1. Background
With one main dimension of less than 100 mm as a minimum, nanomaterials are endowed with novel properties. Their high potential for further applications also characterizes them compared with their original forms (1). Despite numerous uses of such materials, few research studies have focused on their effects on humans and the environment. Accordingly, investigating toxic effects and their mechanisms is of utmost importance since they can generate harmless products (2). Among nanoparticles, carbon nanotubes (CNTs) are approving of interest for their electrical, mechanical, and magnetic properties. In this respect, single-walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs) have been recognized as two forms of nanotubes (3). However, there are few studies concerning their toxicity, whose results are often inconsistent. It has also been reported that such materials can enter the working environment as suspended solids in absorbing quantities and possibly, cause industrial hazards (4). In addition, it has been stated that they can produce CNTs in sizes < 2.5 µm due to the burning of methane and propane of natural gas in ordinary stoves inside and outside houses (4). Studies on the toxic effects of CNTs merely center on the toxicity of these substances on inhalation and epidermal contacts because the greatest possible contacts with nanomaterials are through the skin or the respiratory tract (5, 6). It has been found that CNTs can be absorbed into the circulatory system after entering the lungs and then move into other organs, including the spleen and the kidney, or even the brain (7). It has also been reported that these substances can induce asbestos-like pathogenicity with the risk of carcinogenesis (8). In this regard, Wang et al. confirmed that mitochondrial toxicity could play an important role in apoptosis created by CNTs (9). Mitochondria also have a significant role in oxidation, apoptosis, cell cycle regulation, cell growth, and metabolism (10). Changing the structure of mitochondria can thus induce a significant decrease in cellular metabolism and lead to cell death (11). It is accepted that one of the main causes of damage to the kidney cells is elevated oxidative stress.
2. Objectives
Moreover, studies have shown that CNTs can result in inflammation, apoptosis, and cytotoxicity by inducing oxidative stress, thereby lowering cell viability (12). Nevertheless, no study was found on MWCNT-induced toxicity by oxidative stress in renal mitochondria. Accordingly, this study investigated the effects of MWCNTs on oxidative stress and survival in mitotic mitochondria in rats.
Caffeic acid is a natural phenolic compound in coffee, some fruits, and traditional Chinese medicine (13). This substance and its analogs have a variety of pharmaceutical activities, i.e., anti-oxidant, anti-viral, anti-inflammatory, and anti-cancer properties (14). Therefore, CA was selected as an antioxidant to reduce oxidative stress produced by MWCNTs.
3. Methods
3.1. Materials
MWCNTs (> %95, OD: 5 - 15 nm), CA, and other chemicals, solvents, and reagents (Sigma Chemical Co.) were purchased from Pars Biochemistry Store (Ahvaz, Iran).
3.2. Animals
For the given tests, Wistar male rats (about 200 - 250 g) were received from Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran, and all experimental methods were fulfilled in accordance with ethical protocols introduced by the Animal Ethics Committee at Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran (IR.AJUMS.REC.1396.45).
3.3. Preparation of Nanoparticles
Stoke suspension (600 mg/mL) of MWCNTs was made using distilled water. Three drops of Triton X-100 were added to the sample to describe the particles better. It was then shaken for 20 seconds using a vortex and dispersed using a Sonicator Probe (Ultra Sonic VCX-750 W model) at 4°C and 60 cycles for 30 minutes. The suspension was diluted with distilled water to make the desired concentration and then dispersed again by a Sonicator Probe for 10 minutes (2).
3.4. Size and Morphology of Nanoparticles
3.4.1. Isolation of Mitochondria
The mitochondria of the tissues were isolated by differential centrifugation. First, the kidneys were cleaved into small pieces and then homogenized in mitochondria separation buffer (cold mannitol solution, 225 mM (and subsequently followed for separation of nuclei, unopened cells, and other noncellular tissues by centrifugation at 1500 x g and a temperature of 4°C for 10 minutes. The upper layers were centrifuged at 4°C at 12000 x g for 10 minutes. After that, the upper layer was cast off. The mitochondria were re-suspended in a separation medium (75 mM sucrose, pH 7.4, 0.2 mM EDTA, and 0.225 M D-mannitol) and once again centrifuged for 10 minutes at 12000 x g. Ultimately, the mitochondria were suspended at 4°C in a Tris buffer with 0.05 M Tris-HCl, 0.25 M sucrose, 20 M KCl, 2.0 mM MgCl2, pH 4, and 1.0 mM Na2HPO4. In this study, mitochondria were newly provided for each test. They were then used after 5 hours, except for mitochondria employed in ROS tests and MMP suspended in respiration buffer (10 mM Tris, 0.5 mM MgCl2, 5 mM sodium succinate, 0.32 mM sucrose, 50 µM EGTA, 0.1 mM KH2PO4, 20 mM Mops), and buffer (68 mM D-mannitol, 220 mM sucrose, 10 mM KCl, 2 µM rotenone, 50 µM EGTA, 5 mM KH2PO4, 10 mM HEPES, 5 mM sodium succinate, and 2 mM MgCl2) (15, 16). To ensure high-quality mitochondrial separation, all steps were performed on ice.
3.4.2. Protein Measurements
The mitochondrial protein concentration was established by binding protein molecules to Coomassie brilliant blue and using a BSA as a standard (17). To meet normalization in all the mitochondrial tests, mitochondrial samples were also utilized.
3.5. Dose Assessment of MWCNTs
To select the best dose, the given doses were exposed to mitochondria for one hour in an incubator based on previous reports (2, 18). To this end, the mitochondria were divided into six groups: (1) control (negative control); (2, 3, 4, 5, and 6) MWCNTs (20, 40, 80, 160, and 320 µg/mL). Then, the mitochondrial viability rate was measured using the MTT assay according to the reduction of yellow tetrazole to purple formazone due to mitochondrial respiration in live cells. Moreover, the viability rate was shown as a percentage control, which is 100% assumed. After selecting the effective dose of nanoparticles, the mitochondria were divided into six groups: control group; mitochondria group exposed initially in the environment for 1 hour and then exposed to the CA (80 µM) and subsequently exposed to the environment for 1 hour; groups 3, 4, and 5, exposed to CA (160, 80, 40 and 20 µM) for 1 hour and then exposed to MWCNTs (320 µg/mL) for 1 hour; and group 6, exposed to MWCNTs for 1 hour (320 µg/mL) (19).
3.6. Determination of Complex II Activity Using MTT Assay
The MTT assay is known as a sensitive and reliable indicator of the activity of mitochondrial complex II. In brief; for 1 minute, 1 mL of mitochondrial suspensions of the kidney was centrifuged in 10000 x g. Mitochondria were then suspended in 970 µL of isolation buffer and 500 µL of 0.5 µM MTT solution and incubated at 37°C for 45 minutes. Then, they were dissolved in 800 µL DMSO to dissolve the created purple formazan. The absorbance was also determined at 570 nm through a spectrophotometer (UV-1650 PC, Shimadzu, Japan) (3, 18, 20).
3.7. Mitochondrial ROS Level Assay
Employing a fluorescent DCF-DA probe, the ROS rate in mitochondria was also measured. In sum, isolated mitochondria (protein 0.5 mg/mL) were washed with buffer phosphate (PBS) and incubated for 10 minutes with 1.6 mM DCM-DA at 37°C. Fluorescence was then measured via a fluorescence spectrometry of Perkin Elmer LS-50B (California, USA) at 500 and 520 nm emission and excitation wavelength, respectively (21, 22).
3.8. Measurement of Malondialdehyde Levels
Determining the thiobarbituric acid reactive substances (TBARS), MDA levels of renal mitochondria were assessed. To this end, 500 µL of mitochondrial suspension was added to 1.5 mL of TBARS (10%). Then, 2 mL of TBARS (0.67%) was mixed with the supernatant (1.5 mL) and placed for 15 minutes in a boiling water bath. Next, butanol (2 mL) was added and then centrifuged (4000 x g in 15 minutes) after cooling the samples, and the absorbance was assessed at 535 nm. Using various dilutions of 1, 1, 3, and 3-tetramethoxpropane, the standard MDA curve was prepared (23, 24).
3.9. Measurement of GSH Level
To determine the amount of GSH mitochondria, firstly, 5-sulfosalicylic acid (5%) was added to the samples. To remove the precipitated protein, the samples were centrifuged for 15 minutes at 13000 x g and 4°C, and subsequently, the upper layer was collected. Afterward, 5-Dithiobis (2-nitrobenzoic acid; DTNB) was added to the upper layer, and the total TNB was measured using a spectrophotometer at a wavelength of 412 nm (25).
3.10. Determination of CAT Activity
The CAT activity was investigated using the L.Goth method. For this purpose, 500 µL Tris-HCl 0.05 mM, 1 mL H2O2, and 50 µL of mitochondrial suppression were mixed and incubated for 10 minutes. Then the reaction was blocked by adding 500 mL of 4% ammonium molybdate solution. It should be noted that the absorbance was read at 410 nm, and the result was expressed as protein U/mg (22).
3.11. MMP Measurement
Rhodamine 123 (Rh123), as the mitochondrial absorption of cationic fluorescent dye, was utilized to evaluate the MMP. Rh123 is recognized as a lipophilic cation that can bind to parts of the mitochondrial membrane selectively and with a negative charge. Following the membrane hyperpolarization, the Rh123 enters the mitochondria and reduces fluorescence. The mitochondrial suspension (0.5 mg protein/mL) containing 10 µM Rh123 was reacted in MMP buffer. Then, using a Shimadzu RF-5000U Florescence Spectrophotometer, fluorescence was also examined in λEx = 490 nm and λEm = 535 nm (26, 27).
3.12. Statistical Analyses
Descriptive statistics, i.e., mean ± SD, were used to report the results. The statistical analyses were performed through one-way analysis of variance (ANOVA) using Prism Software (version 8). Using Tukey’s post-hoc test, group differences were also calculated.
4. Results
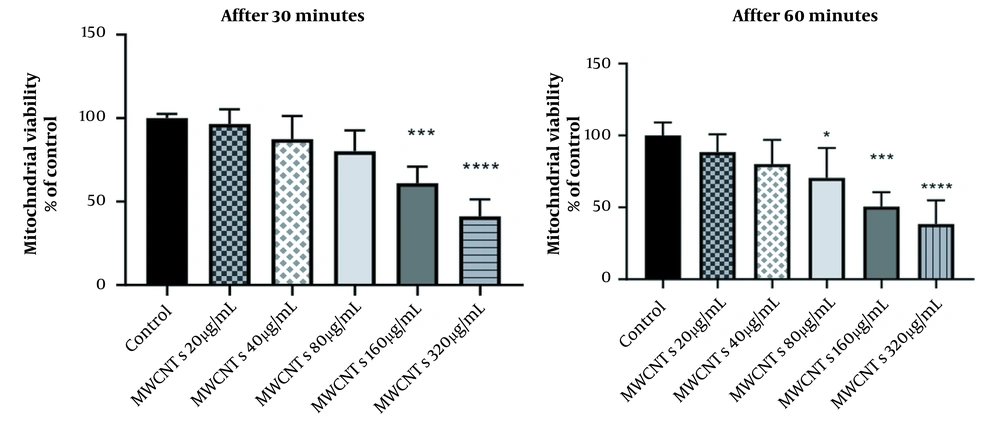
4.1. Dose Optimization of MWCNTs
To select the best dose of MWCNTs, mitochondria were exposed to various concentrations of MWCNTs (20, 40, 80, 160, and 320 µg/mL) for 1 hour; after that, the viability rate was tested using the MTT assay. The viability of the groups compared with H2O2 50 µM as control was also assumed to be equal to 100%. When mitochondria were exposed to 160 µg/mL of MWCNTs, the reduction in viability was approximately 50% compared with mitochondria treated with 50 µM H2O2 (P < 0.01). Therefore, 160 µg/mL of MWCNTs were used in the next experiments (Figure 1).
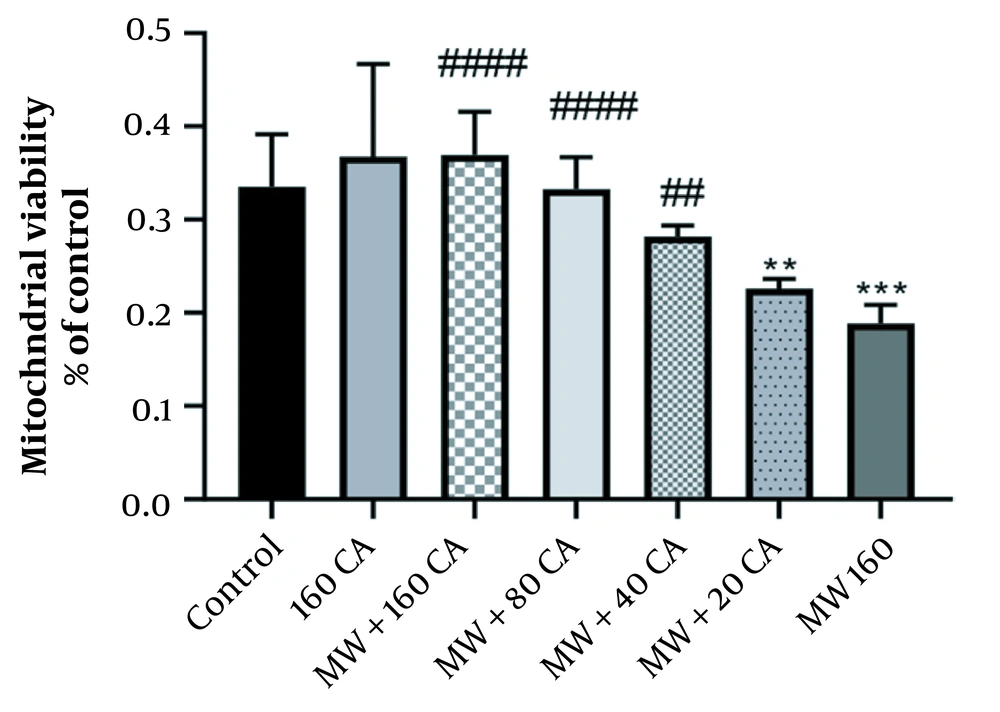
4.2. Effect of CA on Viability Rate of Mitochondria Exposed to MWCNTs
Cell viability was decreased to about 45% when the mitochondria were exposed to 160 µg/mL of MWCNTs for 1 hour. Then, the effect of different doses of CA on the viability rate was investigated. MTT assay showed that pre-treatment of mitochondria with 80 µM of CA could significantly increase the viability rate (P < 0.0001) (See Figure 2). However, there was no significant change in cell viability compared to the control group following 1-hour exposure to 80 µM CA.
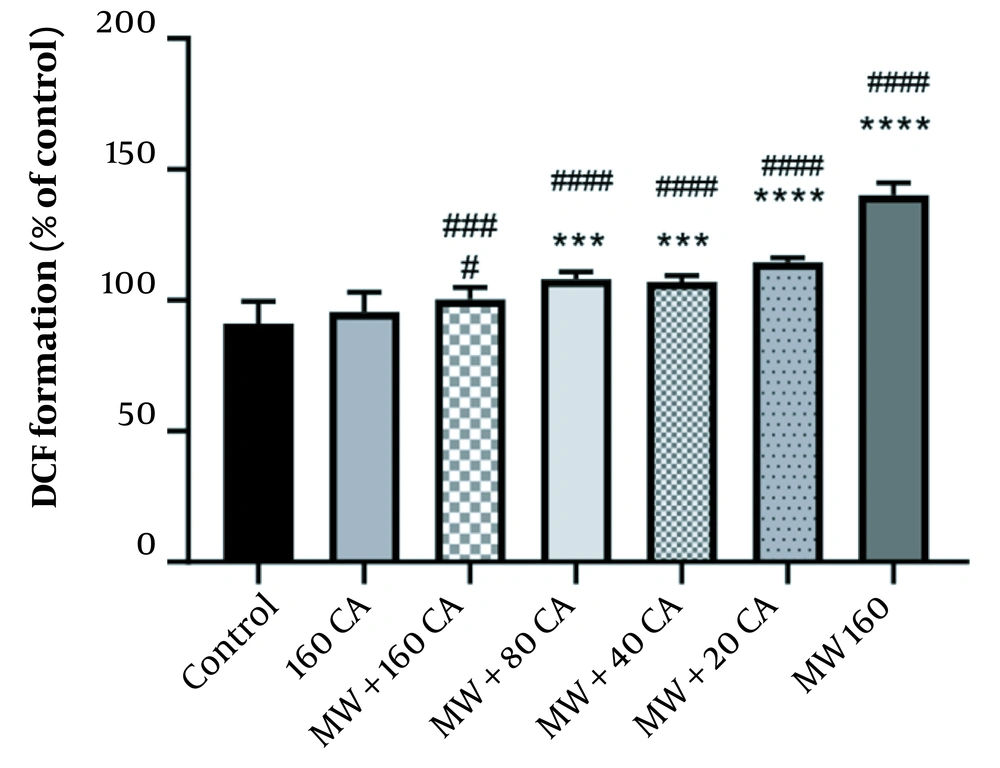
4.3. Effect of CA on Oxidative Stress in MWCNT-Treated Mitochondria
The study findings showed significant ROS production as mitochondria were exposed to 160 mM of MWCNTs (P < 0.05) (See Figure 3). However, pre-treatment with 80 µM of CA could significantly inhibit the production of ROS by MWCNTs (P < 0.0001). Pre-treatment with 80 µM of CA also showed significant changes compared with the control group (P < 0.001).
4.4. Effect of CA on MDA Levels in Mitochondria Exposed to MWCNTs
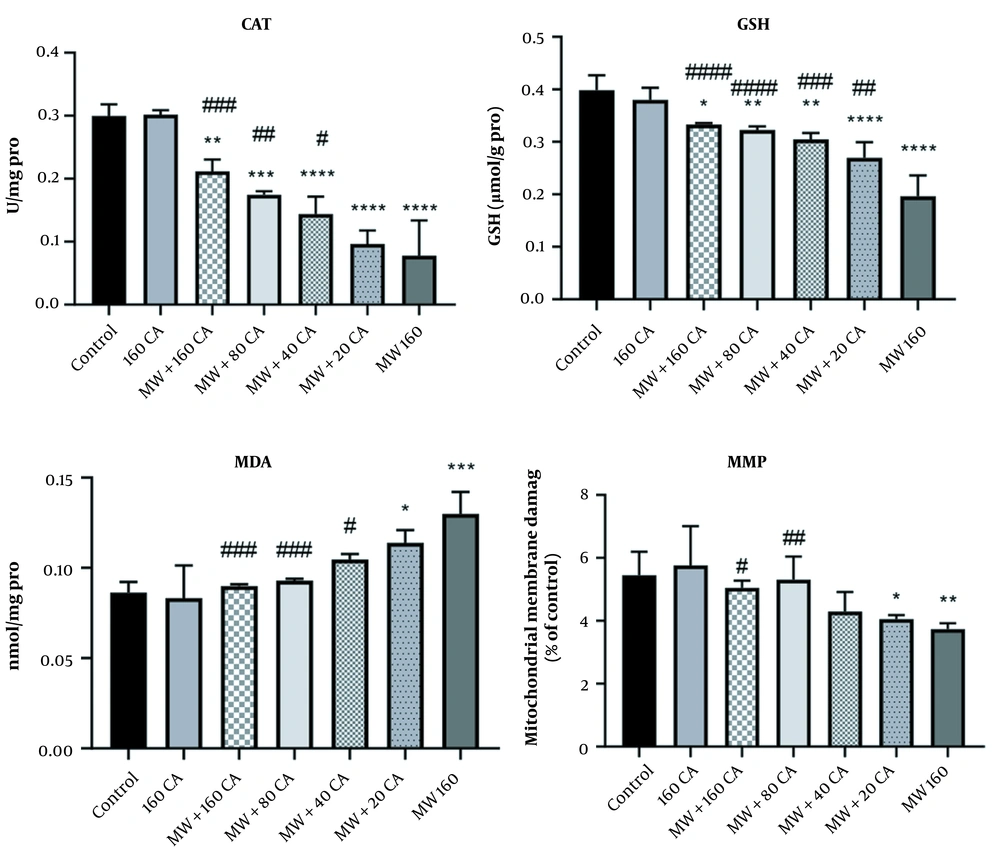
After incubating mitochondria with 160 µg/mL MWCNTs, MDA level (nmol/mg protein) augmented significantly compared with that in the control group (P < 0.001). Additionally, the 1-hour treatment of mitochondria with 40 µM of CA mitigated MDA level compared to that in the MWCNTs group (See Figure 4).
4.5. Effect of CA on GSH in Mitochondria Exposed to MWCNTs
The results of the GSH evaluation revealed a significant decline in the MWCNTs group compared with that in the control group (P < 0.0001). Additionally, 1-hour mitochondrial treatment with 80 µM of CA could prevent this reduction in GSH levels in mitochondria treated with MWCNTs (P < 0.001) (See Figure 4).
4.6. Effect of CA on CAT Activity in Mitochondria Exposed to MWCNTs
According to the study findings, the activity of CAT in mitochondria (unit/mg protein) declined significantly after adding MWCNTs to the mitochondria compared with that in the control group (P < 0.0001). Moreover, a significant increase was also reported in CAT activity after pre-treatment with 80 µM of CA (P < 0.01) compared with the MWCNTs group (See Figure 4).
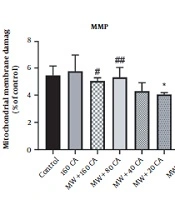
4.7. Effect of CA on MMP in Mitochondria Exposed to MWCNTs
The cationic fluorochrome R123 pigment was utilized to assess MMP. In this respect, MWCNTs could significantly lower MMP in mitochondria (P < 0.01) (See Figure 4), but the 1-hour treatment of mitochondria with 80 µM of CA increased MMP compared with MWCNTs (P < 0.05).
Effect of caffeic acid on the mitochondrial MDA, GSH, and catalase activity in rat Kidney mitochondria exposed to MWCNTs. Results of treatment groups were compared with the control group ** P < 0.01, *** P < 0.001, **** P < 0.0001) and MWCNTs 160 (#P < 0.05, ## P < 0.01, ### P < 0.001). Values are expressed as mean ± SD.
5. Discussion
Mitochondria are dynamic organelles with numerous functions of metabolism and homeostasis of the living cells. Moreover, they play a central role in generating cellular energy through oxidative phosphorylation, calcium homeostasis, caspase-dependent apoptosis initiation, and a cellular stress response (28). In this regard, it is required to find mitochondrial accuracy for the special functioning of the oxidative phosphorylation system. Mitochondria have five complexes along with two electron carriers that are combined into the oxidative phosphorylation system to reduce ROS levels (29). To regulate the production of cellular energy, mitochondria are thus able to send signals (mitochondrial ROS (mtROS) and transmembrane potential) to other cell organelles. Accordingly, excessive mtROS production is responsible for cellular pathology that can directly affect bio-molecules (30). CNTs can also cause oxidative stress in cells. As reported in the related literature, CNTs can directly interfere with cell membranes, activate inflammasomes, and also lead to ROS production (31). A number of research have further reported that MWCNTs cause tissue damage via ROS generation based on dose and time and consequently induce caspase three activity (30, 32). It has even been reported that MWCNTs have induced oxidative stress and reduced cell viability (12). In the present study, the protective effects of CA in renal mitochondria in rats against MWCNTs-induced oxidative stress damage were investigated. The present study also reported that exposure of mitochondria to MWCNTs had diminished cell viability based on doses. Then cell viability decreased up to 3 folds at the highest concentration (160 µg/mL). Correlated with ROS formation, there was also an increase in fluorescence in the MWCNTs group. The contemporary treatment of CA and MWCNT similarly boosted viability in a dose-dependent manner and showed its protective effect above 80 µM of CA. In this study, the effects of MWCNTs on the MMP of samples were similarly examined. In this respect, MWCNTs decreased MMP, as cited in the study by Guo et al. demonstrating that MWCNT could lead to a drop in MMP of cells consistent with cellular apoptosis (33). In this study, MMP treated with MWCNTs was lower than that of mitochondria treated with CA.
Additionally, MDA, GSH, and CAT activity were investigated in all groups. The results of this study imply that MWCNTs can significantly change GSH activity and diminish GSH in renal mitochondria. Decreased CAT levels were also observed in the MWCNTs groups, likely because of the high concentration of MWCNTs (160 mg/kg) employed in this study.
Formed within oxidative stress conditions, MDA is a major peroxidation product that can be used as a lipid peroxidation indicator (34). It should be noted that the instability of the plasma membrane originates from active oxygen atoms produced by peroxide (35). ROS detoxification is also of importance for cell viability. If ROS are not detoxificated by antioxidant materials, peroxides are produced by lipid peroxidation, and toxic aldehydes such as MDA can disrupt cell function (36). Nevertheless, significant rising trends were observed in mitochondrial MDA levels in the MWCNTs groups in this study, and MDA content in the MWCNTs was higher than in other groups. Together with the given changes in antioxidant enzyme activity, the MDA results indicated that the highest level of oxidative stress had occurred in the renal mitochondria of the MWCNTs-treated group.
It should be noted that CA is endowed with numerous biological activities, including ROS scavenging, immune stimulation, and lipid peroxidation quenching (37). The study results in this respect showed that antioxidant enzyme activities had significantly amplified in CA-treated groups compared with the MWCNTs group, suggesting the anti-oxidative effect of CA on mitochondria. The major finding of this research is that CA showed a protective effect against MWCNTs-induced mitochondrial toxicity. Using reactive oxygen metabolites, CA can also protect biopolymers and reduce oxidative DNA damage (38). The probable mechanism of this effect is that CA can act by increasing both the nuclear translocation of Nrf2 and the expression of antioxidant enzymes, decreasing oxidative stress (39). Previous research in this domain revealed that CA administration to rats affected with breast cancer had improved macro-molecular structure, including total protein content in the liver tissues, breast, and serums, indicating maintained cell structure and integrity along with modulated nucleic acids. Furthermore, CA mitigates membrane-bound marker enzyme and peroxidation reactions and increases the enzymic responses of the tricarboxylic acid cycle, adenosine triphosphatases, and antioxidants. These results indicate that mitochondria are a good candidate for the study of toxicity associated with high-dose MWCNTs.
5.1. Conclusions
To investigate toxicological effects induced by MWCNTs in rat mitochondria, a number of anti-oxidative defense system parameters were examined. According to the study results, antioxidant enzyme machinery could play an important role in coping with excessive ROS and protecting mitochondria against oxidative damage, even though they were inhibited after exposure to the highest concentrations of MWCNTs. Besides, CA could reduce oxidative stress and improve antioxidant status in mitochondria. There is a need to conduct further research studies to evaluate CA treatment efficiency against the effects of MWCNTs in animals.