1. Background
Grazing animals are continually uncovered to helminth infections, which include numerous trematode species that cause several diseases. Therefore, infections with the most common liver diseases, fascioliasis, and dicrocoeliasis, can be related to hepatic problems, such as the impairment of carbohydrates, proteins, fat metabolism, and chronic wasting (1). Fasciolosis and dicrocoeliasis belong to the group of foodborne trematode infections. Two species of the genus Fasciola and Dicrocoelium, including F. hepatics and D. dendriticum are the most zoonosis parasitic disease. They have a widespread geographic distribution. In endemic regions, Fasciola and Dicrocoelium infections constitute great problems in livestock production and appreciably threaten public health.
In veterinary medicine, the severity of animal fascioliasis and dicrocoeliasis influences animals to an extraordinary extent, relying on the host and parasite burden. It might be an asymptomatic or severe disease, together with death, which ends up in heavy financial losses. These essential species are fundamental parasites of livestock that can be transmitted to humans, and infections bring about large financial losses (2-6). Fasciolosis is endemic all through the world, infecting over 6 hundred million domestic ruminants, and causes an annual economic loss of around US$3 billion, according to available estimates. Moreover, its incidence in 61 countries is about 17 million; nevertheless, nearly 180 million are at risk of infection. In addition, the disability-adjusted life years estimated for fasciolosis is 90,000 (7-10).
To date, due to a global increase in the prevalence of fascioliasis in humans, it ought to be taken into consideration as a significant human parasitic disease (4). Moreover, called liver flukes, F. hepatica and D. dendriticum are maintained in an indirect life cycle with an invertebrate host (snails) and a vertebrate host (livestock). Fasciolosis and dicrocoeliasis cause a heavy burden (i.e., morbidity and mortality) in endemic regions, mainly by affecting stockbreeding. Adult worms cause chronic infection, with several negative effects on milk, weight, and wool. On the other hand, liver burrowing of the juvenile parasites results in acute diseases, leading to the death of some animals (11, 12). It seems that praziquantel and triclabendazole treatments have no effect on fasciolosis. Therefore, the preferred option is a halogenated benzimidazole derivative. Nevertheless, some studies indicated that parasites isolated from livestock were insensitive to benzimidazole. Cure changes are great, at the same time as destructive reactions following treatment are typically temporary and mild (10), except that the extensive use of triclabendazole for decades caused the improvement of resistant Fasciola populations (4). Since 1995 when the first report of resistance was reported, triclabendazole resistance has been increasingly observed in some countries in the past decades. Albendazole, closantel, and oxyclozanide are other drugs administered to treat Fasciola-infected livestock, only useful for mature (not juvenile) flukes (12). Concerning the treatment of dicrocoeliasis, the effectiveness of higher doses of benzimidazoles and pro-benzimidazole derivates, such as albendazole, against nematodes is proved (5).
Anthelmintic drugs are the prevalent method to control fasciolosis and dicrocoeliasis. Nevertheless, their effectiveness has reduced over time due to haphazard administration, which led to parasite resistance. Moreover, chemical drugs might be poisonous to animals. In addition, the problem of chemical residue should be addressed (6, 13). Triclabendazole is the prevalent option to treat chronic fasciolosis due to its high validity against adult and immature worms (14, 15). Therefore, the use of new safe alternative drugs, such as herbal medications, is an option to grapple with these dilemmas.
Ferula assa-foetida is a plant with the potential to treat various diseases. It belongs to the Apiaceae family, which is obtained from the exudates of the living underground rhizome or taproots of the plant. There are examples of using this plant as raw material for medicine and cosmetics. A study showed the relaxant effect of F. assa-foetida on the smooth muscle in the tracheal chain and suggested the related mechanism responsible (16). Several authors investigated the anti-parasitic properties of F. assa-foetida, such as activity against Trichomonas vaginalis (17), Schistosoma mansoni (18), and Strongylus spp. (19). According to the evidence, naturally-occurring plant products, such as diterpenes, phenolics, and sulfur-containing compounds, have anti-Leishmania properties (20). Nevertheless, there is not enough evidence regarding the anthelmintic properties of this plant, especially concerning liver flukes.
2. Objectives
This study was performed to assess the ability of the F. assa-foetida extract new compound against fasciolosis and dicrocoeliasis using in vitro assay.
3. Methods
3.1. Extraction and Fractionation
The leaves of F. assa-foetida were collected from the market of Kashan, Isfahan, Iran. The leaves of F. assa-foetida were dried at room temperature. An electric processor was used to prepare the powder, which was then soaked for 3 days in an aqueous-methanol 30:70 suspension in order to prepare crude aqueous-methanol extracts (CAME). Afterward, it was filtered using a muslin cloth and filter paper. The entire method was repeated thrice, and after that, by the utilization of a rotary evaporator at 40 °C and low pressure, the combined filtrate was evaporated to get CAME (21).
3.2. Collection of the Parasites
Adult live F. hepatica and D. dendriticum were collected from the livers of sheep slaughtered at Kashan abattoir, central Iran, and examined quickly to avoid any disruption.
3.3. Anthelmintic and Adult Motility Assay
A plant-based study at concentrations of 2000, 4000, 6000, and 8000 µg/mL for F. hepatica and 400, 600, 8000, and 1000 µg/mL for D. dendriticum with triclabendazole and closantel (positive control) and RPMI medium (negative control) was prepared. A minimum of five worms were separated into individual wells of culture plates (12-well plates) and exposed to the above-mentioned extract. Each treatment was repeated thrice at a 5% CO2 incubator, and the number of lively and dead worms was watched at 0, 12, and 24 hours after the treatment.
3.4. Mortality Time
The mortality time was estimated by observing the motility of parasites using a microscope. The motion of the parasites was followed until they appeared to have no motion.
3.5. Cytotoxicity Assay by MTT Method
The MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide, a tetrazole) assay was carried out to evaluate the cell viability in culture media. For this purpose, first, HeLa cells were cultured in Dulbecco’s Modified Eagle Medium containing 10% fetal bovine serum at 37 °C in a 5% CO2 atmosphere. Then, 105 cells were cultured in each well until 24 hours on a 48-well plate. Afterward, 20 µL of MTT solution was added. The plates containing cells and drugs were incubated for 3 - 5 hours at 37°C. Then, 100 µL of dimethyl sulfoxide was added to each sink. After 15 minutes, optical absorption was read using an enzyme-linked immunosorbent assay reader (Model 680, Bio-Rad Laboratories, Inc., USA) at a wavelength of 570 nm.
4. Results
The test by MTT assay on D. dendriticum and F. hepatic showed, at concentrations of 1000 and 8000 µg/mL of hydroalcoholic extract of F. assa-foetida, 85.7% and 3% of the HeLa cells were alive; therefore, the percentages of toxicity were 14.3% and 97% in 24 hours, respectively (Table 1).
| Concentration (µg/mL) | Alive (%) | Toxicity (%) |
|---|---|---|
| Fasciola hepatica | ||
| 2000 | 31 | 69 |
| 4000 | 8 | 92 |
| 6000 | 5 | 95 |
| 8000 | 3 | 97 |
| Dicrocoelium dendriticum | ||
| 400 | 100 | 0 |
| 600 | 97.2 | 2.8 |
| 800 | 91.3 | 8.7 |
| 1000 | 85.7 | 14.3 |
This study confirmed that F. assa-foetida hydroalcoholic extract at concentrations of 400 and 600 µg/mL in addition to 2000 and 4000 µg/mL after 12 hours had no impact in opposition to D. dendriticum and F. hepatica flukes, and all worms were alive. With increasing the concentration, the extract decreased the number of parasites, and all became stained with 0.1% eosin vital dye (Table 2).
| Concentration (µg/mL) | 12 Hours | 24 Hours | P-Value | ||
|---|---|---|---|---|---|
| Alive Worm | Dead Worm | Alive Worm | Dead Worm | ||
| Dicrocoelium dendriticum | < 0.001 | ||||
| 400 | 5 | 0 | 5 | 0 | |
| 600 | 5 | 0 | 3 | 2 | |
| 800 | 1 | 4 | 0 | 5 | |
| 1000 | 0 | 5 | 0 | 5 | |
| Negative control | 5 | 0 | 5 | 0 | |
| Fasciola hepatica | |||||
| 2000 | 5 | 0 | 5 | 0 | |
| 4000 | 5 | 0 | 4 | 1 | |
| 6000 | 3 | 2 | 0 | 5 | |
| 8000 | 0 | 5 | 0 | 5 | |
| Control | 5 | 0 | 5 | 0 | |
The results of the present study confirmed that the highest and the lowest lethal dose (LD50) levels at 12 and 24 hours were 758.5 and 615.2 µg/mL in addition to 6.4 and 5 µg/mL in D. dendriticum and F. hepatica, respectively (Table 3).
| Hour | LD (µg/mL) | ||||
|---|---|---|---|---|---|
| LD10 | LD25 | LD50 | LD75 | LD90 | |
| Dicrocoelium dendriticum | |||||
| 12 | 697.1 | 726.2 | 758.5 | 790.9 | 819.9 |
| 24 | 542.8 | 557.1 | 615.2 | 653.3 | 687.6 |
| Fasciola hepatica | |||||
| 12 | 4.4 | 5.4 | 6.4 | 6.9 | 7.4 |
| 24 | 3.4 | 4.4 | 5 | 5.3 | 5.8 |
Abbreviation: LD, lethal dose.
This study showed closantel and triclabendazole at concentrations of 50 and 5 µg/mL 12 hours after exposure to the adult both worms had no anthelmintic effects, respectively; however, with increasing the concentration of the drugs, the number of live parasites in the culture medium decreased, the worms were motionless, and all became stained with 0.1% eosin vital dye that indicated their death (Table 4).
| Concentration (µg/mL) | 12 Hours | 24 Hours | P-Value | ||
|---|---|---|---|---|---|
| Alive Worm | Dead Worm | Alive Worm | Dead Worm | ||
| Closantel | < 0.001 | ||||
| 50 | 5 | 0 | 5 | 0 | |
| 100 | 4 | 1 | 3 | 2 | |
| 150 | 1 | 4 | 0 | 5 | |
| 200 | 0 | 5 | 0 | 5 | |
| Negative control | 5 | 0 | 5 | 0 | |
| Triclabendazole | |||||
| 5 | 5 | 0 | 5 | 0 | |
| 10 | 3 | 2 | 1 | 4 | |
| 15 | 2 | 3 | 0 | 5 | |
| 20 | 0 | 5 | 0 | 5 | |
| Negative control | 5 | 0 | 5 | 0 | |
Table 5 shows the comparison of suitable concentrations of closantel and triclabendazole to kill different percentages of D. dendriticum and F. hepatica 12 and 24 hours after treatment. The highest and lowest LD50 levels of both drugs were 125 and 102.9 µg/mL and 12.6 and 8.4 µg/mL, respectively.
| Hour | LD10 | LD25 | LD50 | LD75 | LD90 |
|---|---|---|---|---|---|
| Closantel LD (µg/mL) | |||||
| 12 | 89.5 | 106.3 | 125 | 143.6 | 160.4 |
| 24 | 87.7 | 95 | 102.9 | 110.9 | 118 |
| Triclabendazole LD (µg/mL) | |||||
| 12 | 8.6 | 9 | 12.6 | 16.6 | 18.6 |
| 24 | 6.2 | 7.7 | 8.4 | 9.3 | 11.3 |
Abbreviation: LD, lethal dose
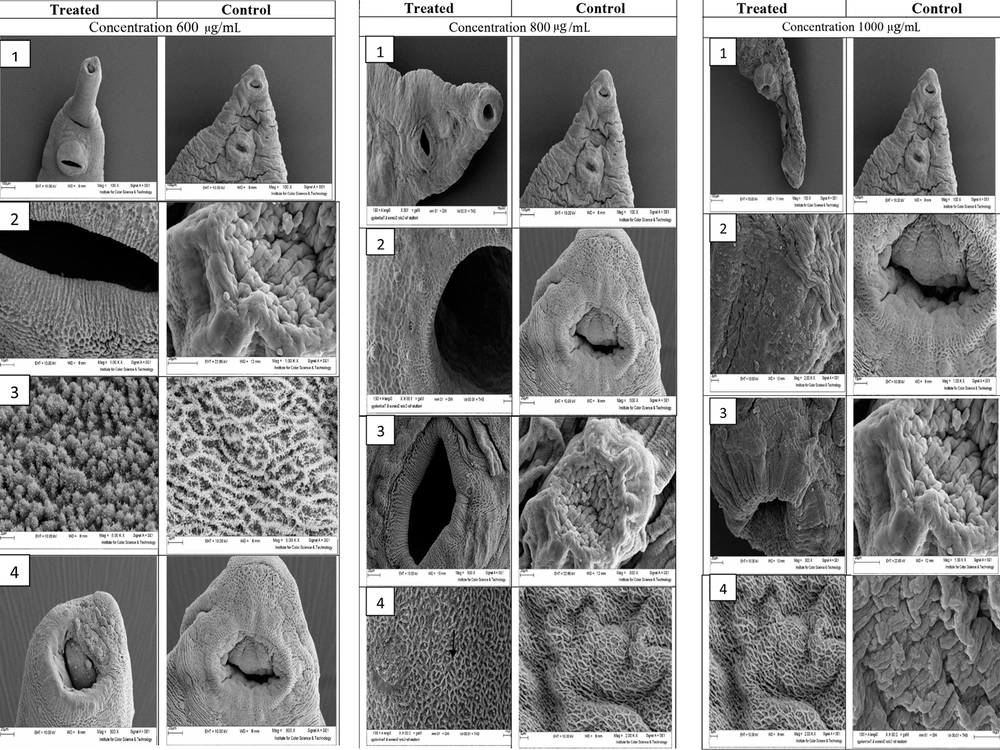
Figure 1 illustrates the ultrastructural changes of D. dendriticum adult after exposure to 600, 800, and 1000 µg/mL F. assa-foetida hydroalcoholic extract 24 hours after treatment using scanning electron microscopy (SEM).
Ultrastructural changes of D. dendriticum adult after exposure to 600, 800, and 1000 µg/mL F. assa-foetida Hydroalcoholic extract 24 hours after treatment using scanning electron microscopy. In section 1, at a concentration of 600 µg/mL, no swelling or blisters are observed in the treated worm. In section 2, sensory papillae can be observed in the worm treated at the edge of the abdominal sucker, and the tegument around the abdominal sucker is completely intact. Section 3 shows the level of the parasite tegument. In the treated worm, the embossed lattice structure is formed with a mass of tegument vesicles that do not change in structure, compared to the control sample. Section 4 shows that the morphology of the mouth worm has changed so that some sensory papillae at the edge of this site have been destroyed, and the area of the tegument has been worn, and to some extent, the vesicle. In some areas of the parasite’s tegument at a concentration of 800 µg/mL, swelling and blister forms are observed. Section 2 shows the sensory papillae and radial grooves around the oral sucker. Section 3 shows the edges of the abdominal sucker, which does not show any change in the tegument surface around the treated worm compared to the control group, and only a small hole is observed in the tegument surface. Section 4 of the tegument shows the surface of the parasite, which is damaged in most areas, and the tegument vesicles are either destroyed or swollen and enlarged.
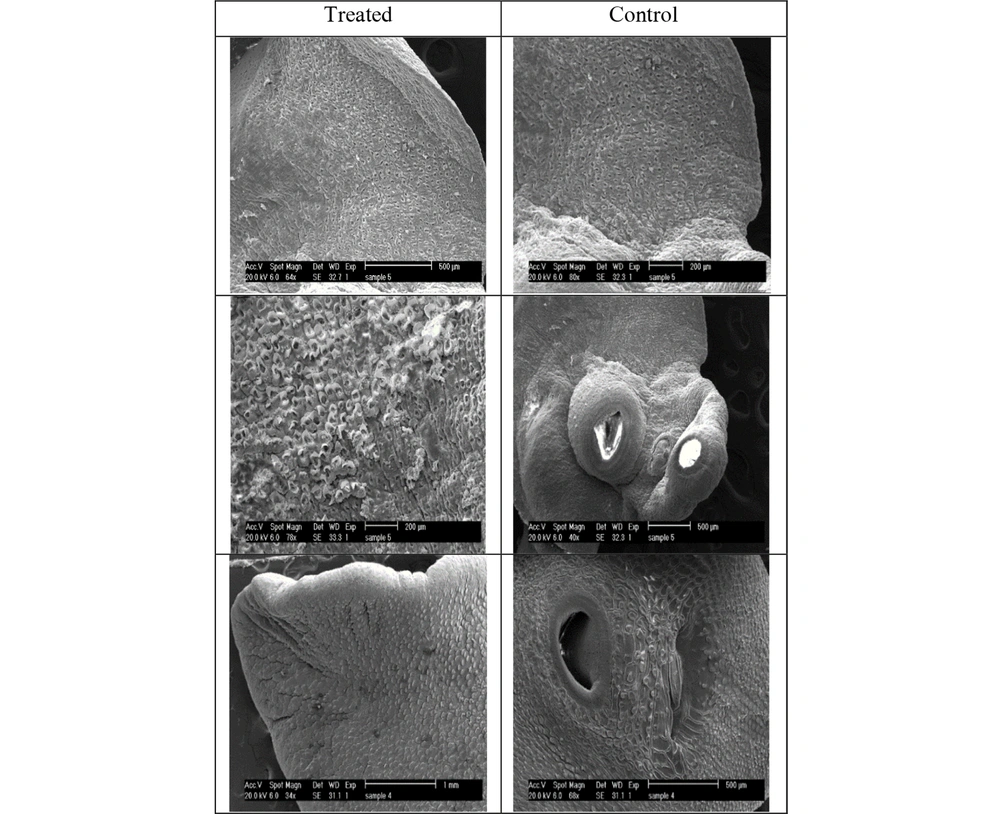
Figure 2 illustrates the ultrastructural changes of F. hepatica adult after exposure to 8000 µg/mL F. assa-foetida hydroalcoholic extract 24 hours after treatment using SEM. The whole images of the control group show that no significant changes occurred, and the surface of the tegument, suckers, sensory papillae, vesicles, and tegument spines have remained unchanged. Moreover, swelling, shrinkage, cavities, rupture, and blisters were not also observed.
Ultrastructural changes of F. hepatica treated with F. assa-foetida extract at a concentration of 8000 µg/mL at 24 hours; tegument shrinkage around the suckers and loss of sensory papillae and spines; presence of numerous blisters and pores on the surface of the tegument indicating damage to the tegument
5. Discussion
With the arrival of drug resistance in opposition to the present formulations, there should be a focus on finding new methods to address the problem of helminth parasites that cause major medical problems. Dicrocoeliasis and fascioliasis are foodborne parasitic diseases of the human biliary tract, resulting from Dicrocoelium dendriticum and Fasciola hepatica causing extensive financial losses and serious health problems by reducing production and viscera condemnation in ruminants. Due to low-performance medications, drug delivery is a tremendous project to improve interventions available for these diseases. This study aimed to determine the anthelmintic properties of Ferula assa-foetida hydroalcoholic extract as an herb in dicrocoeliasis and fascioliasis treatment using in vitro assay.
Helminths of ruminants confer with a set of complicated parasites that are infective to animals and humans, resulting in critical financial and public health concerns in countries. Lack of sufficient veterinary and medical care inspires such concerns, not to mention insufficient regulations on disease control among many different factors (22, 23).
The negative influences of helminths on farm animal productiveness are considered a critical challenge for the livestock industry worldwide (24) in spite of the projected improved dependence on agriculture in the near future (25). These parasites bring about heavy financial losses every year, for instance, through a decline in growth rate and lower production of milk, meat, and wool (26-28).
F. assa-foetida has historically been used for its anthelminthic properties in numerous regions, wherein it has been administered for the treatment of an infection with intestinal parasites. The hydroalcoholic extract of F. assa-foetida at concentrations of 10, 50, and 100 mg/mL confirmed greater than 90% lethality in the larval stages of Strongylus species after 24 hours (19).
Ramadan et al. investigated the impact of F. assa-foetida on S. mansoni load and the egg count in infected mice. Ultrastructural and histopathological adjustments additionally verify the anti-parasitic properties of F. assa-foetida (18). In the current study, the hydroalcoholic extract of F. assa-foetida with a concentration of 800 µg/mL in 24 hours had the maximum lethality regarding D. dendriticum. Kumar and Singh showed the in vitro anthelmintic efficacy of an ethanolic extract of F. assa-foetida against F. gigantica. Kumar and Singh mentioned that the activity of this plant relies upon the time and used concentration, and LD50 was equal to 3.94 mg/mL (29).
Naturally-occurring plant products, which include phenolics, diterpenes, and sulfur-containing compounds, have anti-Leishmania properties (20). The anti-Leishmania effect of F. assa-foetida extract on Leishmania major was proven in the study of Bafghi et al.. In this study, F. assa-foetida extract was used in concentrations of 2.5, 5, 10, and 20 µg/mL. The results of this observation suggest that the survival rate of parasites decreases considerably after 48 and after 72 hours, and the growth of parasites in all doses is inhibited in the logarithmic and regular phases (30).
Historically, F. assa-foetida is an ancient traditional phytomedicine that has been administered to treat various diseases, such as rheumatoid arthritis, stomach pain, weak digestion (31, 32), and influenza H1N1 (33). New studies showed that asafoetida extracts have a neuroprotective effect on oxidative stress-induced apoptosis to foster the prevention of Alzheimer’s disease through the PI3K/Akt/GSK3β/Nrf2/HO‑1 pathway (34). In addition, some new pharmacological and biological research showed several activities and medicinal properties, such as antidiabetic, antihyperlipidemic (32), antifungal (35), molluscicidal (36), antibacterial (37), and cancer chemopreventive (38).
Plant-origin anthelmintic products are getting popular due to the fact they are less expensive and safer than their artificial counterparts due to their biodegradable rate (37, 39). Along with those biological surveys, the phytochemical investigations of asafetida were detected. Asafoetida contains the three most important fractions, including resin, gum, and essential oil.
The resin portion is known to contain aresinotannols A and B, ferulic acid, umbelliferone, and four unidentified compounds. Ferulic acid esters, including resin, gum fraction, including glucose, galactose, l-arabinose, rhamnose, and glucuronic acid, volatile oils, including sulfur-containing compounds, free ferulic acid, coumarin derivatives (e.g., umbelliferone), and different monoterpenes are different components of the plant (19, 37). According to modern phytochemical and pharmacological research, umbelliprenin is an important component of asafetida with high lipoxygenase inhibitory activity (IC50 = 0.0725 M) (40).
Different mechanisms appear to affect this activity, including radical scavenging activity of sulfur-containing compounds, lipoxygenase inhibition by umbelliprenin and its derivatives, enhanced function of endogenous antioxidants, and declined oxidative parameters. A study showed that asafoetida inhibits the microsomal activation-based mutagenicity of 2-acetamidoflourene. The findings showed that asafoetida might also additionally ameliorate the impact of environmental mutagens, in particular present in the food (41). It is well proved that umbelliprenin has incredible cancer chemoprevention, according to both in vitro and in vivo studies, via the administration of a two-stage carcinogenesis assay of mouse skin tumors triggered through peroxynitrite as an initiator and 12-O-tetradecanoylphorbol-13-acetate as a promoter (42, 43).
Blocking the enzyme 5-lipoxygenase might account for at least a proportion of the observed effect of umbelliprenin. Therefore, it can be argued that umbelliprenin is a prominent compound for synthesizing new derivatives with higher efficiency and safety. An in vivo study has proven an antispasmodic activity, which paves the way for the normal administration of asafoetida as an antispasmodic agent (44). Among the examined sesquiterpene coumarins, galbanic acid, farnesiferol C, and epi-conferdione showed a pleasant efficiency, similar to amantadine as an antiviral standard. These compounds might be promising to create new pharmaceutical interventions against viral infections, specifically influenza and the common cold (33).
Asafoetida is a complicated aggregate of those compounds and might have more pronounced impacts in comparison to individual compounds. Further studies are needed to extend our knowledge regarding the antiviral activity of asafoetida, which contains different antiviral compounds. Nevertheless, such evidence can be used as a basis for the conventional administration of asafoetida to treat upper respiratory diseases. Noteworthy, several pharmacological surveys executed on asafoetida employed a water extract of asafetida, which is not the most frequent administration type of asafoetida. As previously mentioned, there are uncertainties regarding the existence of non-polar components in the aqueous extract, or there might be some active components of the complete oleo-gum resin. Only one case report has solely investigated the capability toxicity of asafoetida (45).
A study by Farhadi and Youssefi investigated the antifouling and antifungal effect of F. assa-foetida in a mouse model. In this study, in one control group, piperazine at a dose of 20 mg/kg and praziquantel at 25 mg/kg were used. Infected mice were treated with concentrations of 2.5%, 5%, and 10% of methanolic extract of F. assa-foetida for 2 weeks. The result showed that the treatment of nematode infestation (Syphaciaobvelata) with F. assa-foetida extract did not reduce the number of eggs and parasites (P > 0.05); however, in the group infected with cestode (Hymenolepis nana), the treatment with F. assa-foetida in all doses showed a significant decrease in the number of eggs and worms in comparison to controls (P < 0.05) (46).
Phytochemical screening of F. assa-foetida extract confirmed the presence of flavonoids and polyphenolic compounds as the primary chemical components. Polyphenolic compounds show anthelmintic activity. One of these polyphenolic compounds is tannins (47). Tannins disrupt energy production in worm parasites by disrupting the oxidation process of phosphorylation (48). Other compounds whose anti-parasitic impact on this extract has been demonstrated consist of ferulic acid and coumarins, mainly sesquiterpenes coumarins (30, 33).
The ultrastructural examination of adult D. dendriticum worm confirmed that the finest detrimental impact on parasite tegument was associated with the concentration of 1000 µg/mL at 24 hours. Compared to the control group, the treated worms confirmed that there there was excessive tegumental damage, and there were no traces of prominent streaks, tegumentary vesicles, and sensory papillae in the treated cream. Additionally, the LD50 level of F. assa-foetida hydroalcoholic extract in 24 hours was confirmed to be 615.2 µg/mL. Different fractions of F. assa-foetida were isolated, including gum, resins, volatile oils, coumarin derivatives, diverse monoterpenes, ferulic acid, farnesiferoles, disulfides, symmetric trisulfides, and tetrasulfide (49). The resin of F. assa-foetida has numerous effects, consisting of anticoagulants, smooth muscle relaxants, antidiabetic, anticarcinogenic, antioxidant, antispasmodic, antihepatotoxic, antiulcerogenic, anticholesterolemic, anti-inflammatory, antifertility, antifungal, and anthelmintic (50, 51). The gum extract of F. assa-foetidais was employed to treat diarrhea, constipation, abdominal pain, and parasitic infections (52).
The comparative efficacy of plants against pathogens Staphylococcus aureus has additionally been suggested by the gum extract of F. assa-foetida (49). Gundamaraju mentioned the considerable anthelmintic activity of F. assa-foetida at a concentration of 100 mg.mL-1 (53). At the concentration of 100 mg.mL-1, paralysis and the lethality of an aqueous extract of F. assa-foetida were comparable to piperazine citrate. The major phytochemical ingredients of crude extracts are polyphenolic compounds and flavonoids. In addition, polyphenolic compounds, such as tannins, are reported as anthelmintics (54). The possible anthelmintic property of F. assa-foetida can be attributed to the interference with energy generation in parasites by uncoupling oxidative phosphorylation or through the presence of tannins in the extracts, which can bind to glycoprotein at the cuticle of the parasite, leading to death (55).
The present study investigated the anthelminthic properties of F. assa-foetida extract, compared to control, closantel, and triclabendazole. The SEM images of treated liver flukes confirmed excessive damage, which includes an entire lack of sensory papillae and destruction of distinguished network structures and tegument vesicles. Based on the MTT assay, the toxicity of F. assa-foetida at 800 µg/mL concentration was 8.7%. It can be concluded this herbal medicine had anthelmintic properties. The present study was carried out in an in vitro condition. In order to obtain further accurate information, it is suggested to carry out similar animal studies in order to investigate the effect of herbal medicines on deoxyribonucleic acid and the level of tegument enzymes.
5.1. Conclusions
The current study demonstrated that the F. assa-foetida extract, as an anthelmintic, can be used to treat fasciolosis and dicrocoeliasis using in vitro assay. Noteworthy, the extracts are combinations of several components and are not pure. Therefore, the findings only indicate the efficiency of these extracts. However, this discovery that the plant extracts can be administered as an available source of herbal anthelmintic from plants is promising, as it leads to the introduction of phytomedicine to cope with parasites. The extract could affect tegument breakage, which is of crucial importance for the absorption of nutrients. It is required to investigate the toxicity and protection profiling of plants. Nevertheless, distinct animal toxicity studies of F. assa-foetida and their bioactive compounds are required earlier than clinical trials.