1. Background
Cystic Echinococcosis (CE), commonly known as hydatidosis, is one of the most serious helminthic zoonotic infections affecting both domestic animals and people. Hydatid cysts develop inside the internal organs of intermediate hosts in this disease, which is brought on by the larval form of the parasite Echinococcus granulosus (1, 2). Cystic echinococcosis develops when an intermediate host ingests food or water contaminated with parasite eggs and hydatid cysts. These cysts replace the larval stage of the parasite and can be found in various organs, including the liver, lungs, and other viscera (3). In South America, Asia, Australia, and North Africa, CE is a major public health concern and a common parasitic disease (4, 5). According to estimates, human CE causes the loss of 1 - 3 million DALYs (disability-adjusted life years) and costs the livestock industry up to $2 billion annually. The mortality rate due to CE is about 2 – 4% (3).
The selective treatments for CE are surgery, chemotherapy, and PAIR (Puncture, Aspiration, Injection, Respiration). Since the hydatid cyst is under pressure, rupture, and release of hydatid fluid containing protoscoleces are not unusual during surgery, resulting in the reappearance of CE and secondary infection. Therefore, effective scolicidal drugs are compulsory during surgery (6, 7).
To date, a variety of scolicidal agents have been utilized to inactivate the hydatid cyst content, including formalin, silver nitrate, hypertonic saline, cetrimide, ethyl alcohol, and H2O2 but an appropriate, completely effective, and safe anti-protoscoleces has not been identified. However, these scolicidal chemical agents are accompanied by several side effects, for example, sclerosing cholangitis, liver necrosis, and bile duct fibrosis (8, 9). Therefore, finding new scolicidal agents with fewer side effects, greater efficacy, and lower cost are an urgent need for surgeons (10).
In recent years, herbal medicines and their pure components have been a rich source for new antimicrobial agent development. This is due to the high safety and few side effects of herbal medicines. The structural diversity of plant compounds and the unlimited resources of plants have introduced them as a useful source of new therapeutic compounds (11). In Iran, various plant derivatives, such as Allium noeanum (12), Capparis spinosa (13), Peganum harmala (14), and Ephedra major methanol extracts (15), have been investigated for their effects on hydatid cyst protoscoleces. Alhagi maurorum belongs to the plant family Leguminosae (Papilionaceous) and is commonly known as camel thorn. This plant grows in North Africa, Arabia, Palestine, Syria, Iraq, Turkmenistan, Pakistan, Caucasus, and Central Asia (16). Experimental studies show that the Alhagi maurorum plant has antioxidant, antitumor, anti-diarrheal, and antimicrobial effects. This plant is beneficial against urinary and digestive disorders, rheumatism, hemorrhoids, and liver diseases (17-19).
2. Objectives
This study investigated a hydroalcoholic extract of Alhagi maurorum for preventing secondary hydatid cysts in BALB/c mice.
3. Methods
3.1. Collection of Protoscoleces
Echinococcus granulosus protoscoleces of naturally infected sheep were extracted aseptically from their liver hydatid cysts in two abattoirs in Zahedan and Zabol, Southeast Iran. Initially, hydatid liquid was extracted with a 50-mL syringe and aseptically transferred to a flask for 30 minutes to allow protoscoleces to settle. Protoscoleces were washed thrice in sterile Phosphate-buffered Saline (PBS) enhanced with 40 mg/mL gentamicin after collection. Before inoculation, protoscoleces viability was evaluated using inverted microscopy to observe body movement and vital staining with 0.1% eosin. All samples had viability > 98% at the time of the experiment. Finally, 28 BALB/c mice were administered 2,000 viable protoscoleces suspended in 0.5 mL of sterile PBS intraperitoneally (20).
3.2. Alhagi maurorum Hydroalcoholic Extract Preparation
Leaves and aerial parts of Alhagi maurorum were collected in May 2020 from Zabol in southwestern Iran and identified by the Botanical Section, Zabol University of Medical Sciences (Herbarium number 472). Then, 100 g of air-dried Alhagi maurorum plant was extracted separately using the percolation method and 70% alcohol over 72 hours at room temperature. The extracts were passed through filter paper (Whatman No. 3, Sigma, Germany) to remove plant debris. The extracts were eventually concentrated in a vacuum at 37°C using a rotary evaporator (Heidolph, Germany) and stored at −20°C until use.
3.3. Treatment of Mice
Six- to eight-week-old female BALB/c mice weighing 21 to 23 g were purchased from the Pasteur Institute (Algiers, Algeria). The mice were kept inside an environment with a 12:12 h light/dark cycle, constant temperature of 24 – 30°C, relative humidity of 50 ± 10%, and free access to food and drink. The infected mice were randomly separated into four groups one month after infection. Each mouse in the control group (group 1) received a daily oral injection of PBS, while the second group received a daily oral injection of albendazole (150 mg/kg). Each mouse in the third group received an oral injection of Alhagi maurorum hydroalcoholic extract daily at a dosage of 500 mg/kg, whereas mice in the fourth group received an oral injection of Alhagi maurorum hydroalcoholic extract daily at a dosage of 250 mg/kg. The research was approved by the Local Ethics Committee of Zabol University of Medical Sciences in accordance with the ethics guidelines of "Principles of Laboratory Animal Care." After 6 weeks of treatment, the size (mm), weight (g), number, and fertility of hydatid cysts were measured after the euthanasia and necropsy of all mice.
3.4. Viability Test
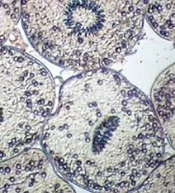
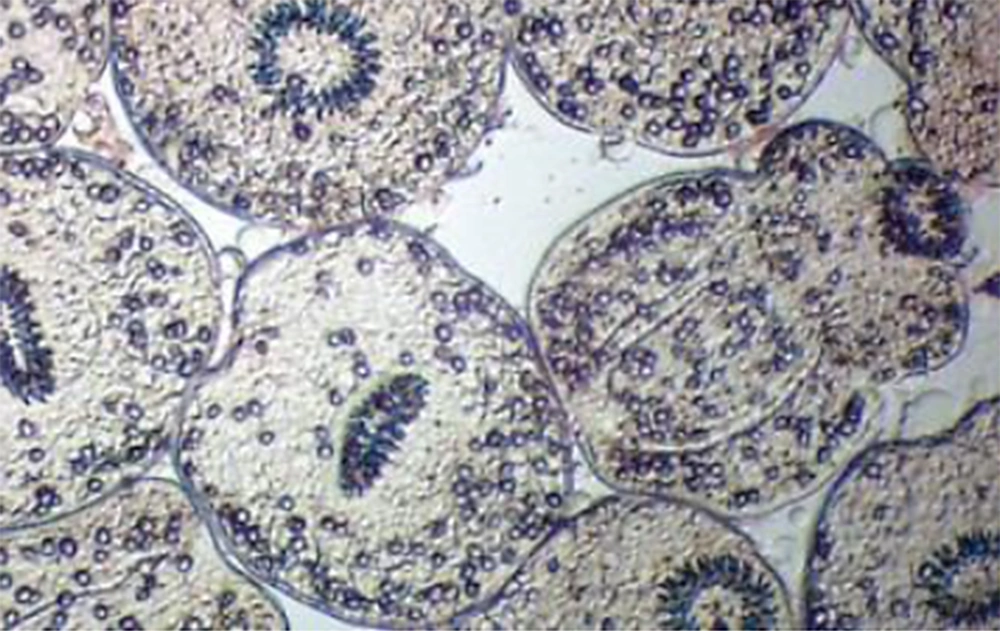
The viability of hydatid cyst protoscoleces was determined using an eosin omission examination.
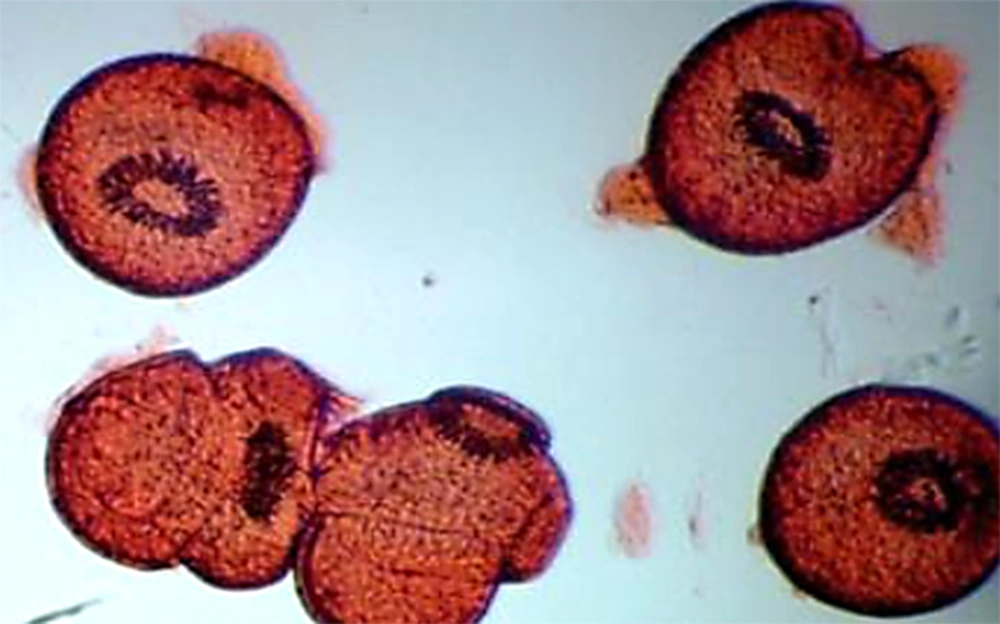
After contact with 0.1% eosin solution (1 g of eosin powder in 1000 mL distilled water), live protoscoleces stayed neutral and exhibited characteristic muscular and flame cell activity (Figure 1), while dead protoscoleces immersed in eosin became red (Figure 2) (21).
3.5. Assessment of Cyst Development
Three months after infection, all mice were euthanized. The peritoneal cavity was opened after the necropsy, and the internal organs were checked for hydatid cysts. The hydatid cysts were carefully removed. The size (mm), weight (g), number, and fertility percentage were measured. A scaled ruler measured the cyst size, and a digital scale indicated the cyst weight (Kern & Sohn GmbH, Balingen, Germany). To evaluate the fertility of the cysts, the cyst fluid was aseptically extracted using a syringe. The extracted fluid was then collected individually in a graduated glass container for each sample. Clear fluid without protoscoleces indicated an infertile cyst.
3.6. Statistical Analysis
Data were analyzed using SPSS 20.0 (SPSS Inc., Chicago, IL, USA). Differences between groups were analyzed by one-way Analysis of Variance (ANOVA), followed by the Tukey test, and P < 0.05 was considered statistically significant.
4. Results
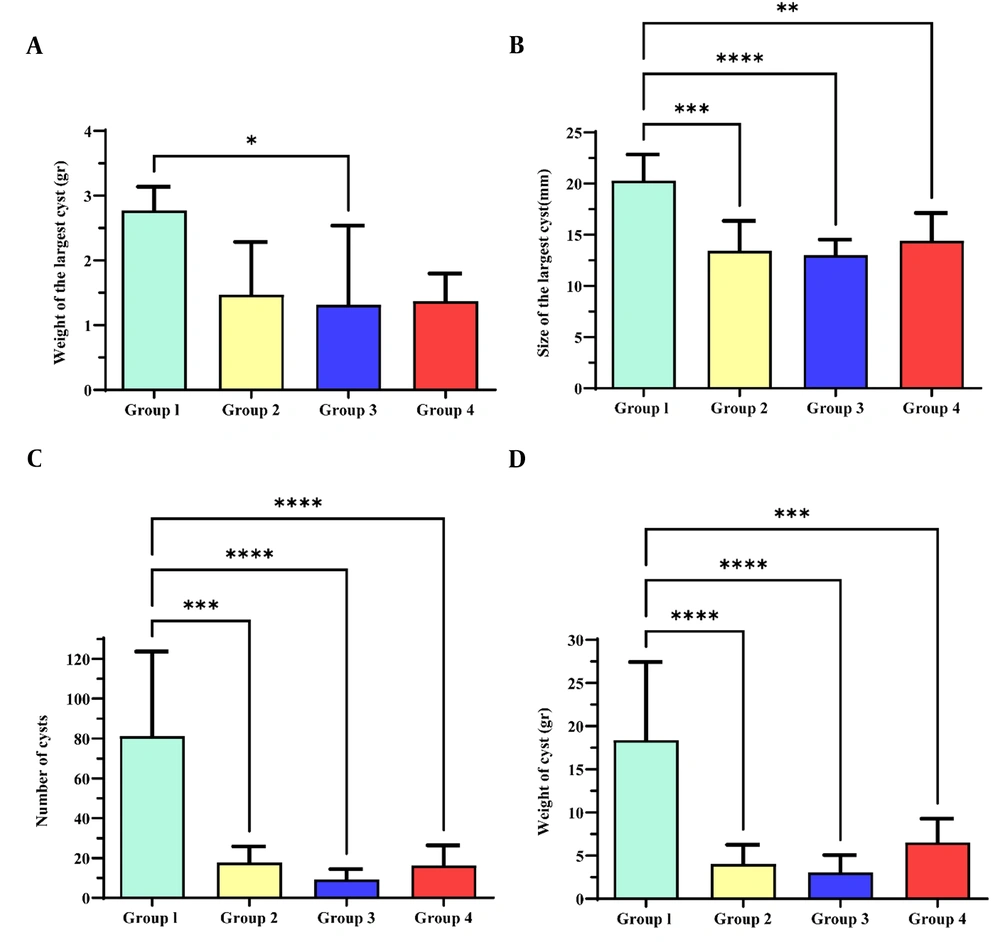
The weight (g), size (mm), and number of cysts recovered from each mouse were compared between the groups in the present study. Parasitological parameters (number, size, and weight) were significantly lower in 500 mg/kg Alhagi maurorum hydroalcoholic extract (group 3), 250 mg/kg Alhagi maurorum hydroalcoholic extract (group 4), and 150 mg/kg albendazole (group 2) than in the control group (group 1) (P < 0.05). Moreover, there were no statistically significant differences in the parasitological parameters of hydatid cysts between treatment groups 1, 2, and 3 (P > 0.05). The fertility percentage of hydatid cysts did not significantly differ between the groups (P > 0.05, Table 1). A significant difference in the weight of the largest cysts was found between group 3 and the control group (P < 0.05, Figure 3A). Furthermore, the size of the largest cysts (mm), the number, and the weight of the cysts (g) showed a significant difference between the group control and groups 2, 3, and 4 (P < 0.05, Figure 3B, C, and D).
| Group | Total Number of Cysts | Number of Fertile Cysts | Fertility Percentage (%) |
|---|---|---|---|
| Control | 568 | 500 | 89 |
| Albendazole, 150 mg/kg | 124 | 106 | 86 |
| Alhagi maurorum extract, 500 mg/kg | 65 | 54 | 84 |
| Alhagi maurorum extract, 250 mg/kg | 113 | 88 | 78 |
Anti-protoscoleces effect of Alhagi maurorum hydroalcoholic extract on parasitological parameters (weight, size, and number of cysts) in BALB/c mice in different treatment groups. The difference in the weight of the largest cyst (g) between group 3 and the control group was significant (P < 0.05, Figure 3A). The size of the largest cysts (mm) showed a significant difference between the control group and groups 2, 3, and 4 (P < 0.05, Figure 3B). The number of cysts showed a significant difference between the three treatment groups and the control group (P < 0.05, Figure 3C). The weight of cysts (g) was higher in the control group than in the other groups (P < 0.05, Figure 3D). *P-value < 0.01; ** P-value < 0.001 *** P-value < 0.0001; **** P-value < 0.00001
5. Discussion
This study showed that treating hydatid cysts with Alhagi maurorum hydroalcoholic extract is effective in mice. The plant extracts and their purified natural crops provide infinite possibilities for new therapeutic discoveries (22). A suitable scolicidal agent should have minimal side effects, be affordable and accessible, demonstrate high efficacy, and be safe and stable in hydatid fluid. So far, an ideal substance with the mentioned properties has not been identified (23). For thousands of years, Alhagi maurorum has been used as herbal medicine for various medical purposes. Furthermore, previous studies have shown the anti-fungal effect of Alhagi maurorum extract against Alternari alternata, Candida albicans, and Cladosporium cladosporoides (24). Studies on the antibacterial activity of aqueous, ethanol, methanol, and acetone extracts of camel thorn (Alhagi pseudalhagi) against gram-positive and gram-negative bacteria revealed that Alhagi pseudalhagi extract has antibacterial effects at high concentrations (25). Research on Alhagi maurorum indicates sterol, fatty acid, alkaloid, and flavonoid content (26, 27). Plants containing flavonoids have anti-protoscoleces effects (12, 13). In addition, previous studies have shown that herbal extracts can induce the apoptotic pathway. Because apoptosis is a process of cellular self-destruction, it provides a convenient way to eliminate parasitic agents, such as Echinococcus protoscoleces (14). Therefore, this study, for the first time, investigated the effect of the Alhagi maurorum hydroalcoholic extract against secondary hydatid cysts in BALB/c mice. Our study did not precisely determine which compound of the Alhagi maurorum plant has an anti-protoscoleces effect. Further studies are needed to accurately assess the impact of this plant on hydatid cysts.
5.1. Conclusions
A hydroalcoholic extract of Alhagi maurorum exhibited an anti-protoscoleces effect in BALB/c mice. However, cellular and molecular research should be carried out to gain a deeper understanding of the various effects of this extract for the treatment of hydatid cysts. Future studies should investigate the effects of Alhagi maurorum extract alone or combined with albendazole on the changes in liver enzymes and blood factors in laboratory animals.