1. Background
Myocardial infarction (MI), the major subtype of ischemic heart disease (IHD), is the most prevalent cause of death in the 21st century. This condition results from the rupture of a vulnerable plaque, leading to the thrombotic occlusion of coronary arteries (1). Prolonged myocardial ischemia is accompanied by an increase in reactive oxygen species (ROS) and oxidant factors (2). On the other hand, the reperfusion phase leads to mitochondrial alterations, followed by the apoptosis and necrosis of cardiomyocytes in infarcted areas (3). Despite the presence of established MI treatment guidelines, remodeling complications remain to be the foremost leading cause of death.
Among various treatments, herbal medications have been brought to the horizon regarding their availability, low cost, and minimal adverse effects and drug interactions (4). Growing evidence supports the high efficacy of herbal medicines, causing them to receive the attention of physicians as add-on treatments for cardiovascular diseases (CVDs) (5).
The Scrophularia genus, a flowering plant with more than 300 species, has a wide distribution in the north and south of America and east of Asia (6). It is well-known for its bilateral or rarely radial symmetric flowers reaching a height of 10-50 cm, as well as thick, hard, and green leaves (7). In recent studies, the Scrophularia genus has been linked to CVDs. The anti-apoptotic and antioxidant effects of Scrophularia on cardiomyocytes have indicated its cardioprotective potential (8). Further, Scrophularia has been shown to improve hypertension and arrhythmias, making it a beneficial therapeutic choice for MI.
2. Objectives
According to the aforementioned, we here aimed to assess the effects of an indigenous plant species belonging to this genus, S. atropatana, on MI-associated tissue injury in a rat model.
3. Methods
3.1. Animals
Thirty-six male Wistar rats weighing 280 - 300 g were obtained from the animal house of the Faculty of Pharmacy, Tabriz University of Medical Sciences (TBZMED). The procedures were in accordance with the ‘Principles of Laboratory Animal Care’ (NIH publication 82-23, revised in 1985 and further amendments in 1996). This study was approved under the ethical approval code of IR.TBZMED.REC.1395.1272. Rats were kept in polypropylene cages under a 12-hour light/dark cycle at room temperature (20 ± 2°C) without any restrictions on access to food and water.
3.2. Plant Extract Preparation
The chopped aerial parts of S. atropatana were collected from altitudes above 1600 m in the Spiran region of the East Azerbaijan province in May 2013. A voucher specimen was deposited in the herbarium of the Faculty of Pharmacy, TBZMED, Iran, under the accession code TBZ-FPH 8962.
The air-dried parts of S. atropatana were extracted with 200 mL methanol and separately concentrated using a rotary evaporator at a maximum temperature of 45°C. Finally, 1 g of the extract was obtained.
3.3. MI Induction
Isoproterenol (ISO) (Sigma) was dissolved in normal saline (0.1 mL) and was injected (100 mg/kg) subcutaneously in the neck area for two days (every 24 hours) (9).
3.4. Experimental Groups
The rats were randomly classified into 6 groups (n = 6): (1) control; (2) ISO; (3) ISO + S. atropatana (SAT) (5 mg/kg); (4) ISO + SAT (10 mg/kg); (5) ISO + SAT (20 mg/kg); and (6) SAT. The control group received 0.1 mL of normal saline intradermally for 2 days (every 24 hours) and 0.3 mL of normal saline orally for 3 days (every 24 hours) (the first dose was administered by gavage 2 hours after the first saline injection). In the ISO group, MI was induced, and the animals received 0.3 mL oral normal saline for 3 days (every 24 hours). In ISO + SAT groups, after MI induction, the rats received oral SAT (5, 10, and 20 mg/kg) for three days. Finally, in the SAT group, the animals were infused with 0.1 mL normal saline intradermally for 2 days (every 24 hours) and then fed with oral SAT (20 mg/kg) for 3 days.
3.5. Hemodynamic Parameters
Rats were anesthetized by ketamine (100 mg/kg IP) and xylazine (10 mg/kg IP) 48 hours after the last intervention. Systolic arterial pressure (SAP) and diastolic arterial pressure (DAP) were measured through a Millar Micro Tip catheter placed into the isolated right carotid artery. Median arterial pressure (MAP) was calculated based on SAP and DAP, and the heart rate was measured by the instrument power lab/4sp-AD electrocardiogram (ECG) (10). The catheter was placed into the left ventricle to measure the left ventricular systolic pressure (LVSP), left ventricle end-diastolic pressure (LVEDP), and minimum and maximum increase in the left ventricular pressure (LV dp/dt Min, LV dp/dt Max) (10).
3.6. Tissue Sample Collection
Blood samples were obtained and centrifuged at 13000 rpm, 4°C. The collected serum samples were kept at -80°C for biochemical tests. Then the heart tissue was isolated and weighed to measure the hypertrophy rate. Finally, heart tissue samples were kept in 10% formaldehyde for histopathology evaluations.
3.7. Malondialdehyde, Total Antioxidant Status, and Lactate Measurement
In this study, malondialdehyde (MDA) was selected due to potential oxidant activity. Trichloroacetic acid was added to serum samples, followed by centrifugation, to separate serum proteins. Then thiobarbituric acid was applied to the samples at 95°C for 50 minutes, and the colorful complex was quantified by reading absorption at 532 nm (11).
The total antioxidant capacity of the serum was measured by fluorescence recovery after photobleaching (FRAP) (12, 13). In this process, the reducing capacity of the plasma was evaluated by spectrophotometry following the reduction of Fe3+ to Fe2+, generating a blue color measured at 593 nm.
Serum lactate level was measured using the Lactate Mono-Liquid kit (BiorexFars). First, harvested tissues were homogenized by a lactate assay buffer at 4°C, and the supernatant was obtained. Endogenous LDH was inactivated by adding deproteinizing solutions. Then the plate was prepared by adding a lactate standard and samples into the wells. Finally, OD was measured at 450 nm using a microplate reader. The assay was performed in duplicates.
3.8. Histopathology Evaluation
Heart tissues were horizontally sectioned and stained by hematoxylin and eosin (H&E). A semi-quantitative scoring system was used for evaluating hypertrophy, edema, and necrosis in cardiac sections ((0) No pathologic change; (1) mild pathologic changes; (2) moderate pathologic changes; and (3) severe pathologic changes) (14).
3.9. Statistical Analysis
The data were analyzed by GraphPad Prism 6 using an Unpaired t-test (control vs. ISO, control vs. SAT (20 mg/kg)) and one-way analysis of variance (ANOVA) with Tukey’s post-hoc test (ISO vs. ISO + SAT). A P value < 0.05 was considered to designate statistically significant differences. The results were presented as mean ± standard error of the mean (SEM).
4. Results
4.1. The Effect of Scrophularia atropatana on Arterial Pressure
As shown in Table 1, MAP significantly decreased after ISO injection compared to the control group. All three doses of S. atropatana significantly increased MAP compared to the ISO group. There was also a significant decrease in SAP in the ISO group, showing a significant elevation after SAT treatment. In addition, DAP decreased following ISO injection and in all three treatment groups, showing a significant increase compared to the ISO group. The unpaired t-test results showed that SAP, DAP, and MAP did not change significantly in the SAT (20 mg/kg) group compared to the control group.
4.2. The Effects of Scrophularia atropatana on Cardiac Parameters
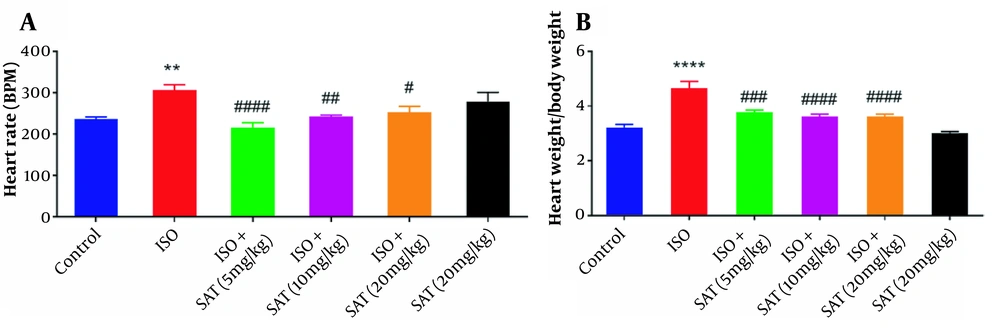
Regarding Figure 1A, ISO administration raised the heart rate, while treatment with the 5, 10, and 20 mg/kg doses of S. atropatana reduced the heart rate significantly. Also, in the SAT group, the heart rate did not significantly change in comparison with the control group.
As shown in Figure 1B, marked cardiac hypertrophy was observed following ISO injection, as evidenced by an increased HW/BW ratio. In contrast, S. atropatana (5, 10, and 20 mg/kg) significantly reduced this ratio. There was no significant change in the HW/BW ratio in the SAT group in comparison with the control group.
4.3. The Effect of Scrophularia atropatana on Left Ventricular Pressure
As shown in Table 2, LVSP significantly decreased after ISO injection in comparison with the control group, and treatment with 20 mg/kg S. atropatana increased LVSP compared to the ISO group. Moreover, LVEDP increased significantly after ISO injection and showed a noticeable decline in the ISO + SAT (5, 10, and 20 mg/kg) groups.
| Groups | LVSP (mmHg) | LVEDP (mmHg) | LVdp/dt/P (1/sec) |
|---|---|---|---|
| Control | 116 ± 2.42 | 6.7 ± 0.26 | 3.3 ± 0.20 |
| ISO | 62 ± 10.92 b | 12.2 ± 1.23 b | 1.4 ± 0.26 c |
| ISO + SAT (5 mg/kg) | 81 ± 9.23 | 3.9 ± 0.82 d | 3.7 ± 0.83 e |
| ISO + SAT (10 mg/kg) | 89 ± 8.12 | 4.70 ± 0.73 d | 3.4 ± 0.42 f |
| ISO + SAT (20 mg/kg) | 104 ± 19.51 e | 3.66 ± 1.19 d | 3.9 ± 0.53 e |
| SAT (20 mg/kg) | 83 ± 2.12 c | 9.07 ± 0.65 | 4.3 ± 0.77 |
The Effects of Scrophularia atropatana on LVSP, LVEDP, and LVdP/dt/P a
There were significant reductions in LVdp/dtMax and LVdP/dtMin following ISO injection. In all three ISO + SAT groups (5, 10, and 20 mg/kg), LVdP/dt/P increased significantly (Table 2). Finally, LVSP revealed a significant decrease in the SAT group, but no significant alterations were witnessed in LVEDP and LVdp/dt levels in this group.
4.4. The Effect of Scrophularia atropatana on MDA and TAS Levels
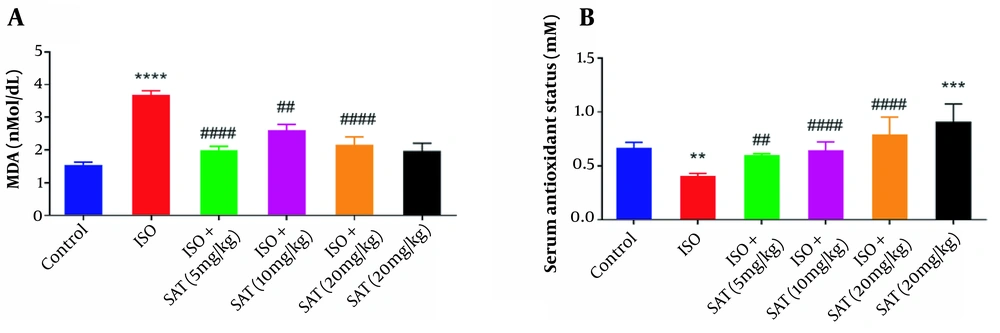
Following ISO injection, a significant increase in MDA level was observed (P < 0.001). Treatment with S. atropatana extract (5, 10, and 20 mg/kg) reduced MDA level significantly (P < 0.0001, P < 0.01, P < 0.0001, Figure 2A), but MDA level showed no significant difference between the SAT and control groups.
Also, TAS showed a significant reduction in the ISO group (P < 0.01), which was reversed after treatment with S. atropatana extract (5, 10, and 20 mg/kg) (P < 0.01, P < 0.0001, and P < 0.0001, respectively, Figure 2B).
According to the results of the unpaired t-test, receiving S. atropatana (i.e., SAT group) significantly raised TAS compared to the control group (P < 0.001).
4.5. The Effect of Scrophularia atropatana on Serum Lactate Level
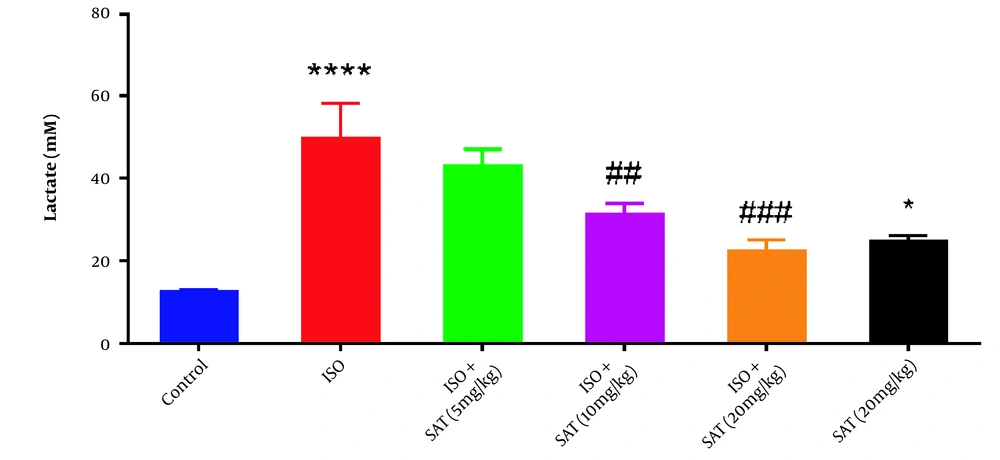
Serum lactate level increased significantly following ISO injection. On the other hand, administration of 10 or 20 mg/kg of S. atropatana significantly reduced serum lactate levels. Similarly, a significant increase in lactate level was observed in the SAT (20 mg/kg) group compared to the control group (Figure 3).
4.6. Histopathologic Effects of Scrophularia atropatana on the Heart
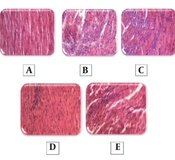
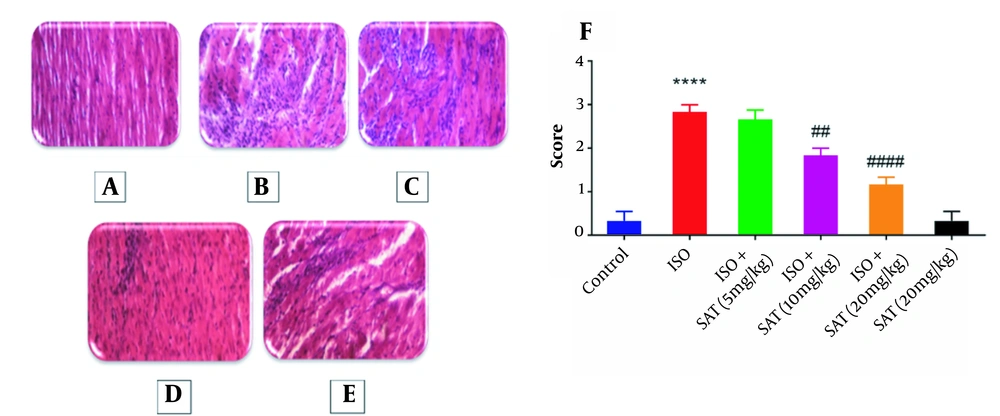
Figure 4 shows the results of the cardiac histopathology examination and relevant quantitative assessments. There was no significant necrosis or other histopathologic changes in the cardiac sections obtained from the animals in the control group. However, significant edema and necrosis were noticeable in cardiomyocytes in the ISO group. Administration of 10 or 20 mg/kg methanolic extract of S. atropatana significantly reduced the edema and necrosis in the heart tissue caused by ISO.
The histopathologic effects of Scrophularia atropatana on the heart tissue. Photo-micrographic sections of rat hearts at 400X magnification (hematoxylin and eosin (H&E)) and comparisons between groups have been shown. Histologic sections of the heart in A, the control; B, ISO; C, ISO + SAT (5 mg/kg); D, ISO + SAT (10 mg/kg); and D, ISO + SAT (20 mg/kg) groups. F, Quantitative illustration of histopathologic changes, data are expressed as mean ± SEM (n = 6). (**** P < 0.0001 vs. control group; ## P < 0.01, #### P < 0.0001 vs. ISO group).
5. Discussion
In the present study, we evaluated the effects of S. atropatana methanolic extract on cardiac functional parameters and histological features in rat models of ISO-induced MI. The main purpose of MI management is to prevent the harmful aftereffects of the ischemia-reperfusion phase (15). Recently, herbal medicines have gained considerable approval as complementary treatments due to their high efficacy, minimal drug interactions, and low cost (16). Here, we intended to evaluate the potential cardioprotective effects of S. atropatana extract in a rat model of MI.
In this study, MI induction was associated with a reduction in MAP. Although blood pressure may elevate in the initial phases of MI, the reduction of SAP, DAP, and LVSP levels in this study confirmed the creation of an infarcted area and decreased post-MI contractility (17, 18). In this study, the treatment of rat models of MI with S. atropatana extract reversed blood pressure variations and improved blood flow. These results were similar to the effects of Scrophularia frigida, which improved the blood flow and ventricular blood pressure and alleviated arrhythmias (6).
Myocardial infarction is usually accompanied by increased heart rate, which is secondary to sympathoadrenal discharge or ventricular/supraventricular arrhythmias (19). In this study, the heart rate was observed to elevate following MI induction. However, S. atropatana administration reduced the heart rate, which might be related to a reduction in MI-induced dysrhythmias. Similarly, Scrophularia nodosa was reported to decrease arterial pressure in a previous study (20).
Following MI, the stiffness of the left ventricle, along with reduced compliance relaxation, leads to an elevation in LVDEP (21). Elevated LVEDP is accompanied by cardiac hypertrophy, which is associated with an elevated HW/BW ratio following MI (22). A reduction in LVEDP level after S. atropatana treatment suggested a protective role for this plant against cardiac hypertrophy.
During ischemia, the mitochondrial electron transport chain shows a deceleration (i.e., reduced oxidative phosphorylation); however, in the reperfusion phase, oxygen availability accentuates ROS and MDA formation (23). An elevation in MDA level promotes necrosis in cardiomyocytes, which was confirmed by histopathology evaluations (24). In contrast, S. atropatana extract reduced MDA levels and improved edema and necrosis in cardiomyocytes.
Our results also showed a decline in TAS in the MI group. However, exposure to S. atropatana enhanced cardioprotective effects by increasing TAS. ROS-induced expression of the beta-myosin heavy chain in myocytes has been noted to play a role in myocardial hypertrophy (25). According to our findings, S. atropatana can prevent cardiac hypertrophy by reducing LVEDP and augmenting cardiac antioxidant capacity.
Lactate rapidly elevates following MI, which is due to poor reperfusion and oxygen deficiency, suggesting this biomarker as a prognostic factor in MI patients (26, 27). In this study, S. atropatana administration reduced lactate levels in rat models of MI, indicating improved cardiac blood flow and oxygen delivery.
The Scrophularia genus is rich in phenolic compounds that have shown considerable cardioprotective effects in previous studies. Polyphenols are known to have anti-inflammatory and antioxidant effects, leading to a reduction in cardiac hypertrophy (28). Therefore, the cardioprotective effects of S. atropatana may be related to its polyphenolic compounds.
5.1. Conclusions
Scrophularia atropatana improved the cardiac hemodynamic and histopathologic status in rat models of MI and can be suggested as a beneficial complementary medication for patients experiencing ischemia-reperfusion post-MI, acute cardiac dysrhythmias, or coronary artery interventions. Future studies will pave the path to understanding the exact mechanisms of the cardioprotective effects of S. atropatana.