1. Context
The successful development of proteomics and genomics techniques led to recognition that peptides are important biological mediators due to their potency, low toxicity, and selectivity. However, peptides have several limitations, including low oral bioavailability, short circulation time, and low plasma stability. Therefore, the pharmaceutical industry viewed peptides with little interest because of these factors and the high costs associated with large-scale production (1). In the late 1980s, peptides were used as receptor subtype-specific probes and lead compounds in a wave of peptide drug development. Peptide medications have become increasingly popular since recombinant human insulin was approved in 1985. As a result, almost twice as many peptides entered clinical trials during 2000 - 2010 as in the 1990s (2). Recently, peptides and proteins have been considered for medicine production by researchers and pharmacological companies (1). Since 2015, a significant number of medications approved by the FDA have been peptide drugs, and the percentage of medicines approved by the FDA for all drugs has been increasing. Table 1 shows the number of approved medications sorted by total and peptide drugs, as well as the percentage of peptide drugs to total medicines during 2015 - 2022 (Table 1). About 10% of the total number of medications approved by the FDA during 2015 - 2022 (Table 1) were peptides (36 vs. 350) (https://www.fda.gov/).
| Year | Total FDA-approved Medications | Number of Peptide Therapeutics | Percentage of FDA-Approved Peptides, % |
|---|---|---|---|
| 2022 | 28 | 6 | 21.5 |
| 2021 | 50 | 10 | 20 |
| 2020 | 53 | 4 | 7.5 |
| 2019 | 48 | 6 | 12.5 |
| 2018 | 59 | 1 | 1.7 |
| 2017 | 46 | 6 | 13 |
| 2016 | 22 | 1 | 4.5 |
| 2015 | 44 | 2 | 4.5 |
| Total | 350 | 36 | 10.2 |
Peptide drugs can be classified into three major categories: native, analog, and heterologous. Native peptides have precisely the same sequence as the live creature from which they have been identified (3). Additionally, some native peptides have been modified or substituted to improve pharmacological properties. Therefore, they have some differences from the original peptide from which they have been derived. These are known as analog peptides (4). Some other peptides have been discovered from the natural peptides through phage display, synthetic library screening, or other methods. They were named heterologous peptides (5).
Conjugation has been considered a helpful mechanism to change or improve the properties of peptides and proteins, which are candidates for drug design. Conjugation to the Fc fragments of antibodies has been used as a half-life extension strategy in peptide drug design. These peptides are known as antibody-drug conjugates (ADCs) (6). The ADCs are taken into all consideration of peptide drugs in this paper.
The peptide medications approved during 2015 - 2021, along with their indication, administration, therapeutic target, and route, are summarized in Table 2.
| Active Ingredient (Trade Name) | Indication | Therapeutic Target | Administration Route |
|---|---|---|---|
| Year 2021 | |||
| Vosoritide (Voxzogo™) | Achondroplasia | Natriuretic peptide receptor B | SC |
| Melphalan flufenamide (PepaxtoTM) | Multiple myeloma and amyloid light-chain amyloidosis | Aminopeptidases overexpressed in multiple myeloma cells | IV |
| Voclosporin (Lupkynis™) | Lupus nephritis | T-cells | PO |
| Pegcetacoplan (Empaveli™) | Adult patients with paroxysmal nocturnal hemoglobinuria | Complement protein C3, its activation C3b | SC |
| Dasiglucagon (ZegalogueTM) | Hypoglycemia in diabetic patients over the age of six | Glucagon-receptor | SC |
| Piflufolastat-F18 (PylarifyTM) | Prostate cancer patients with PSMA-positive lesions undergoing positron emission tomography | PSMA | IV |
| Difelikefalin (KorsuvaTM) | Hemodialysis patients with chronic kidney disease associated with pruritus | Kappa opioid receptor | IV |
| Odevixibat (BylvayTM) | Progressive familial intrahepatic cholestasis and patients with pruritus over 3 months of age | Ileal bile acid transporter | PO |
| Tisotumab vedotin-tftv (TIVDAK™)* | Recurrent or metastatic cervical cancer, during or after chemotherapy | Tissue factor (TF-011) | IV |
| Loncastuximab tesirine-lpyl (ZynlontaTM)* | Relapsed or refractory diffuse large B-cell lymphoma in adults | B-cell lymphoma, CD19 | IV |
| Year 2020 | |||
| Setmelanotide (ImcivreeTM) | Chronic weight management (obesity) | Melanocortin-4 receptor (MC4R) | SC |
| 64Cu-DOTATATE (DetectnetTM) | Peptide scintigraphic imaging | Somatostatin receptor | IV |
| 68Ga-PSMA-11 | Peptide diagnosis of recurrent prostate carcinoma | Prostate-specific membrane antigen (PSMA) | IV |
| Belantamab mafodotin-blmf (BlenrepTM)* | Relapsed or refractory multiple myeloma | B-cell maturation antigen (BCMA) | IV |
| Year 2019 | |||
| 68Ga-DOTATOC | Scintigraphic imaging | Somatostatin receptor | IV |
| Afamelanotide ScenesseTM | Erythropoietic protoporphyria | Melanocyte-stimulating hormone receptor | SC |
| Bremelanotide VyleesiTM | Hypoactive sexual desire disorder | Melanocyte-stimulating hormone receptor | SC |
| PADCEV® (enfortumab vedotin-ejfv)* | Urothelial cancers | Nectin-4 receptor | IV |
| POLIVY® (polatuzamab vedotin-piiq)* | Refractory diffuse large B-cell lymphoma | CD79b receptor expressed in mature B-cells | IV |
| ENHERTU® (fam-trastuzumab deruxtecan-nxki) * | Unresectable or metastatic HER2-positive breast cancer | Human epidermal growth factor receptor-2 (HER2) | IV |
| Year 2018 | |||
| [177Lu]Lu-DOTA-TATE | Used in theranostic radiopharmaceuticals | Somatostatin receptor | IV |
| Year 2017 | |||
| Plecanatide (Trulance) | Activation of guanylate cyclase-C | Gastrointestinal laxative | PO |
| Etelcalcetide (Parsabiv) | Activation of CaSR on parathyroid chief cells | Hemodialysis patients with chronic kidney disease and secondary hyperparathyroidism | IV |
| Abaloparatide (Tymlos) | Selective activation of the parathyroid hormone one receptor | Osteoporosis | SC |
| Semaglutide (Ozempic) | Agonist of glucagon-like peptide-1 | Treatment for type 2 diabetes mellitus | SC |
| Macimorelin (Macrilen) | Mimic the endogenous ligand for the secretagogue (Ghrelin) | For the diagnosis of adult growth hormone deficiency | PO |
| Angiotensin II (Giapreza) | Increases the production of ADH acting on the CNS | Control of blood pressure in adults with sepsis or other critical conditions | IV |
| Year 2016 | |||
| Adlyxin Lixisenatide® | Diabetes | 44 aa GLP-1 peptide with (Lys)6 at the C-terminal | SC |
| Year 2015 | |||
| Insulin degludec Tresiba® | Diabetes | Modified insulin, an amino acid deletion, and hexadecanedioic acid via -Glu at the Lys (B29) | SC |
| Ixazomib Ninlar® | Multiple myeloma | N-Acylated, C-boronic acid dipeptide | PO |
Abbreviations: IV, Intravenous; SC, Subcutaneous; PO, Oral.
a Antibody-drug conjugates are marked with a star above the drug name.
Some previous studies have reviewed the pharmaceutical peptides approved by the FDA until 2021 (7-9). Here, we will discuss the peptides approved in 2022 in detail.
2. FDA-Approved Peptide Therapeutics in 2022
Over the last eight years (since 2015), the FDA has approved 350 new medications, including 28 in 2022. A total of six peptides have been approved this year (https://www.fda.gov/). Year 2022, with 21.5% peptide medication approvals, had the highest rate in the percentage of FDA- approved peptides (Table 3) (7-9).
| Active Ingredient (Trade Name) | Indication | Therapeutic Target | Administration Route |
|---|---|---|---|
| Terlivaz (terlipressin) | Improve kidney function | Vasopressin V1 receptors versus V2 receptors | IV |
| DAXXIFY™ (DaxibotulinumtoxinA-lanm) | Improvement in the appearance of glabellar lines along with corrugator and/or procerus muscle activity | SNAP25 protein | IM |
| Xenpozyme™ (olipudase alfa-rpcp) | Adult and pediatric patients with non-CNS manifestations of acid sphingomyelinase deficiency | Sphingomyelin | IV |
| KIMMTRAK (tebentafusp-tebn) | Melanoma | CD3 T cell | IV |
| ENJAYMO™ (sutimlimab-jome) | Cold agglutinin disease | C1s in the classical complement pathway, | IV |
| VABYSMO™ (faricimab-svoa) | Neovascular (wet) age-related macular degeneration and diabetic macular edema | Vascular endothelial growth factor-A and angiopoietin 2 (Ang-2) | Intravitreal |
Abbreviations: IV, intravenous; IM, intramuscular.
2.1. Terlivaz (Terlipressin)
Terlivaz contains terlipressin, which targets vasopressin receptor subtypes V1 and V2, with twice the selectivity for V1 receptors (10). Terlipressin is a peptide composed of 12 amino acids with the chemical name N-[N-(N-glycylglycyl)glycyl]-8-L-lysinevasopressin and a molecular weight of 1227.38 kDa (11). It is a synthetic analog of vasopressin that revealed more pharmaceutical activity and fewer side effects than vasopressin (12). It is effective in treating two of the common complications of liver diseases, including hepatorenal syndrome (HRS) and acute variceal bleeding (AVB) (13). Terlivaz is administered intravenously. It is recommended to inject 0.85 mg (1 vial) of Terlivaz every 6 h on days 1 - 3. On the fourth day, if serum creatinine (SCr) decreases versus the baseline level by at least 30%, continue using the medication as in the previous days. However, if SCr decreases by less than 30% compared to baseline, the dose may be increased to 1.7 mg (2 vials) intravenously every 6 h (14).
An initial randomized controlled trial in patients with hepatorenal syndrome type 1 (HRS-1) revealed that terlipressin significantly improved renal dysfunction and survival compared to placebo (15). Several other studies provided further evidence of the effect of terlipressin and albumin in patients with HRS (13).
In patients with HRS-1, following the administration of a single dose (0.85 mg) of terlipressin, changes in cardiovascular, splanchnic, hepatic, and renal circulation were evident as follows: systemic vascular resistance and mean arterial pressure increased. At the same time, the heart rate and cardiac output decreased, and myocardial perfusion and stroke volume did not alter. In splanchnic circulation, terlipressin counteracts nitric oxide-induced vasodilatation and induces splenic vasoconstriction, resulting in diminished portal vein blood flow. Terlipressin also raises the hepatic arterial resistance and reduces the pressure gradient of hepatic venous. In addition, renal arterial resistance and renal perfusion pressure reduce. The sum of all these events causes an effective circulatory volume increase, which counteracts the activation of the renin-angiotensin-aldosterone system, and finally, hyperdynamic circulation improves (13).
Terlipressin was approved for the treatment of HRS and AVB. There is no evidence that terlipressin inhibits or induces any of the CYP enzymes in human liver microsomes directly, time-dependently, or metabolism-dependently. No significant drug-drug interactions are predicted for Terlivaz.
2.2. DAXXIFY™ (DaxibotulinumtoxinA-lanm)
DaxibotulinumtoxinA-lanm is the first neuromodulator that blocks cholinergic transmission at the neuromuscular junction by inhibiting acetylcholine release. This agent temporarily improves the appearance of moderate to severe glabellar lines in adults by relaxing the facial muscles that cause glabellar lines (16). Following the injection of DAXXIFY into skeletal muscle, it internalizes into nerve terminals, translocates into the neuronal cytosol, and cleaves SNAP25, a protein required for docking synaptic vesicle membranes. As a result, acetylcholine is released, which decreases muscle function dose-dependently. Recovery of function occurs gradually due to the degradation of neurotoxin light chains in neurons and the formation of axonal sprouts. A slow reversal of DAXXIFY's pharmacological effects occurs due to muscle re-innervation (17).
DaxibotulinumtoxinA-lanm is a 150 kDa botulinum toxin product stabilized with peptide exchange technology that lasts longer (6 - 9 months) than conventional neuromodulators, such as Botox (3 - 4 months). It is also free of animal-based components and human serum albumin (17). DAXXIFY™ is administered 0.1 mL (8 units) intramuscularly into five sites on the forehead and around the eyebrows. The FDA approved it on September 2022 (18).
2.3. Xenpozyme™ (Olipudase alfa-rpcp)
Xenopus is the first disease-specific peptide approved by the FDA for treating the non-central nervous system (CNS) manifestations of acid sphingomyelinase deficiencies in adult and pediatric patients (19). Over time, deficiency in acid sphingomyelinase leads to decreased lung function, enlarged liver and/or spleen, and growth delay in children (20). Xenopus delivers olipudase alfa-rpcp, an enzyme replacement therapy, to cells to reduce sphingomyelin production by providing acid sphingomyelinase that allows sphingolipids to be destroyed (21). Olipudase alfa-rpcp is a hydrolytic lysosomal sphingomyelin-specific enzyme composed of 570 amino acids with a molecular weight of 76 kDa, produced in a Chinese hamster ovary cell line using recombinant DNA technology (20). XENPOZYME (olipudase alfa-rpcp) was approved in Japan on 28 March 2022 for the first time. Regulatory review in the USA is underway. XENPOZYME is supplied for intravenous injection in vials containing 20 mg olipudase alfa-rpcp, 4.47 mg dibasic sodium phosphate, 74.6 mg methionine, 8.17 mg monobasic sodium phosphate, and 250 mg sucrose (19).
2.4. KIMMTRAK (Tebentafusp-tebn)
KIMMTRAK is a new immunotherapy indicated for adult patients with HLA-A*02:01–positive uveal melanoma that has metastasized to other parts of the body or cannot be removed by surgery. This new treatment is designed so that KIMMTRAK causes the patient's T-cells to be activated and fight uveal melanoma tumor cells (22, 23). KIMMTRAK (tebentafusp-tebn) is a 77 kDa bispecific gp100 peptide-HLA-directed T cell receptor CD3 T-cell engager produced by recombinant DNA technology in Escherichia coli. HLA-A*02:01/gp100 is a marker often found on the surface of uveal melanoma tumor cells. Binding of tebentafusp-tebn to the HLA-A*02:01/gp100 complex leads to T-cell activation. Activated T-cells recognize, attach, and kill uveal melanoma tumor cells (24, 25).
In February 2022, KIMMTRAK (tebentafusp-tebn) was given a positive opinion by the EU Committee for Medicinal Products for Human Use for treating uveal melanoma. In the UK, Australia, and Canada, KIMMTRAK is under review by the regulatory authorities for the treatment of metastatic uveal melanoma. KIMMTRAK is supplied for intravenous injection in a single-dose vial; each contains 100 mcg tebentafusp-tebn, 0.95 mg citric acid monohydrate, 2.91 mg di-sodium hydrogen phosphate, 5 mg mannitol, 0.1 mg polysorbate 20, 25 mg trehalose, and water for injection, with a pH of 6.5 (26).
2.5. ENJAYMO™ (Sutimlimab-jome; Sutimlimab)
Sutimlimab was developed for monoclonal antibody therapy in a patient with cold agglutinin disease (CAD) (27). Sutimlimab is an immunoglobulin G (IgG-4) monoclonal antibody, which binds specifically to complement protein component 1, s subcomponent (C1s), and cleaves complement protein component 4. When C1s is inhibited, complement opsonins are not deposited on RBC surfaces, which inhibits hemolysis in patients with CAD (28). Sutimlimab comprises 445 amino acids in each heavy chain (H chain) and 216 amino acids in each light chain (L-chain). Sutimlimab-jome has a molecular weight of 147 kDa (29).
In February 2022, ENJAYMO received its first approval in the USA. ENJAYMO has been prepared for intravenous infusion only. In each ENJAYMO vial, a single dose is intended. For patients with CAD, ENJAYMO dosage is determined by body weight. The recommended dose is 6,500 mg for patients weighing 39 kg to less than 75 kg and 7,500 mg for patients weighing 75 kg or more. ENJAYMO should be administered intravenously every 2 weeks for the first 2 weeks, and every 4 weeks thereafter (29).
2.6. VABYSMO™ (Faricimab-svoa, Faricimab)
Faricimab (faricimab-svoa; Vabysmo™) is a bispecific antibody with a molecular weight of approximately 149 kDa that binds to and inhibits both vascular endothelial growth factor (VEGF)-A and angiopoietin-2 (Ang-2). Roche/Genentech is producing VABYSMO by recombinant DNA technology using mammalian Chinese Hamster Ovary (CHO) cell culture to treat retinal vascular diseases via intravitreal injection (30). Faricimab received its first approval in January 2022, in the USA, for treating patients with neovascular (wet) age-related macular degeneration (nAMD) or diabetic macular edema (DME). It has also been recently approved in Japan and is currently under regulatory review in the EU for use in the treatment of nAMD and DME (31). Several countries worldwide are conducting Phase III clinical trials of faricimab for treating nAMD, DME, and macular edema caused by retinal vein occlusion (32).
Faricimab, by inhibiting the VEGF-A, suppresses endothelial cell proliferation, vascular permeability, and neovascularization. Through inhibiting Ang-2, Faricimab promotes vascular stability and desensitizes blood vessels to VEGF-A effects. Levels of Ang-2 are elevated in some of the patients with nAMD and DME. It is not yet known whether Ang-2 inhibition contributes to the therapeutic effect and clinical response in nAMD and DME (33). Faricimab is prescribed in two different regimens for nAMD and DME. Each vial of Faricimab has been described as a single dose containing 0.05 mL (50 µL) of the solution, including 6 mg faricimab-svoa, 155 mcg L-histidine, 52.2 mcg of L-methionine, 20 mcg polysorbate,73.1 mcg sodium chloride, and 2.74 mg D-sucrose (31).
3. Scorpion Venom Peptides with Therapeutic Abilities
Most of the peptide therapeutics in the market, as well as the medications undergoing clinical trials, are analogs in order to improve the effectiveness of the drugs and reduce their side effects (3). Nevertheless, natural sources still inspire the production and development of peptide therapeutics. In an estimation, around 40% of the therapeutics come from nature (2).
Peptides identified in the venom of different venomous animals, including snakes, toads, spiders, leeches, lizards, snails, and scorpions, when isolated or synthesized as a single compound and used at appropriate concentrations, can become helpful medications (34). As a result of the urgent need to discover or improve treatment regimens for broad-spectrum diseases, the pharmacological applications of venomous peptides have gained enormous attention from pharmaceutical industries and experts (2). Currently, there are not many venom-based peptide therapeutics approved by the FDA. Some of the most important ones are captopril, atracurium, ziconotide, and eptifibatide, and some others are undergoing clinical or preclinical trials. However, these animals have considerable potential for developing more therapeutics (2). There are tremendous increasing advances in modern technologies, including genomics, proteomics, genomics, and transcriptomics which help to discover new venom-based medicines (35).
Among the venomous animals, scorpion venom contains a wide range of bioactive molecules, including peptides, proteins, enzymes, and other compounds with beneficial properties for drug design and development. Scorpion venom peptides are highly specific and have a good affinity for macromolecules in humans (2).
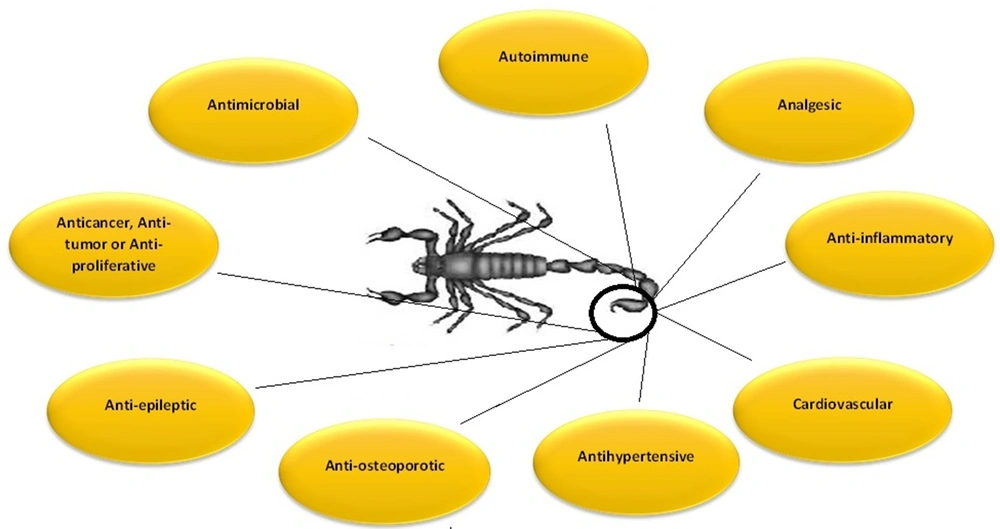
Before the last two decades, scorpions were known for stings and threats to human life, but research showed that venom toxicity is related to a few toxins within the venom. Despite their name, most toxins are not toxic, while some have beneficial therapeutic applications (36). Medicinal compounds identified in scorpion venoms have diverse abilities. They can target a wide range of diseases, including cardiovascular disorders, epilepsy (37), autoimmune problems (38), hypertension (39), cancers (36, 37), pain (40), as well as inflammatory (41) and microbial diseases (42-44) (Figure 1).
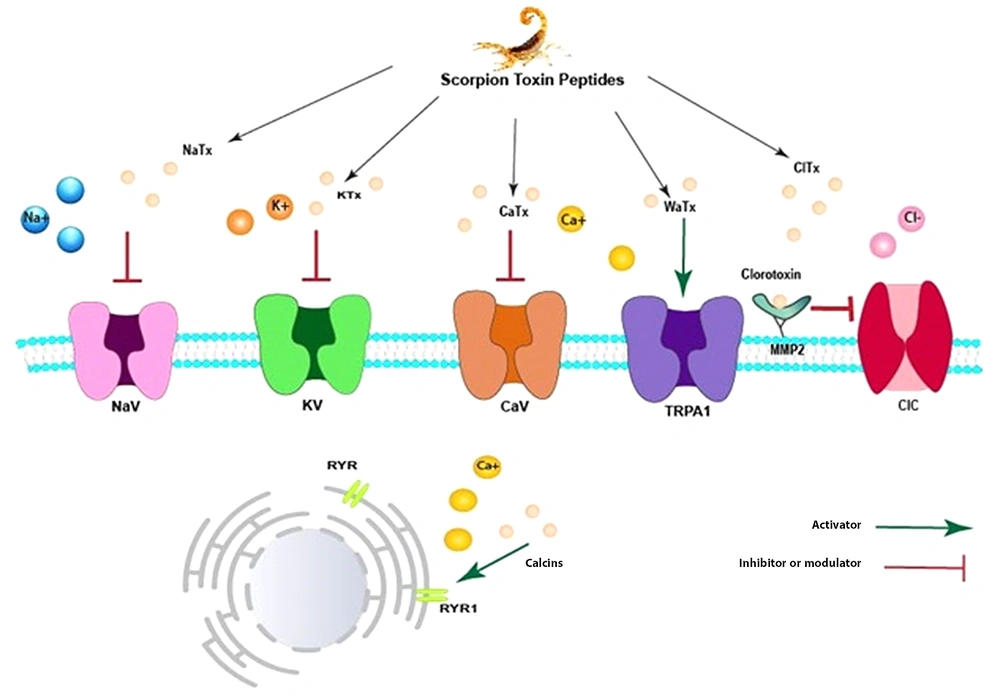
Almost 40% of marketed medications target ion channels (Na+, K+, Ca2+, and Cl-) and G protein-coupled receptors. These receptors are identified as druggable receptors. Some scorpion venom toxins target different ion channels by blocking or modifying them. Therefore, it is unsurprising that many scorpion toxins have been considered for pharmaceutical properties. Figure 2 illustrates a schematic picture of scorpion venom components on different types of receptors (Figure 2). Some venom peptides, namely NaTx peptides, specifically modulate the opening and closing of Na+ ion channels (45). However, KTx peptides typically are pore-blocking agents. They usually block the passage of potassium ions by inserting a key residue into the pore of the channel (46), gating modifying (47), or turret blocking (48). CaTx peptides represent a different action. Some of them, such as Kurtoxin, can inhibit voltage-gated calcium channels. However, calcins can bind to RyRs located in the endo/sarcoplasmic reticulum, leading to increased intracellular Ca+2 (49). Wasabi Receptor Toxin (WRTx) is capable of irritating Transient Receptor Potential (TRPA1) (50). Chlorotoxins, which specifically change the function of Cl- channels, exert their effect by binding to the MMP-2 receptor (Figure 2). The MMP-2 receptor is located next to the Cl- channel on the extracellular surface of the membrane. After binding to the M receptor, chlorotoxins change their conformation to inhibit the Cl- transportation (51).
Schematic illustration of Scorpion venom peptides' effects on the membrane ion channels: NaTx peptides specifically modulate the opening and closing kinetic mechanism of Na+ ion channels, while KTx peptides typically are pore-blacking agents. Some CaTx peptides, including Kurtoxin, could inhibit voltage-gated calcium channels. However, calcins can bind RyRs in the endo/sarcoplasmic reticulum, increasing intracellular Ca+2. Wasabi Receptor Toxin (WRTx)-like calcins, can irritate Transient Receptor Potential (TRPA1). Clorotoxin binds its receptor MMP-2 and can inhibit a voltage-gated chloride channel.
At the same time that researchers became aware of the function and capabilities of scorpion toxins mentioned above, the attention of pharmacology researchers and pharmaceutical industries was directed towards this small gland full of benefits. The richness, specialization, and efficiency of the venom components make the tiny venoms attractive to researchers worldwide. As identification approaches continue to identify new potent peptides in the venom gland of different species of scorpion, the more active venom-derived peptides are found for application as therapeutics, cosmetics, and insecticides (2). Although several scorpion peptides have been identified with great therapeutic potential, many have not yet reached the clinical trial stages. Here, we will describe those that have passed a significant part of their initial research and currently are in clinical or preclinical states.
3.1. Scorpion-derived Peptides Currently in a Clinical or Preclinical State
3.1.1. BLZ-100; Tumor Paint®; Tozuleristide
BLZ-100, known as tumor paint, is a chlorotoxin (CTX)-indocyanine green conjugate capable of specifically binding solid tumors and fluorescing near infrared wavelengths, minimizing light scatter and signal attenuation (52). The CTX used in this composition is a 36-amino acid chloride channel-blocking toxin initially identified in the venom gland of the scorpion Leiurus quinquestriatus (53). The CTX has demonstrated specificity for tumor targeting in various forms, including radiolabeled, fluorescent-tagged, or nanoparticle-encapsulated types (54). Surgery is a definitive way to treat many cancers. In these cases, actionable contrast between the tumor tissue and surrounding healthy tissue is an important and helpful principle for the surgeon to remove the cancerous tissue without damaging the healthy tissue. BLZ-100 emits light when it binds to cancerous tissue. Thus, a surgeon can detect and remove the cancerous tissue entirely (52). Tumor paint binds both primary tumors and metastatic lesions in transgenic and xenograft mouse models of glioma, prostate cancer, medulloblastoma, colorectal cancer, and sarcoma (55). A preclinical test also validated the utility of BLZ-100 in providing contrast for imaging canine adenocarcinomas, squamous cell carcinomas, and mast cell tumors (52). The CTX is attractive as a targeted imaging agent for cancer because of these unique properties (56). BLZ-100 is undergoing human clinical trials and has shown negligible toxicity in humans in all clinical trials conducted to date (56, 57).
In Phase 1 clinical trial of BLZ-100 on 17 subjects, patients received 3 - 30 mg of intravenous tozuleristide 3 - 29 h before surgery. Based on the results of this study, Tozuleristide imaging may be helpful for Fluorescence-guided surgery of gliomas at doses up to 30 mg (57). A Phase 2/3 study is undergoing. Surprisingly, in two patients with preoperatively suspected gliomas, cavernous vascular malformations were present after resection. It is postulated that tozuleristide fluorescence is caused by binding to matrix metalloproteinase-2 and annexin A2. According to the literature, multiple cerebrovascular lesions, including cavernous malformations, express both of these ligands. As a result, it suggested that the binding of BLZ-100 to cerebral vascular malformations is a potentially novel application of this compound (58).
A phase 2 and 3 randomized, blinded study of BLZ-100 on pediatric primary CNS tumors was conducted on 35 cases during neurosurgical resection with a single dose of 15 mg/m2 administrated 1-2 h prior to surgery and using Canvas Imaging System to display the cancerous tissues in the monitor. The safety of BLZ-100 was confirmed, and according to the Safety Monitoring Committee (SMC), the trial should proceed as planned (59). The first human study of BLZ-100 on 21 adult skin cancer patients who were administered intravenous BLZ-100 also demonstrated the tolerability and safety of BLZ-100 (56). Some CTX-like peptides have also been identified in the venom gland of different scorpion species that may be a good candidate for further in vivo and in vitro studies (51, 60, 61).
3.1.2. Margatoxin
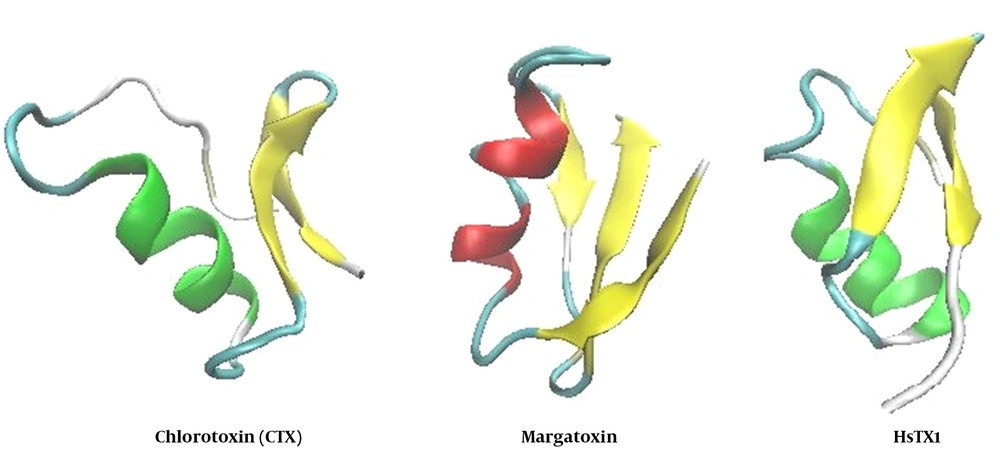
Voltage-gated potassium Kv1.3 channel is highly expressed in human T and B lymphocytes, and a Kv1.3-mediated efflux is required for activating these types of cells. Since T cells are the primary mediators in autoimmune disease, the blockers of Kv1.3 can be an attractive target for drug development (62, 63). Research on the venom gland of scorpion Centruroides margaritatus led to the discovery of margatoxin (MgTx) (Figure 3), which was an active peptide against kv1.3 MgTx that blocked the KV.13 channel in pM concentrations (64). This toxin performs the blocking action by inserting a lysine residue into the channel pore (65).
Merck et al. (cited in Koo et al.) developed MgTx for preclinical use and demonstrated its efficacy in a mini-pig model of delayed-type hypersensitivity (DTH). They demonstrated that the blockade of Kv1.3 by MgTx in vivo inhibits DTH and antibody response to an allogeneic challenge (69). A fluorescent conjugate of MgTx (GFP-MgTx) was constructed. Using GFP-MgTx, it became possible to identify peptide pore blockers and evaluate their affinity for the Kv1.3 channel. As potential drug candidates, GFP-MgTx can be utilized in screening and as a pre-selection tool for potassium channel blockers (70). Another conjugated form of MgTx with luminescent quantum dots (QDs), known as QD-MgTx, was utilized to assess its potency to block Shaker channels Kv1.1 to Kv1.7 using patch-clamp electrophysiology in HEK293 cells. The results showed that MgTx could provide a valuable tool to deliver ion channel inhibitors to targeted tissues in vivo (71).
Blocking of Kv1.3 in mice carbon tetrachloride (CCl4) induced hepatic fibrosis models, regulating macrophage polarization and cytokine secretion, was assessed as a potential treatment for MgTx. MgTX prevented the mice from developing liver fibrosis due to CCl4. MgTX also reduced pro-inflammatory cytokines and increased interleukin-10 production in the serum of mice with HF. Overall, the results of this study suggested that MgTX reduces CCl4-induced HF in mice by polarizing macrophages, secreting cytokines, and activating STAT (72).
3.1.3. HsTX1
HsTX1 is a peptide composed of 34 amino acids which originated from the venom gland of the scorpion Heterometrus spinnifer (Figure 3). This is another blocker of the Kv1.3 channel (73). It has been demonstrated that KV1.3 blockers are excellent candidates for treating autoimmune diseases, as described above. A recently designed mutant analog of HsTX1, HsTX1[R14A], and HsTX1[R14Abu] confirmed the greater selectivity and affinity for Kv1.3. A low pM range affinity and a selectivity of more than 2000-fold for Kv1.3 over Kv1.1 were reported for both mutants (74).
Buccal mucosa (75) and pulmonary (76) administration of HsTX1 [R14A] to rats and mice, respectively, were revealed to be effective in delivering the plasma levels of the peptide well more than those required for effective therapy. Therefore, the pulmonary and buccal administration of HsTX1 [R14A] is suggested as a promising alternative treatment for autoimmune diseases. Furthermore, a PEDylated analog of HsTX1 [R14A] was demonstrated to decrease inflammation in an active DTH model and the arthritis model of rheumatoid arthritis induced by pristane (77).
4. Conclusions
Peptide therapeutics have recently received more attention as a significant part of the medications approved by the FDA are peptide drugs. The number of peptide therapeutics approved in 2022 compared to the total number of medicines approved this year accounts for the highest percentage compared to previous years. Many peptide drugs originate from nature. The venom glands of venomous animals are an important source of peptide compounds with medicinal potential. Peptides of the venom glands of scorpions have received particular attention due to their high specificity and affinity to the macromolecules of human cells. An extensive and increasing study is being conducted to identify and use the compounds of scorpion venom glands for medication production. Although researchers have already discovered some of the vital potentials of scorpion venom, much remains to be discovered about it and its therapeutic effects. This review reports the scorpion peptide compounds in the clinical and preclinical stages.