1. Background
As a chronic metabolic disease, diabetes is closely associated with cardiovascular disease and premature death. According to the International Diabetes Federation (2006), the number of individuals with diabetes is expected to reach 380 million by 2025. The standard treatment of diabetes often proves ineffective in many patients due to complications and non-compliance. The use of herbal medicines or medicinal plants as a form of treatment has gained widespread acceptance and prevalence in today's world. These products have a safe profile and a simple prescription process, making it necessary to study their blood sugar and lipid-lowering properties, as well as other properties, in patients with type 2 diabetes (1).
Nowadays, using natural compounds as an alternative or supplement to treatment has been widely considered, and among these herbal compounds is NS. On the other hand, this plant is used to prevent and treat diseases such as asthma, diarrhea, and dyslipidemia (2, 3).
A systematic review was conducted to investigate the effects of NS on metabolic parameters in diabetic patients, which found that NS could affect dyslipidemia and increase blood sugar by various potential mechanisms. These effects include antioxidant properties, effects on insulin secretion and glucose uptake, gluconeogenesis, and modulation of gene expression. Finally, NS can improve the glycemic status and fat profile in diabetic patients (4).
A recent comprehensive review study found that thymoquinone, the major phenolic terpene found in NS, could alleviate and improve the vascular complications of diabetes. NS exerts these effects through mechanisms such as reducing hyperglycemia, improving hyperlipidemia, anti-inflammatory effects, anti-platelet aggregation and antithrombotic effects, antioxidant effects, as well as regulating the expression of genes involved in vascular endothelial disorders and reducing gene expression and secretion of some cytokines such as monocyte chemoattractant protein 1 (MCP-1), interleukin-1 beta, tumor necrosing factor alpha (TNF-α) and cyclooxygenase 2 (Cox-2) (5).
A study was conducted in Saudi Arabia to evaluate the effect of NS on fat profile in type 2 diabetic patients. This study showed that consumption of NS at a rate of 2 g per day could significantly reduce the level of total cholesterol, triglycerides, and low-density lipoprotein (LDL) cholesterol and increase the high-density lipoproteins (HDL) cholesterol level compared to the control group. Therefore, this herb treats dyslipidemia in diabetic patients and is a natural protective factor against cardiovascular disease in patients (6).
Islam et al., in a review article, concluded that NS had improved health in some non-clinical and clinical studies due to strong evidence and may be used as one of the best sources for modern herbal medicine (7). Hamdan et al., at the University of Kuala Lumpur, conducted a systematic review to provide comprehensive information on the effects of NS on laboratory parameters of hyperglycemia in diabetic patients. They showed that NS could be used as an adjunct to oral hypoglycemic drugs in the control of diabetic patients by significantly lowering fasting blood sugar (FBS), 2-hour postprandial blood sugar (2hppBS), and glycosylated hemoglobin (HbA1c), and insulin resistance (8).
2. Objectives
Based on the texts, and scientific and research documents, the effects of NS medicinal plants on glucose and fat parameters in diabetic patients are not yet definite, and the need for continuous studies in this field is still felt. Therefore, in this study, we intend to investigate the effects of NS on these laboratory parameters of glucose and fat in type 2 diabetic patients in the form of a clinical trial.
3. Methods
A double-blind, randomized, controlled clinical trial (RCT) was conducted in which 80 patients diagnosed with type 2 diabetes were randomly assigned to either the control or intervention group. The research environment was the Diabetes Clinic located in Imam Khomeini Hospital in Khomein City, and the samples of this study were selected from the population of diabetic patients referred to this clinic by considering the inclusion criteria. Inclusion criteria included: Diagnosis of type 2 diabetes according to ADA (American Diabetes Association) criteria, being treated with oral hypoglycemic agents (OHA), no change in the dose of blood lipid-lowering drugs during the last eight weeks, declaration of readiness for participation in the study and fill out the informed consent form, age over 18 years old and under 70 years old, at least six months have passed since the diagnosis of their diabetes, not suffering from mental illness or incurable disease and not taking any medicine in this field (HbA1c ≥ 7%), the patient condition is abnormal in terms of blood lipid profile. Exclusion criteria include the existence of complications associated with cardiovascular disease (IHD, HF, cardiac arrhythmia), kidney disorder, any liver disease, unwanted side effects after the use of herbal medicine, unwillingness to cooperate in any stages of the research, not taking the desired herbal medicine according to the instructions, i.e., using less than 90% of the provided herbal capsules, having secondary causes of hyperlipidemia such as chronic renal failure, endocrine disorders, triglyceride (TG) above 400, changes in the patient's standard medications during the study.
The patients initially completed the informed consent form. Then the demographic characteristics questionnaire, which included questions about age, gender, marital status, duration of diabetes, type of medication, and other relevant factors, was completed. The patients were then referred to the hospital laboratory, where blood samples were obtained to check the baseline profile of blood lipids, including cholesterol, triglycerides, LDL, and HDL, and also the baseline level of glycemic indicators, including FBS, 2hppBS and HbA1C.
After this step, the random allocation or randomization method was used to match the experimental and control groups. In this way, patients were randomly assigned to one of the intervention and control groups by block randomization with quadruple blocks. Patients and therapists were unaware of allocating study participants between the control and intervention groups, so the study was double-blind. Then, the patients in the experimental group received 500 mg of ground NS for eight weeks (1 capsule per day). During this period, the patients in the control group received one placebo capsule of the same composition and shape. All patients completed the study, and none experienced any side effects from taking this herbal capsule. At the end of 8 weeks (approximately two months), blood samples were taken from patients again, and serum lipids and blood glucose levels were re-evaluated. Serum lipid and blood glucose levels were also measured in the laboratory using an auto analyzer. The blood sample prepared by the patient was poured into a clot tube and placed at 37°C until the blood was completely clotted. Then the tube was centrifuged (at 3000 rpm for 10 minutes), and the serum was tested once removed.
The ethical points of the design were carefully complied with. Written consent was filled out by patients. Any patient could leave the study at any time by informing the researcher. The confidentiality of all information obtained from the participants was guaranteed, and for this purpose, their names and surnames were not written on the questionnaire sheets. The researcher adhered to the provisions of the Helsinki Declaration and the code of ethics approved by the Ministry of Health at all stages. This research was submitted to www.irct.ir as a clinical trial design with trial registration code (IRCT20170501033751N3). The research project number of the Vice Chancellor for Research of Khomein School of Medical Sciences (KUMS) was 98000012. Statistical analysis was conducted by SPSS software (version 21, IBM Corporation). Quantitative data were expressed as means ± SD and qualitative as frequency. The level of significance was P < 0.05. The normal distribution of the variables was checked by Kolmogorov-Smirnov Test. The Repeated Measure ANOVA test made comparison between the groups.
4. Results
The mean age of participants was 55.12 ± 6.47 and 53.82 ± 5.67 in the control and experimental groups, respectively. Regarding gender frequency, 30 were male (37.5%), 50 were female (62.5%), and marital status, 80 were married (100%). The primary education level had the highest frequency among samples, with 27 people (33.8%), and in terms of jobs, 48 people were housewives (60%). The demographic characteristics of the two groups did not show any statistically significant difference, which indicated the similarity (Table 1).
| Variable | Control Group | Test Group | P-Value a |
|---|---|---|---|
| Age (y) | 55.12 ± 6.47 | 53.82 ± 5.67 | 0.34 b |
| BMI | 26.74 ± 4.03 | 27.93 ± 3.49 | 0.16 b |
| Duration of diabetes | 5.22 ± 2.40 | 4.17 ± 2.68 | 0.06 b |
| Gender | 0.35 c | ||
| Male | 13 (32.5) | 17 (42.5) | |
| Female | 27 (67.5) | 23 (57.5) | |
| marital status | |||
| Single | 0 (0) | 0 (0) | |
| Married | 40 (100) | 40 (100) | |
| Widowed or divorced | 0 (0) | 0 (0) | |
| Education | 0.27 c | ||
| illiterate | 10 (25) | 12 (30) | |
| Primary | 17 (42.5) | 10 (25) | |
| Secondary | 7 (17.5) | 5 (12.5) | |
| Diploma | 4 (10) | 10 (25) | |
| University | 2 (5) | 3 (7.5) | |
| Employment status | 0.43 c | ||
| Employee | 0 (0) | 1 (2.5) | |
| Unemployed | 10 (25) | 9 (22.5) | |
| Housewife | 26 (65) | 22 (55) | |
| Retired | 4 (10) | 6 (15) | |
| Freelancer | 0 (0) | 2 (5) |
a It is statistically significant at 0.05.
bt-Test
c Chi-square test
Mixed models (repeated measure + ANOVA) were used for advanced analysis. The result of these analyses is shown in Tables 2 and 3.
| Variable | Control Group Mean (95% CI) | Intervention Group Mean (95% CI) | F-Statistics | P-Value a |
|---|---|---|---|---|
| FBS | 161.61 (147.26 - 175.96) | 152.53 (138.18 - 166.88) | 3294.22 | 0.376 |
| PPG | 230.65 (206.03 - 255.28) | 226.08 (201.46 - 250.71) | 0.068 | 0.795 |
| HbA1C | 6.98 (6.63 - 7.32) | 6.81 (6.46 - 7.15) | 0.483 | 0.489 |
| TG | 188.56 (164.68 - 212.44) | 184.16 (160.28 - 208.04) | 0.067 | 0.796 |
| Chol | 162.14 (151.31 - 172.97) | 171.02 (160.19 - 181.85) | 1.33 | 0.252 |
| LDL | 90.88 (84.11 - 97.65) | 95.71 (88.94 - 102.48) | 1.006 | 0.319 |
| HDL | 41.65 (39.11 - 44.18) | 40.18 (37.64 - 42.71) | 0.665 | 0.417 |
Abbreviations: Chol, cholesterol; FBS, fasting blood sugar; HbA1C, glycosylated hemoglobin; HDL, high-density lipoproteins; LDL, low-density lipoprotein; PPG, postprandial glucose; TG, triglyceride.
a It is statistically significant at 0.05.
| Variable | (T1) First-time Measurement Mean (95% CI) | (T2) Second-time Measurement Mean (95% CI) | F-Statistics | P-Value |
|---|---|---|---|---|
| FBS | 156.97 (147.43 - 166.51) | 157.17 (145.05 - 169.30) | 0.002 | 0.960 |
| PPG | 222.74 (204.27 - 241.21) | 234.000 (214.81 - 253.18) | 2.44 | 0.122 |
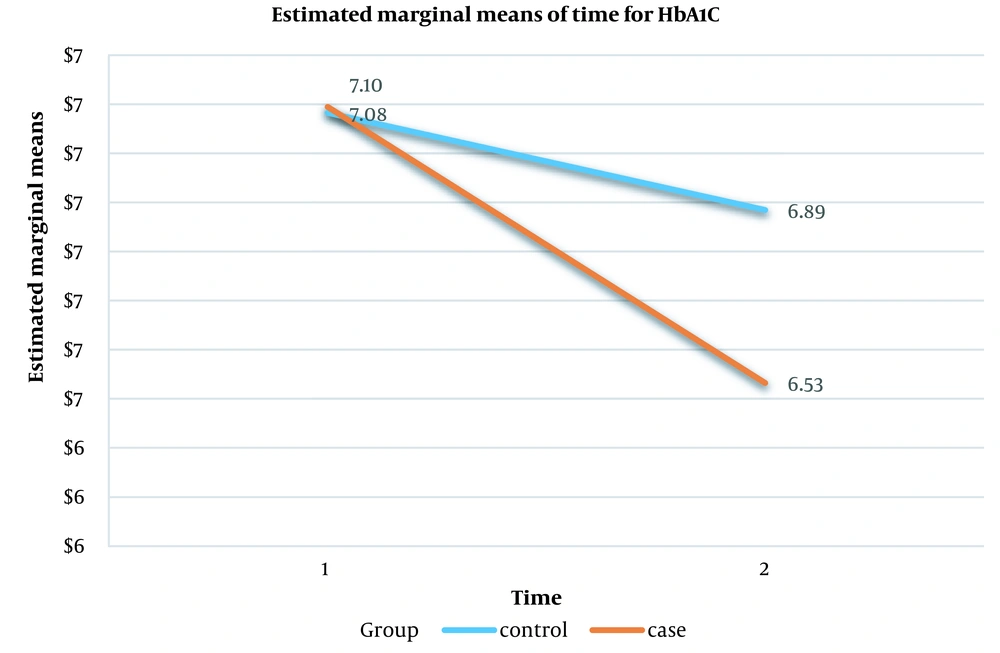
| HbA1C | 7.08 (6.83 - 7.34) | 6.70 (6.43 - 6.98) | 15.89 | 0.0001 a |
| TG | 193.73 (174.73 - 212.74) | 178.98 (160.94 - 197.03) | 3.703 | 0.058 |
| Chol | 174.28 (165.71 - 182.86) | 158.88 (150.24 - 167.51) | 15.27 | 0.0001 a |
| LDL | 95.57 (89.86 - 101.28) | 91.02 (85.94 - 96.10) | 3.25 | 0.075 |
| HDL | 43.40 (41.07 - 45.72) | 38.43 (36.61 - 40.25) | 21.30 | 0.0001 a |
Abbreviations: Chol, cholesterol; FBS, fasting blood sugar; HbA1C, glycosylated hemoglobin; HDL, high-density lipoproteins; LDL, low-density lipoprotein; PPG, postprandial glucose; TG, triglyceride.
a It is statistically significant.
Analysis of between-subject effects (group) using repeated measure ANOVA test showed that none of the studied variables had statistically significant differences between two (group variable) groups (Table 2). This analysis tells us that these differences were not statistically significant despite the apparent difference between the measurements made on the variables both before and after the intervention between the control and intervention groups.
In examining the within-subject effects (time) using repeated measures, the ANOVA test showed that the variables HbA1c, Chol, and HDL had statistically significant differences (P = 0.0001) (Table 3). This analysis indicates a significant difference in these three variables' time-variable measurements taken before and after the intervention period.
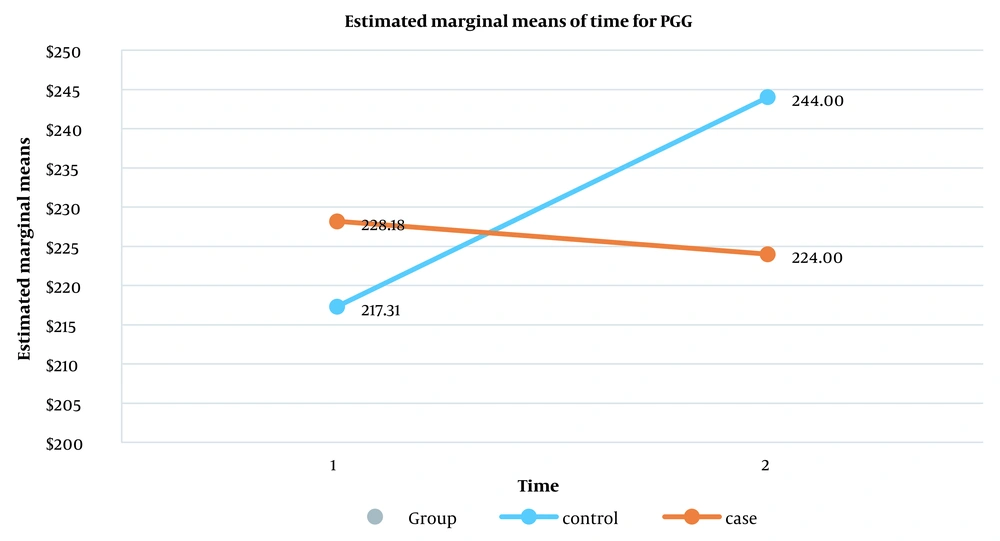
Finally, in the evaluation of the interaction effect between the two variables, i.e., group and time, according to the results of the intragroup test, it was found that the variables FBS, TG, Chol, LDL, and HD were not statistically significant, meaning that no interaction effect was observed between the control and intervention groups before and after the interventions. However, this interaction was statistically significant for the postprandial glucose (PPG) variable (P = 0.035) (Table 4). However, although a 0.36 % difference had been seen for the absolute value of HbA1c between the two groups after interventions, the interaction effect for the HbA1c variable was marginally not statistically significant (P = 0.06) (Table 4). Figure 1 shows the interaction effect between the control and intervention groups in the pre-and post-intervention measurements for the PPG variable. Figure 2 shows the interaction effect between the control and intervention groups in the pre-and post-intervention measurements for the HbA1c variable.
| Type of Variable | Degree of Freedom (df) | Mean (MS) | F -Statistics | P-Value |
|---|---|---|---|---|
| FBS | 1 | 1134.22 | 1.75 | 0.189 |
| PPG | 1 | 9526.48 | 4.59 | 0.035 a |
| HbA1C | 1 | 1.33 | 3.65 | 0.06 |
| TG | 1 | 342.22 | 0.146 | 0.704 |
| Chol | 1 | 177.45 | 0.286 | 0.595 |
| LDL | 1 | 783.22 | 3.082 | 0.083 |
| HDL | 1 | 92.11 | 1.98 | 0.163 |
Abbreviations: Chol, cholesterol; FBS, fasting blood sugar; HbA1C, glycosylated hemoglobin; HDL, high-density lipoproteins; LDL, low-density lipoprotein; PPG, postprandial glucose; TG, triglyceride.
a It is statistically significant at 0.05.
5. Discussion
The results showed no statistically significant difference between the values of each group before and after the interventions were administered. On the contrary, when comparing groups in terms of time measurements, only reductions in HbA1c, total cholesterol (TC), and HDL were statistically significant. Finally, there is an interaction effect only between the PPG and HbA1c variables.
In a study, Sabzghabaee et al. examined 88 individuals over 18 with total cholesterol > 200 mg/dL, which showed significant reductions in TC, low-density lipoprotein (LDL), and TG, and this reduction was more significant for TG concentration. NS had no beneficial effects on FBS and HDL, and the results were somewhat consistent with our study (9).
Bamosa et al. conducted a study to examine the impact of NS on blood sugar control in patients with type 2 diabetes. The study concluded that a daily intake of 2 g of NS significantly reduced FBG, 2hPG, and HbA1C without causing any significant change in body weight. At the end of 12 weeks of treatment, HbA1C decreased by %1.52 (P < 0.0001). Insulin resistance decreased significantly (P < 0.01), while beta cell function increased during 12 weeks of treatment (P < 0.02). The use of NS at a dose of 1 g per day resulted in an improvement in all measured parameters, but the improvement was not statistically significant compared to the baseline. However, no further increase in beneficial response was observed with two and three grams per day. The three doses used in the study did not have a negative effect on kidney and liver function in diabetic patients during the study period. The results of these studies were not completely consistent with the results of our study (10).
A review study by Qidwai and Ashfaq examined the effect of NS supplements on the lipid profile of patients with diabetes. The results were based on the fact that most human and animal experiments performed on humans and animals with diabetes or metabolic syndrome showed weight loss and improved serum fat levels, including reduced total fats, triglycerides, and LDL levels. However, the increase in HDL levels showed questionable results. NS and its various drugs can be adjuncts to fat-lowering drugs to control fats. However, its role as a major therapeutic agent cannot be recommended, and further analysis using standard preparations is recommended with careful consideration of the methods' shortcomings. The results of the above study were in line with our results (11).
Daryabeygi-Khotbehsara et al. in a systematic review and meta-analysis concluded that NS could lower FBS, HbA1c, TC, and LDL levels in diabetic patients. However, the overall effects on TG and HDL were insignificant. In general, the results of this meta-analysis were consistent with the results of the present study in some parameters (HbAlC and TC) and inconsistent in some other parameters (12).
The results of a nonrandomized clinical trial conducted by Badar et al. in Saudi Arabia over one year, which examined the effect of NS on fat profile, blood pressure, and heart rate in type 2 diabetic patients, showed that during the first three months, the differences between the two control and intervention groups were not significant for TG and TC for up to three months, it was significant for LDL-C and were not significant for HDL-C. The results of this study were consistent with our study in terms of HDL-C, TC, and TG indices and were not consistent for LDL-C (13).
In a systematic review conducted to investigate the complementary effect of NS on blood parameters and anthropometric indices, it was found that FBS was significantly reduced by N. Sativa in most studies but not in three studies. It was found that in all trials, NS could significantly reduce the amount of HbAlC. TG was decreased in 7 studies and not significantly decreased in 10 studies, TC was significantly decreased in 10 studies and not significantly decreased in 4 studies, LDL was significantly decreased in 11 studies and not significantly decreased in 3 studies, HDL significantly increased in 6 studies and not significantly increased in 10 studies (14). The results of this systematic review, of course, were somewhat similar to the present study but indicated a significant discrepancy in the effects of NS on blood sugar and blood lipid parameters and showed that its beneficial effects were still not definite and more trials should be conducted in this field.
A systematic review by Amiza Hamdan in 2019 on the effects of NS on type 2 diabetic patients was performed, and finally, seven related articles were reviewed. The results of this study showed that in all initial studies, NS seed could have significantly reduced effects on blood levels of fasting blood sugar (FBG), 2hPPG, and HbA1c reducing insulin resistance and increasing its level in diabetic patients (8).
A systematic review entitled "Effects of Nigella sativa on glycemic control, lipid profiles, and biomarkers of inflammatory and oxidative stress: A systematic review and meta-analysis of randomized controlled clinical trials" was done in 2020. This meta-analysis demonstrated the beneficial effects of NS on fasting glucose, HbA1c, triglycerides, total cholesterol, very low-density lipoprotein (VLDL), and LDL-cholesterol levels. These results are similar for HbA1c and total cholesterol with our study but inconsistent for HDL cholesterol (15).
Our study had limitations, including a smaller sample size and a relatively short intervention period (8 weeks). Although the relatively low dosage of NS capsules (500 mg) used in our research may interpret as a limitation, interestingly, at the same time, it would be as a strong point because there are very rare RCTs that implement low doses of NS in their studies. It is recommended to conduct future studies with a larger sample size, longer course, and variant drug dosages.
5.1. Conclusions
The results of our study showed that consumption of NS in diabetic patients could reduce some fat profiles and also some glycemic ones.