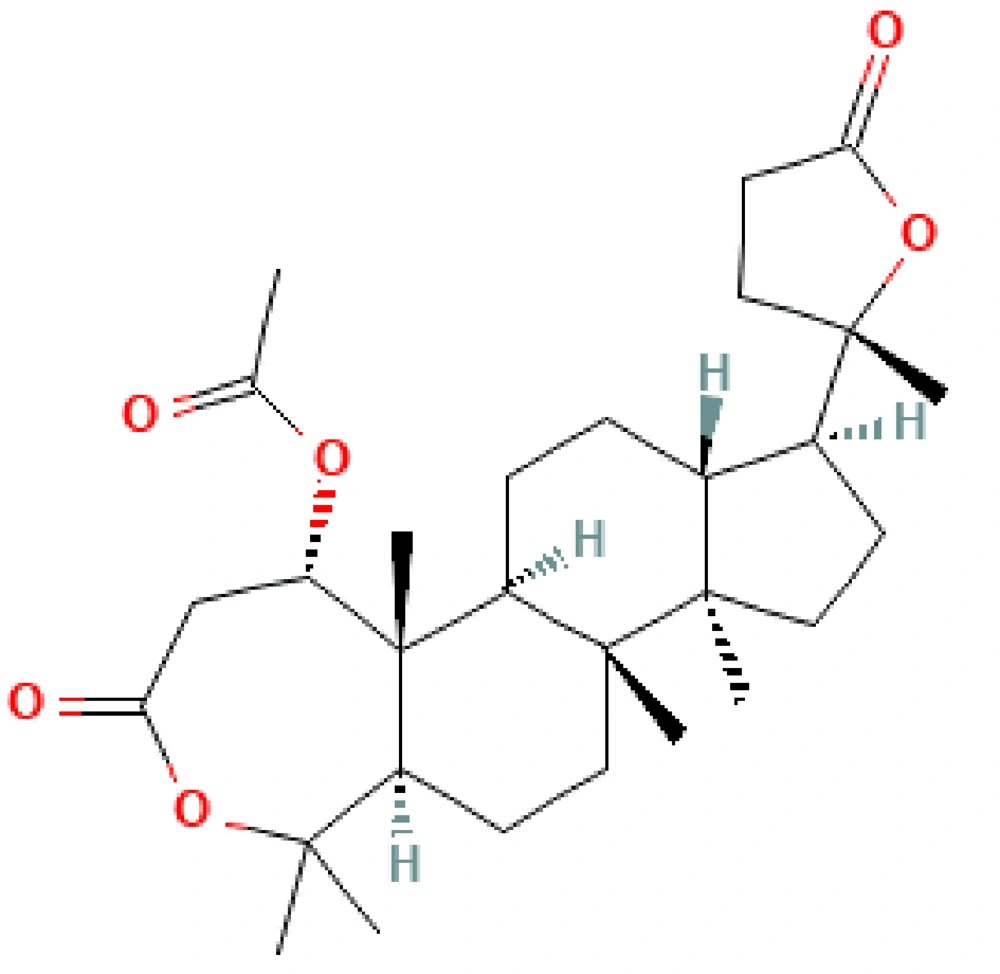
Cleome brachycarpa is a woody herb with yellow flowers (6), mostly cultivated in different areas of India, Iran, and Pakistan. It has miscellaneous usages for improving joint pain (7), soreness, tenderness, itching, leprosy, leucoderma, scabies, intestinal pain (8), and swelling; it also has anti-rheumatic (9, 10), vermicidal, anthelmintic (11), antioxidant (12), and antiemetic effects (13). Cleome brachycarpa have exceptional chemical composition like triterpenoid dilactone, deacetoxy brachycarpone (Figure 1) (14), triterpenoid cleocarbpone, ursolic acid, and cabralealactone. These compounds make the whole plant medicinal and are useful in bronchitis, diarrhea, malaria, heart failure, seizures, immune system deficiencies, jaundice, fever, chills, and treating swollen body parts (15-17). Likewise, sesquiterpenes and flavonoids make Cleome more anti-fungal, anti-bacterial, and antitumor with good cytotoxic effects (5). It strongly kills plant pests and microbes (18) and is a tranquilizer (19). Hence, this species of Cleome is vital in its properties and composition (20-22).
This study evaluated Cleome brachycarpa for its anti-inflammatory and analgesic activity and effects on the hematopoietic system. Inflammation is the protective response to eliminate the initial cause of injury. Different chemicals like prostaglandins, nitric oxide, leukotrienes, and histamines are released throughout inflammation that encourage smooth muscle tightening, boost movement of neutrophils, amplify the permeability of vessels, pain initiation, and damage (23), and increase chemical mediators like COX-2 (24). These changes after acute inflammation cause edema, usually measured by an instrument, e.g., a plethysmometer (25, 26). Following a similar path, the anti-inflammatory activity of Cleome brachycarpa was assessed by producing inflammation and edema by acetic acid (27) compared to a well-known marketed drug, diclofenac sodium, by measuring any change in the paw volume of the edematous rat.
Similarly, the analgesic or pain-combating property of the mentioned Cleome species was evaluated compared to morphine by inducing pain in mice. Hot water is one way to produce pain that increases pain-generating mediators such as Prostaglandin E, bradykinin, and histamine. Pain reaction in rodents was quantified by the tail-flick method, and the efficacy of analgesic medicine was noted by observing the time in which the tail was flicked in response to heat.
2. Objectives
To date, no study has investigated the anti-inflammatory and analgesic properties of Cleome brachycarpa. Thus, this study aimed to evaluate its analgesic and anti-inflammatory properties and effects on different blood parameters and liver enzymes. We assessed WBC, RBC, Hb level, hematocrit value, MCV, MCH, MCVC, platelet count, cholesterol SGPT, and ALP enzymes.
3. Methods
3.1. Compliance with Ethical Standards
All procedures in the study followed the Helsinki Declaration (1964), and the Ethics Committee of Baqai Medical University officially permitted the entire investigational trial with letter no. BMU-EC/05-2022.
3.2. Collection of Plant Material
The plant was collected from the Department of Pharmacognosy, Faculty of Pharmaceutical Sciences, University of Karachi.
3.2.1. Extraction of Plant Material
The entire Cleome brachycarpa plant was first splashed with water to condense the contagious load. Afterward, it was cut into pieces and dehydrated at a high temperature of 50ºC. It was then crushed and extracted. Later, it was soaked in 95% ethanol for three days, then sieved and dried under pressure. A two-time maceration was done, then dried in the evaporator. In this way, 5% Cleome brachycarpa was obtained (28).
3.2.2. Experimental Animals
Three types of lower mammals were pooled, including rats, mice, and rabbits, for anti-inflammatory, analgesic, and hematological evaluation, respectively. A total of 40 Wistar strain albino rats of 200 ± 5 g (10 weeks of age), 30 mice of 30 ± 2 g (10 weeks of age), and 60 rabbits of 1000 - 1500 g (10 months of age) were gathered from a retained and renowned animal house of Baqai Institute of Pharmaceutical Sciences (BIPS). Rabbits were selected for this evaluation because they have similar hematological variations to humans (29). All animals were housed in the animal house for 12/12-hour light/dark cycles at 25 ± 2°C temperature. Well-balanced laboratory feed was provided to all animals, and one week before the study, the weight of all animals was noted.
3.2.3. Test Doses
We used Cleome brachycarpa 200 mg/kg/body weight (21, 28, 30), morphine (31), and diclofenac sodium (32).
3.3. Investigational Plan for Anti-inflammatory Activity
3.3.1. Materials
We used a plethysmometer (UGO BASILE 7140), adult male albino rats (Wistar strain), ethanol extract of Cleome brachycarpa, feeding tube, acetic acid, diclofenic sodium, and distilled water.
3.3.2. Methodology of Anti-inflammatory Activity
Group A: Control animals (n = 10) were administered normal saline orally for 10 days. Group B: Positive control Wistar strain rats (n = 10) were administered acetic acid (2%) in the right hind paw plantar surface to develop inflammation during the trial. Group C: Standard rats (n = 10) were administered a standard drug (Diclofenic sodium, 10 mg/kg/body weight) orally one hour ahead of the injection of acetic acid. Group D: Test rats (n = 10) were orally administered Cleome brachycarpa extract (200 mg/kg) for 10 days.
The volume of the rat paw was measured by a plethysmometer (No. 7140) from zero to the eighth hour after the acetic acid injection. Percentage inhibition was estimated by the Newbould method:
The outcomes were articulated as ± standard error mean (SEM), and the statistical significance of differences among the groups was investigated via ANOVA (Amol and Rajeshkhar, 2011).
3.4. Investigational Plan for Analgesic Activity
3.4.1. Material
We used Cleome brachycarpa 200 mg/kg/body weight, morphine, a feeding tube, a water bath, gloves, and a stopwatch.
3.4.2. Methodology of Analgesic Activity
Group A: Control mice (n = 10) were administered normal saline orally for 10 days. Group B: Standard mice (n = 10) were administered morphine 10 mg/kg/body weight orally on the experiment day. Group C: Test mice (n = 10) were orally administered Cleome brachycarpa extract 200 mg/kg for 10 days.
Analgesic activity was evaluated by the tail-flick method. An appropriate restrainer was utilized to keep each mouse in a suitable position keeping the tail extending out. The tail was immersed in a water bath (51 ± 1°C), and tail flicking time from hot water was noted as the retention time. The observations were taken from zero to the sixth hour. Percentage inhibition was analyzed as follows:
where T1 is post-drug latency, and T0 is pre-drug latency. The data were statistically evaluated by SPSS version 23 (33).
3.5. Investigational Plan for Hematological Analysis
3.5.1. Materials
We used vacutainers containing anticoagulant/gel, syringes, gloves, Labfuge (centrifuge machine), Vitalab Micro® (Merck), Sysmex® KX-21, Fluitest® ALP DGKC kit for alkaline phosphatase (ALP), ALAT (SGPT) (IFCC mod.) kit for alanine aminotransferase (ALAT), and Cholesterol Innoline kit for cholesterol.
3.5.2. Research Methodology
Group A: Control rabbits (n = 30) did not receive treatment. Group B: Treated rabbits (n = 30) received 200 mg/kg ethanol extract of Cleome brachycarpa orally for 10 days. Sample collection: By cardiac puncture, 4 mL of blood samples were drawn from the animals after 10 days of dosing. Serum separation for enzymes: Blood was first centrifuged by Labfuge (model 80 - 2S) at 4°C and 3000 rpm for about 20 minutes. Serum was separated and used for analysis.
3.5.3. Analysis of Hematological Parameters
Complete Blood Count: WBC, RBC, Hb, HCT, MCV, MCH, MCHC, and platelet count were scrutinized via an automated hematology analyzer, Sysmex® KX-21 (Newland, 2007). Liver Enzymes: ALP and SGPT levels were analyzed in treated rabbits with Vitalab Micro® (Merck) and respective kits. Cholesterol was also analyzed with the help of Vitalab Micro® (Merck) using a cholesterol Innoline kit. Statistical analysis of the above parameters was performed using a student t-test at P < 0.05 as significant, P < 0.001 as extra significant, and P < 0.0001 as extremely significant.
4. Results
4.1. Anti-inflammatory Activity
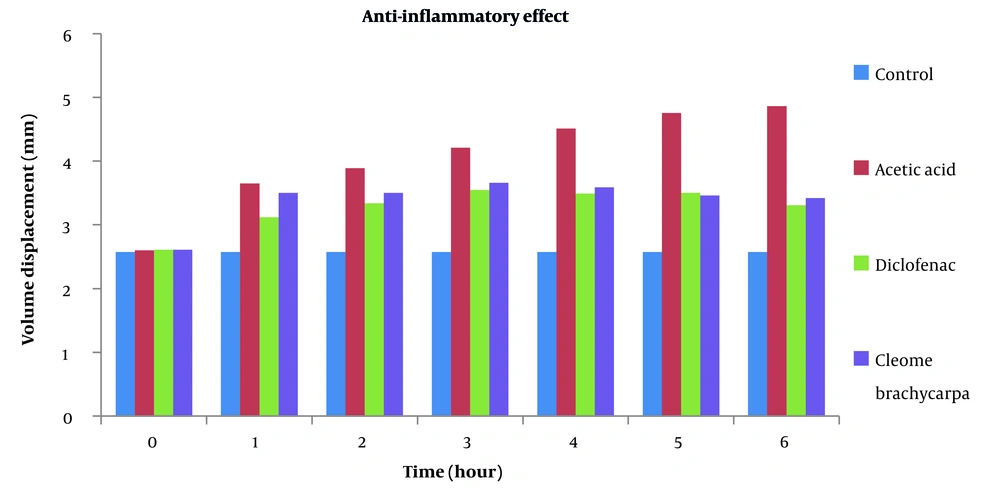
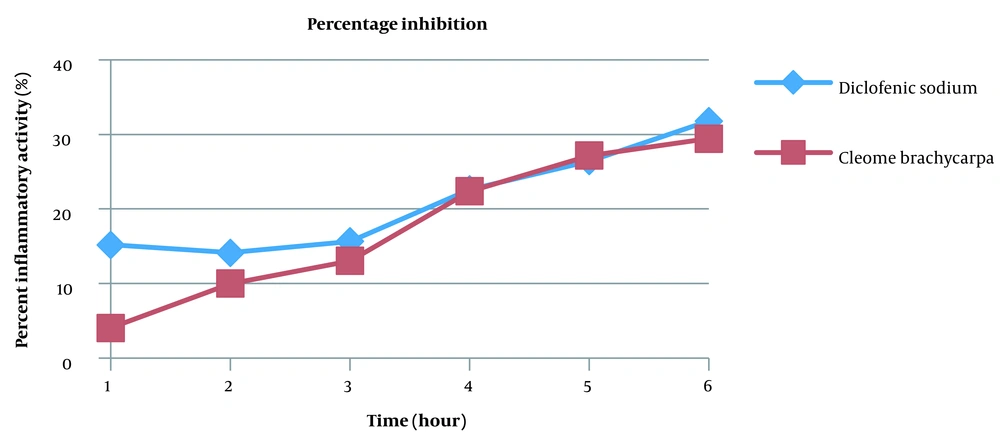
Many anti-inflammatory medicines are available, but they all have many side effects. This research aimed to discover a unique anti-inflammatory agent with minimal unwanted effects (27). Changes in edematous volume by Cleome brachycarpa were measured by a plethysmometer that displayed a significant anti-inflammatory response, as shown in Table 1 and Figure 2. The herb produced very close results to Diclofenac sodium, proving Cleomes' high efficacy in managing inflammation. However, the tested extract showed maximum inhibition of 29.42% in the sixth hour, as shown in Table 2 and Figure 3. Statistical analysis was done by ANOVA at P-value < 0.05 as significant.
| Groups | Time, h | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | |
| Control | 2.57 ± 0.01 | 2.57 ± 0.01 | 2.57 ± 0.01 | 2.57 ± 0.01 | 2.57 ± 0.01 | 2.57 ± 0.01 | 2.57 ± 0.01 |
| Acetic acid | 2.60 ± 0.15 | 3.68 ± 0.05 | 3.89 ± 0.06 | 4.21 ± 0.16 | 4.515 ± 0.16 | 4.755 ± 0.12 | 4.86 ± 0.14 |
| Diclofenic sodium | 2.61 ± 0.13 | 3.12 ± 0.09 | 3.34 ± 0.16*** | 3.55 ± 0.23* | 3.49 ± 0.29*** | 3.48 ± 0.21*** | 3.31 ± 0.17*** |
| Cleome brachycarpa | 2.61 ± 0.003 | 3.53 ± 0.28 | 3.50 ± 0.09 | 3.665 ± 0.07 | 3.50 ± 0.12** | 3.46 ± 0.13*** | 3.43 ± 0.11*** |
Anti-inflammatory Effect of Cleome brachycarpa in Albino Rats a
| Groups | Time, h | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Diclofenic sodium | 15.21 | 14.13 | 15.67 | 22.61 | 26.42 | 31.80 |
| Cleome brachycarpa | 4.07 | 10.02 | 13.06 | 22.39 | 27.15 | 29.42 |
Percentage (%) Inhibition of Cleome brachycarpa
4.2. Analgesic Effect
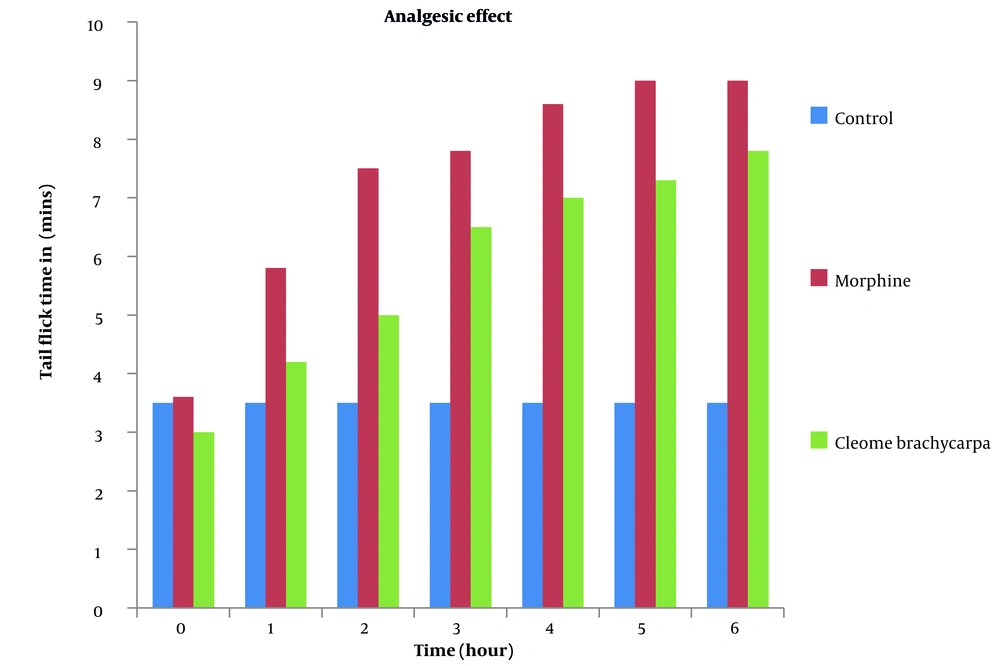
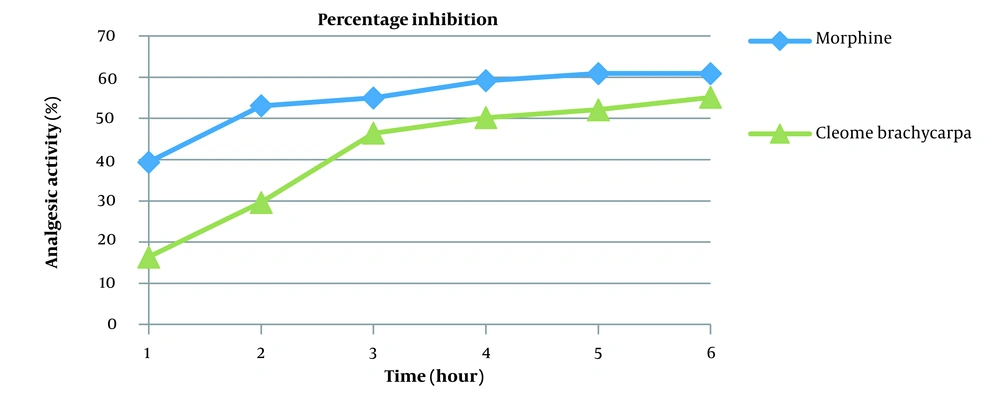
Pain is an emotional and obnoxious event because of the actual or potential tissue injury (34) treated by various analgesics, producing sleep and drowsiness (35). Cleome brachycarpa was carefully chosen to evaluate its analgesic effect (36), and the outcomes were compared with the standard agent, morphine. The results of Cleome brachycarpa were noted compared to morphine. As shown in Table 3 and Figure 4, morphine started to reduce pain in the first hour, while Cleome extracts produced a similar effect in the third hour, which was very close to the standard one. Cleome showed maximum inhibition of 55.10% in the sixth hour, as shown in Table 4 and Figure 5.
| Groups | Time, h | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | |
| Control | 3.52 ± 0.15 | 3.52 ± 0.15 | 3.52 ± 0.15 | 3.52 ± 0.15 | 3.52 ± 0.15 | 3.52 ± 0.15 | 3.52 ± 0.15 |
| Morphine | 3.65 ± 0.20 | 5.81 ± 0.28*** | 7.50 ± 0.36*** | 7.82 ± 0.28*** | 8.6286 ± 0.16*** | 9.00 ± 0.00*** | 9.00 ± 0.00*** |
| Cleome brachycarpa | 3.07 ± 0.22 | 4.21 ± 0.24 | 5.00 ± 0.47* | 6.57 ± 0.49*** | 7.07 ± 0.29*** | 7.35 ± 0.34*** | 7.84 ± 0.20*** |
Analgesic Effect of Cleome brachycarpa in Mice a
| Groups | Time, h | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Morphine | 39.41 | 53.06 | 54.98 | 59.16 | 60.88 | 60.88 |
| Cleome brachycarpa | 16.38 | 29.60 | 46.42 | 50.21 | 52.10 | 55.10 |
Percentage (%) Inhibition of Cleome brachycarpa in Mice
4.3. Hematological Evaluation
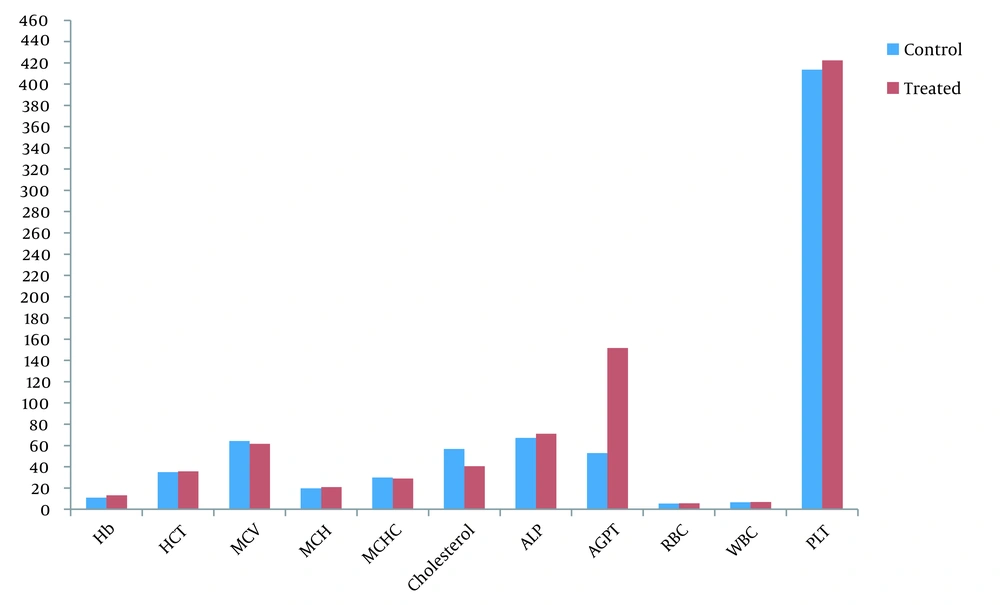
The effects of Cleome brachycarpa on complete blood count, cholesterol, and liver enzyme were statistically evaluated by student t-test. Examination showed little increases in Hb, HCT, MCH, WBC, RBC, PLT, and ALP values, but they were insignificant (P > 0.05). It also produced slight decreases in MCV, MCHC, and cholesterol values but insignificantly. On the other hand, it significantly increased SGPT from 52.83 ± 15.2 to 151.83 ± 8.37 (P = 0.000), as elucidated in Table 5 and Figure 6.
| Variables | Control | Treated | t-test | P-Value |
|---|---|---|---|---|
| Hb | 10.9 ± 0.186 | 13.10 ± 0.242 | 0.655 | 0.527 |
| HCT | 34.91 ± 0.917 | 35.66 ± 0.836 | 0.725 | 0. 485 |
| MCV | 64.26 ± 1.579 | 61.66 ± 1.282 | 0.295 | 0.77 |
| MCH | 19.58 ± 0.411 | 20.83 ± 1.01 | 1.17 | 0.268 |
| MCHC | 29.85 ± 0.299 | 29.00 ± 0.426 | 0.608 | 0.557 |
| RBC | 5.38 ± 0.113 | 5.554 ± 0.15 | 0.350 | 0.734 |
| WBC | 6.56 ± 0.145 | 6.88 ± 0.254 | 1.079 | 0.306 |
| PLT | 413.66 ± 27.78 | 422.33 ± 27.10 | 0.223 | 0.828 |
| Cholesterol | 56.83 ± 21.33 | 40.5 ± 25.31 | 0.937 | 0.361 |
| ALP | 67.16 ± 21.7 | 71.0 ± 14.4 | 0.36 | 0.76 |
| SGPT | 52.83 ± 15.2 | 151.83 ± 8.37 | 13.96 | 0.000 |
Effect of Cleome brachycarpa on Blood Parameters in Rabbits a
Effect of Cleome brachycarpa on blood parameters in rabbits. Hb, hemoglobin in g/dL; HCT, hematocrit in % (percentage); MCV, mean corpuscular volume in fL; MCH, mean corpuscular hemoglobin in pg; MCHC, mean corpuscular hemoglobin concentration in g/dL; Cholesterol, mg/dL; WBC, white blood cells in 103/µL; RBC, red blood cells in 106/µL; SGPT, glutamate pyruvate transaminase U/L; ALP, alkaline phosphatase; Pltelets= 109/µL.
5. Discussion
Since prehistoric times, herbs have been exploited as therapeutic agents because of their salutary efficiency and ease of availability. In the present era, much research is being done on plants. Family Cleomaceae possesses several therapeutic species, including Cleome brachycarpa, a plant with plenty of beneficial effects.
The optimistic pain-challenging ability of this herb could be a great source for treating fever and abdominal and rheumatic pains (37, 38). It has 43 components of essential oils (39) which could be the source of these medicinal properties (16). Our current research evaluated Cleome brachycarpa as a good analgesic and anti-inflammatory herb, showing significant results compared to the marketed drugs.
The anti-inflammatory activity of the methanol extract of Cleome (200 mg/kg) was evaluated by producing edema through sub-plantar injection of 2% acetic acid, and the volume was measured following volume displacement methods. The variation between early and after-treatment paw volumes pointed towards the degree of inflammation and reported the comparable effects of test species (P < 0.001***) to that of standard drug.
At the 200 mg/kg dose, the Cleome extract showed maximum inhibition of the edema (29.42%) compared to standard diclofenac (31.80%) at the sixth hour. The hang-up effects of the Cleome extract might probably be due to cyclooxygenase inhibition, which ultimately decreased prostaglandin production. In our experiment, pre-treated animals with Cleome extract showed a significant edema inhibitory response in the second hour following injection. This result suggests that Cleome extract might suppress the later stage of inflammation via decreasing cyclooxygenase. Consequently, induced paw edema was reduced after using crude extract, representing its inhibitory effects on prostaglandins.
According to the literature review, Cleome is rich in triterpenoids, brachycarpone, deacetoxybrachycarpone, cabralealactone, and ursolic acid (40). Triterpenoids hold analgesic (41), anti-inflammatory (42, 43), and antiemetic (44) possessions. Analgesic and anti-inflammatory behavior of ursolic acid has also been established (45). Many inflammatory diseases are common in society throughout the world, and natural products offer a great hope to discover new and safe bioactive lead compounds.
The analgesic activity of Cleome brachycarpa was compared with morphine. It showed a significant decrease in pain (P < 0.001***), as shown in Table 3 and Figure 4. According to the literature review, different Cleome species are efficient against acetic acid-induced writhing in mice and have been recommended as peripherally acting painkillers and CNS depressants (46) through hindering prostaglandin synthesis and release along with other endogenous GISTs. Similarly, the analgesic mechanism of ethanolic extract of brachycarpa may be linked to the inhibition of cyclooxygenases (47), as it showed significant pain inhibition (55.10%) in the sixth hour comparable to the reference morphine (60.88%) as shown in Table 4 and Figure 5.
Our hematological study showed a little increase in Hb, HCT, MCH, WBC, RBC, PLT, and ALP, showing that the extract might possess some phytochemical moiety that stirred erythropoietin oozing in bone marrow cells. Hemoglobin is reflected as a major oxygen transporter (48); consequently, our extract potentially rallies the oxygen transport as it augmented the Hb value. This outcome might be due to phenolic compounds (49). Also, MCH is hemoglobin per red blood cell in blood as Hb concentration was elevated in our results, so MCH is increased, too. Besides, WBC constitutes an important part of the immune system, protects from infectious diseases, and is important in wound healing. Its increased value from baseline displayed wound-healing properties, possibly, due to ursolic acid as immunosuppression. Our results showed a slight decrease in MCV, possibly because the body's compensatory mechanism was activated by increasing the erythropoietic system.
Platelets usually govern the hemostasis process. In our study, there was little increase in PLT which might be due to the triterpenoid derivatives and could be of great source in preventing bleeding disorders, as also reported by Sarfaraz et al., 2018 that the increase in hematological parameters might be due to any phytochemical entity which boosts up the release of erythropoietin from marrow cells (28).
Cleome brachycarpa could be a great source for pharmaceutical companies to formulate and check the effect of this herb on different doses to evaluate its best dose to treat bleeding disorders and viral diseases like dengue to encounter the problem of low levels of platelet counts. It has been anticipated from the above results that Cleome brachycarpa owns hematopoietic effects that might be advantageous in treating various types of anemia and immunosuppressive disorders. Moreover, the plant extract decreased cholesterol levels which work as the foremost causative feature in the pathogenesis of atherosclerosis and consequently in cardiovascular diseases and hence could be a source of cholesterol-lowering agents in the future, protecting various cardiovascular diseases, as also mentioned by Sarfaraz et al. (50).
Cleome brachycarpa significantly increased SGPT without disturbing ALP levels which should be further evaluated (51). Elevated levels of SGPT are usually reported with several administration drugs like NSAIDs and Anti-TB drugs (52, 53). Thus, monitoring liver physiology remains recommended while using such drugs.
Because of the therapeutic efficacy, low cost, and safety profile, discussion on herbal preparations has become very frequent as a source of medicinal agents. Family Cleomaceae still has not been immensely evaluated, even though it has been publicized for several therapeutic belongings. Our results claim that Cleome brachycarpa is a wonderful anti-inflammatory and analgesic agent that can greatly improve complete blood count.
Pain and inflammatory diseases are the most common conditions that resist and decrease a patient's functional status. Many synthetic products are available, like NSAIDs, taking significant anti-inflammatory and analgesic effects but resulting in numerous side effects. The main factor which limits the use of NSAIDs is their gastrointestinal (GI) toxicity. Epidemiologic studies point out that their use boosts the danger of GI problems. Studies put forward serious clinical gastric events yearly due to NSAID use resulting in a huge economic burden (54).
Still, researchers investigate continuously for improving the pharmacokinetics, pharmacodynamics, efficacy, and safety profile of drugs. Based on this concept, Cleome brachycarpa extract was evaluated and presented for pharmacological effects, especially anti-inflammatory and analgesic activity, with lesser side effects. The current study concluded the well-comparable effects of Cleome brachycarpa extract. It worked as an analgesic mediator, possibly because of hindering Kapa receptors in the brain stem and thalamus, resulting in pain relief and sedation, and Delta receptors widely circulated in the brain, spinal cord, and digestive system, as it gave comparable results to morphine. Further, the results revealed the extract's anti-inflammatory effect, which may be due to inhibiting both leukocyte migration and COX-1 and COX-2 enzymes.
The other perspective may be that any of the components of Cleome brachycarpa has therapeutic and healing properties for inflammation. However, further advanced (clinical) studies are compulsory to approve these effects.
From the clinical point of view, the above study on Cleome brachycarpa at 200 mg/kg is very important, as it could be used to treat many diseases with no alteration in hematological parameters with continuous monitoring of LFT. Further clinical trials are recommended for further authentication. The findings draw attention to Cleome brachycarpa as a possible nominee for research and isolation because of its vital composition, which has pronounced beneficial effects in various diseases.
As a result, healthcare professionals and scientists may deem the Cleome genus in mounting evidence-based alternative remedies to treat a range of pathologies without causing any severe undesirable effects. Future potential research work should isolate specific components as the center of attention to determine the specific pathways involved in various biological activities. In vitro and in vivo analysis of various plant extracts authenticate their conventional use, yet clinical trials must be conducted to establish the translational approach. Furthermore, evaluating the plant phytochemicals is compulsory to determine their therapeutic range for potential drug design, development, and discovery.
5.1. Conclusions
The current study concluded the well-comparable effects of Cleome brachycarpa extract and found it a potent anti-inflammatory and analgesic agent comparable to diclofenac sodium and morphine, respectively. Moreover, various hematological parameters, WBC, RBC, Hb, HCT, MCV, MCH, MCHC, platelet count, cholesterol levels, and liver enzymes, were also evaluated to ensure the safe use of this natural product. It is found that no significant changes were observed in tested parameters, while it is noticeable that it may help improve platelet count and decrease cholesterol level, although the results are not supported to significant levels.