1. Background
The peptic ulcer is a common gastrointestinal disorder of gastric mucosa that is generally induced in the stomach or duodenum. The fundamental cause of ulceration is an imbalance between gastric offensive factors (e.g., hydrochloric acid, pepsin, refluxed bile, leukotrienes, or reactive oxygen species (ROS)) and defensive factor(s) (e.g., mucosa-bicarbonate barrier, surface-active phospholipids, prostaglandins (PGS), mucosal blood flow, cellular regeneration and migration, non-enzymatic and enzymatic antioxidants, or some growth factors) (1). Neutrophils generate superoxide radical anion (O2.-) that pertains to a group of reactive species. Superoxide radical anion interferes with cellular lipids and results in lipid peroxidase generation. This reaction produces malondialdehyde (MDA) and 4-hydroxynoneal (4-HNE) (2).
Nitric oxide (NO) is a strong mediator of mucosal resistance. Moreover, the role of NO is elucidated by its vasodilation effect and for this reason, its local release is essential for the retention of gastric mucosal blood flow (3).
The first purpose of treating gastric disorders is regulating acid secretion using antacids, H2 receptor blockers (e.g., ranitidine and famotidine), anticholinergics drugs (e.g., pirenzapine), and proton pump inhibitors (e.g., omeprazole) (4). However, considering several side effects and limited efficacy of modern drugs, it is necessary to find improved alternatives for gastric disorder treatment (5, 6). Natural products such as plants have been considered the raw materials for drug synthesis and constitute a considerable source of new therapeutic agents (7). Plant extracts are the essential sources of new medicine and have been efficacious in the peptic ulcer treatment (8).
Research in medicinal plants demonstrates that numerous plants have noteworthy medicinal potential. For instance, Pistacia atlantica is widely used for treating abdominal discomfort/pain and dyspepsia and also has antimicrobial, analgesic, and diuretic effects (9-11). Pistacia atlantica oil is also known as a stable and antioxidant material (12, 13). The antioxidant activity of essential oil of Pistacia atlantica was shown previously (14).
2. Methods
2.1. Preparation of Pistacia atlantica Hydroalcoholic Extract
Pistacia atlantica fruits were collected from the mountains of Yasuj, Iran, during autumn 2016. The voucher specimen (No., A1200910FP) is deposited at the herbarium of the Faculty of Pharmacy, Ahvaz Jundishapur University of Medical Sciences (AJUMS). The fruits were minced and 300 g of its powder was soaked in ethanol 70% (v/v) for 72 hours at the room temperature. The extract was filtered by filter papers and concentrated by a rotary evaporator under reduced pressure (40°C). Subsequently, the extract was dried in an oven at 37°C (to give a yield of 3%) and used as a powder.
2.2. Animals
42 adult male Wistar rats weighing 200 - 250 g were purchased from the animal house of AJUMS (Ahvaz, Iran). They were placed in standard single cages and kept under standardized conditions of temperature (22 ± 4°C) and humidity (55°C). They were fed a normal laboratory diet. The animals were fasted for 24 hours before the experiments and allowed water ad libitum. Furthermore, the institutional ethics committee of the medical school, AJUMS, approved all procedures adopted in this study (approval number: B-9531).
2.3. Induction of Acute Gastric Lesion by Ethanol
Gastric ulcer was induced in overnight fasted rats that were kept in mesh cages to prevent coprophagia but were free to access water (15) by administrating 100% ethanol orally (1 mL/200 g). One hour after ethanol application, the animals were anesthetized with ketamine (50 mg/kg) and xylazine (10 mg/kg). The stomachs were dissected and opened along the greater curvature to determine the number and the length of gastric lesions (16, 17).
Part of the stomach was excised and the sample was subsequently homogenized in cold potassium phosphate buffer (0.05 M, pH 7.4). The homogenate was centrifuged at 5000 rpm for l0 minutes. The obtained clear supernatant was stored at -80°C prior to analysis for subsequent measurement of malondialdehyde and nitric oxide (18).
2.4. Experimental Design
Experimental groups were orally treated with different doses of Pistacia atlantica hydroalcoholic extract (100, 200, and 400 mg/kg). Normal saline was orally administered to the ulcer control group (5 mL/kg). In addition, one hour before receiving ethanol (5 mL/kg), the positive control group received ranitidine (50 mg/kg) and the negative control group received normal saline (5 mL/kg). Finally, the last group received Pistacia atlantica extract (400 mg/kg) without ethanol administration (17, 19). Six animals in each group were used for the experiments.
2.5. Detection of Malondialdehyde in Rat Gastric Mucosa
The MDA formation was determined as a measure of peroxidation. The MDA level measurement was done via a commercial chemical colorimetric assay kit according to the manufacturer’s protocol (MDA assay kit; ZellBio GmbH, Ulm, Germany). It utilized the MDA-TBA adduct formed by the reaction of MDA with TBA (thiobarbituric acid) under elevated temperature. MDA is measured in acidic media and elevated temperature (90°C - 100°C) calorimetrically at 535 nm. This method can determine MDA with 0.1 µM sensitivity.
2.6. Detection of NO in Rat Gastric Mucosa
The NO level measurement was done by a commercial chemical colorimetric assay kit according to the manufacturer’s protocol (NO assay kit; ZellBio GmbH, Ulm, Germany) (CAT No. 2B-NO-96A, V406). It is employed to quantitatively assess nitric oxide based on its metabolites (nitrite/nitrate) and Griess reaction.
2.7. Histopathology
For histopathological assessment, the stomachs from all groups were fixed in 10% formalin solution and embedded in paraffin. Sections were then deparaffinized and stained with hematoxylin and eosin.
2.8. Statistical Analysis
The data are presented as the mean ± SD. The statistical significance of differences between the groups was determined by a one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test for multiple comparisons. A probability value of P < 0.05 was assumed to indicate the statistical significance.
3. Results
3.1. Effect of Pistacia atlantica on Ethanol-Induced Gastric Lesions
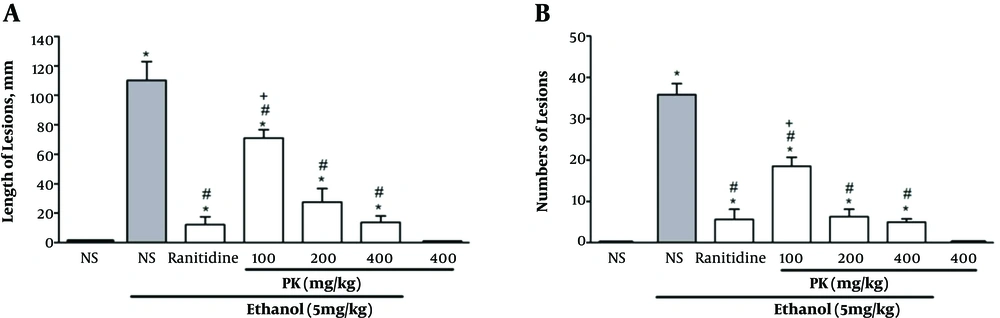
Oral pretreatment with Pistacia atlantica extracts caused a significant reduction (P < 0.05) in the length (Figure 1 and 2A) and the number of gastric lesions (Figure 2B) in a dose-depended manner. Among the tested doses, the highest dose (400 mg/kg) showed the maximum inhibition of the length and number of gastric lesions. The same gastroprotective activity was observed for Pistacia atlantica extract (at 400 and 200 mg/kg doses) and ranitidine as the reference drug (Figure 2). There was no difference between the gastroprotective activity of doses of 200 mg/kg and 400 mg/kg.
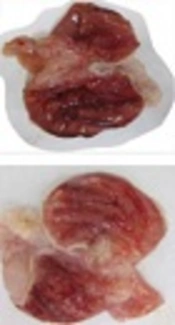
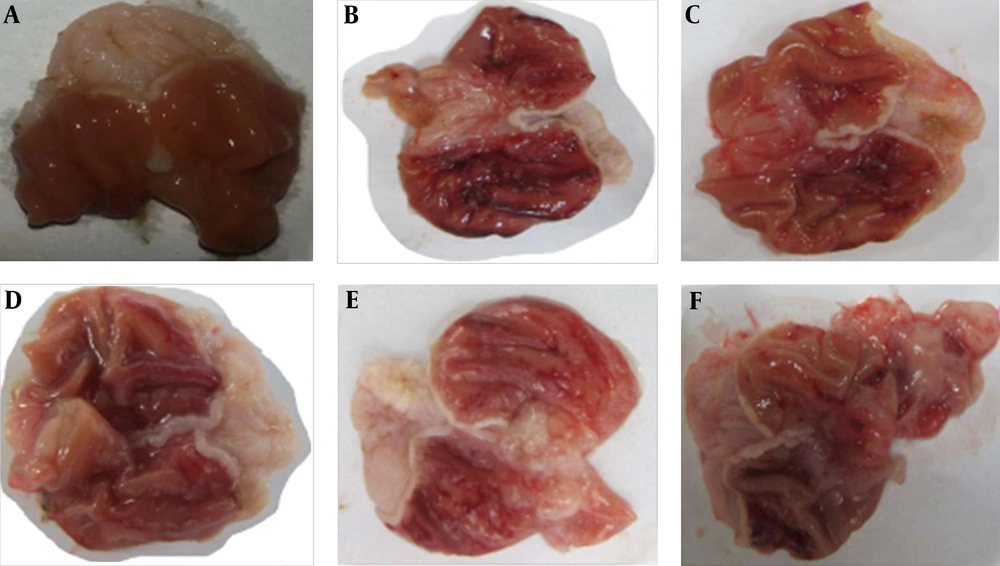
Gross evaluation of stomach from groups of A, normal saline; B, normal saline + ethanol; C, ethanol + Pistacia atlantica (100 mg/kg); D, ethanol + Pistacia atlantica (200 mg/kg); E, ethanol + Pistacia atlantica (400 mg/kg); and F, ethanol + ranitidine (50 mg/kg). The results showed that treatment with Pistacia atlantica at doses of 200 and 400 mg/kg and ranitidine (50 mg/kg) decreased gastric ulcers.
Effect of Pistacia atlantica extracts on A, length and B, numbers of gastric ulcers (in mm) induced by ethanol. Values are expressed as means ± SEM; n = 6. * Significant when compared with respective normal saline (NS) (P ≤ 0.05); # significant when compared with respective normal saline and ethanol (P ≤ 0.05); + significant when compared with respective ranitidine (P ≤ 0.05).
3.2. Stomach MDA
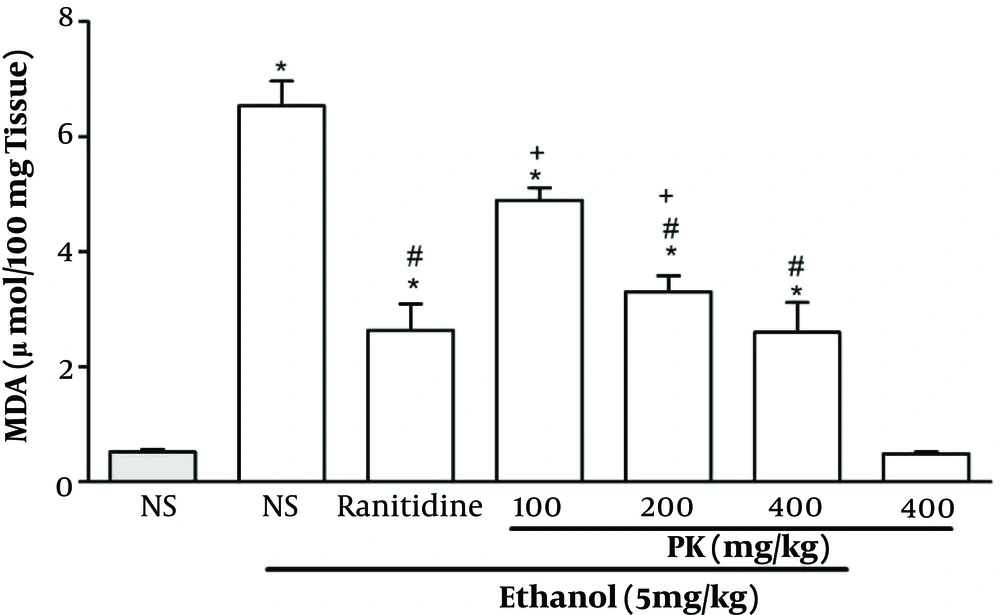
As compared with normal rats, the ethanol-treated group displayed a higher stomach MDA level (P < 0.05) (Figure 3). The administration of Pistacia atlantica extract at doses of 200 and 400 mg/kg exhibited a significant reduction in the MDA level (P < 0.05). There was no significant difference between the effects of doses of 200 and 400 mg/kg.
Effect of Pistacia atlantica extracts on the stomach MDA levels. Values are expressed as means ± SEM; n = 6. * Significant when compared with respective normal saline (NS) (P ≤ 0.05); # significant when compared with respective normal saline and ethanol (P ≤ 0.05); + significant when compared with respective ranitidine (P ≤ 0.05).
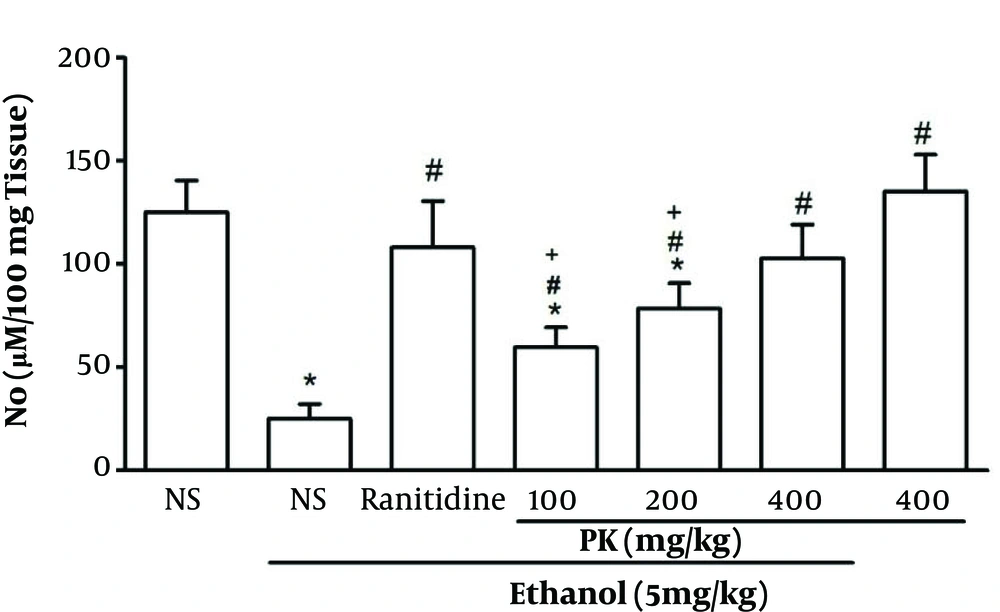
3.3. Stomach NO
The ethanol treated group displayed a lower stomach NO level than all groups, hence leading to oxidative stress (P < 0.05) (Figure 4). The administration of Pistacia atlantica extract at doses of 200 and 400 mg/kg exhibited a comparable increase in the level of NO in a dose-depended manner (P < 0.05) (Figure 4). There was no significant difference between the doses of 200 and 400 mg/kg.
Effect of Pistacia atlantica extract on the stomach NO levels. Values are expressed as means ± SEM; n = 6. * Significant when compared with respective normal saline (NS) (P ≤ 0.05); # significant when compared with respective normal saline and ethanol (P ≤ 0.05); + significant when compared with respective ranitidine (P ≤ 0.05).
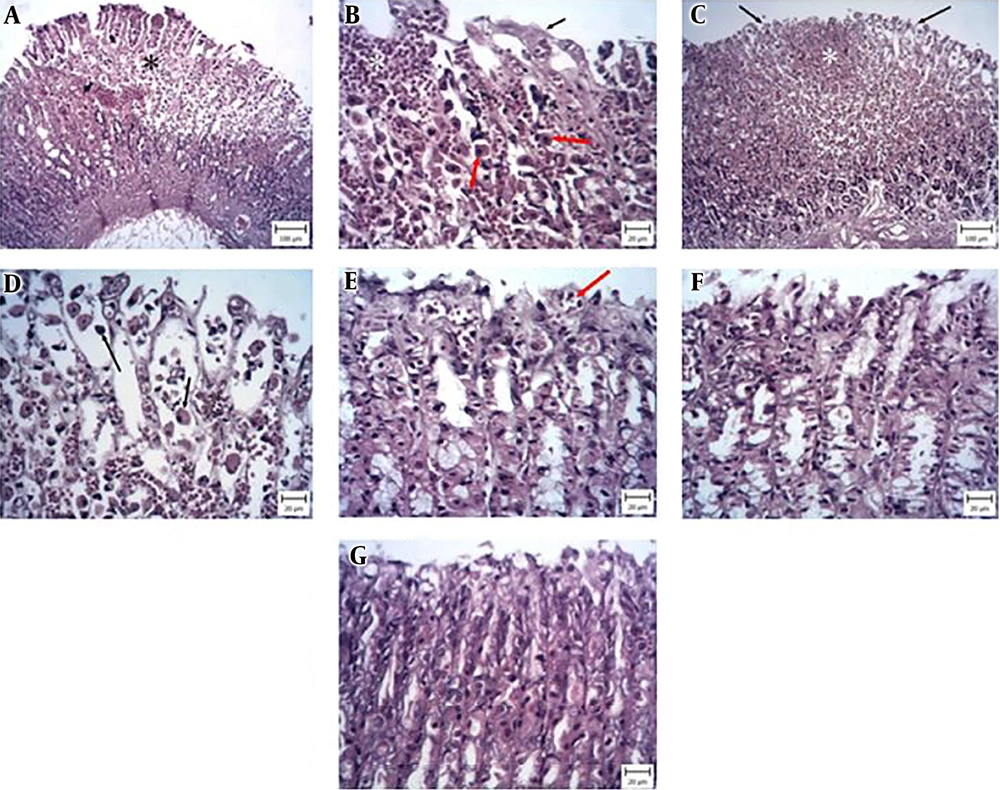
3.4. Histopathology
The histopathological findings of ethanol-treated stomach revealed several vast ulcers in the stomach mucosa (Figure 5A), which were characterized by the necrosis of epithelial and glandular cells and hemorrhage according to many erythrocytes (Figure 5B). The pretreatment of ethanol-induced animals with Pistacia atlantica extract (100 mg/kg) showed different ulcers with necrosis and hemorrhage in the stomach mucosa (Figure 5C and D). The group that received Pistacia atlantica extract (200 and 400 mg/kg) displayed a small area of ulcers and hyperemia (Figure 5E and F). The animals that received Pistacia atlantica extract (400 mg/kg) exhibited a decrease in the size of ulcers. Similar changes occurred in the group receiving ranitidine as the reference drug. Moreover, the ranitidine group showed hyperemia and focal desquamation of epithelial cells.
Obtained stomach tissues stained with H&E. Appearance of rat stomachs in different groups: A, normal saline and ethanol; B, normal saline and ethanol with higher magnification; C, ethanol and pretreatment with 100 mg/kg Pistacia atlantica extract; D, ethanol and pretreatment with 100 mg/kg Pistacia atlantica extract with higher magnification; E, ethanol and pretreatment with 200 mg/kg Pistacia atlantica extract; F, ethanol and pretreatment with 400 mg/kg Pistacia atlantica extract; and G, ethanol and Ranitidine (50 mg/kg). The results showed A, large areas of hemorrhage (asterisk) and necrosis of the epithelium; B, desquamation of epithelial cells (black arrow) and necrotic cells with eosinophilic cytoplasm and dark basophilic nuclei (red arrows). Also, hyperemia and hemorrhage (white asterisks) are seen; C, large areas of hemorrhage (white asterisk) and necrosis of the epithelium (black arrows); D, desquamation of epithelial cells, necrosis (red arrows), and hyperemia (black asterisk); E, hyperemia (red arrow) and desquamation of epithelial cells; F, hyperemia and desquamation of epithelial cells are obvious; and G, the normal structure of stomach.
4. Discussion
Ethanol-induced gastric ulcer is a usual, convenient animal model for the investigation of gastroprotective drugs. The role of ethanol is explained by its ulcerogenic effect. Ethanol progresses disarrangement in mucosal microcirculation and ischemia, produces free radicals, and releases endothelium (20).
The etiology of gastric ulcer is unknown in many patients. This is an indication of the imbalance between aggressive and protective factors leading to a peptic ulcer (21). In the present study, the oral administration of Pistacia atlantica displayed protective effects against ethanol-induced gastric lesions.
Additionally, NO is a major mediator for conduction of acid and alkaline secretion, as well as gastric mucosal secretion, and has considerable effects on gastric mucosal protection (22). In our study, the oral administration of Pistacia atlantica increased the NO level in stomach tissue.
Lipid peroxidation generates MDA as a product of secondary aldehyde formation. Moreover, MDA is a biological index of lipid peroxidation measurement (23). In this study, the oral administration of Pistacia atlantica decreased the MDA level in stomach tissue.
From the ancient times, plants and natural products have been used in folk medicine to cure the peptic ulcer (24). Pistacia atlantica belongs to the Anacardiaceae family (25). Pistacia species have numerous effects such as anti-inflammatory, anti-pyretic, anti-fungal, anti-microbial, antiviral, anti-insect, anti-atherogenic, hypoglycemic, anti-cancer, and hepatoprotective effects (26). Various compounds have been discovered in the essential oil of some pistacia species such as cymene, Linalool, β-caryophyllene, α-thujene, finkone, sabinene, α- phellandrene, cineol, α-finkone, borneol, and α-terpineol (23). Pistacia atlantica has noticeable effects against Helicobacter pylori (27, 28). The oral and rectal administration of Pistacia atlantica fruit extract showed reliable effects on induced colitis physiologically and pathologically (29).
The results of the current study revealed that the oral administration of Pistacia atlantica could significantly protect the gastric mucosa from ulcer induction by ethanol in a dose-depended fashion. The highest dose (400 mg/kg) showed the maximum gastroprotective effect. Pretreatment with Pistacia atlantica extract displayed a significant reduction in the MDA level and a comparable increase in the NO level.
The results obtained in the present research are in line with those of other studies. Gourine et al. studied the antioxidant activity and chemical composition of Pistacia atlantica essential oil, and the results revealed that Pistacia atlantica essential oil has an antioxidant effect and has monoterpene and oxygenated sesquiterpenes like α-pinen and α-thujene (14).
Moreover, Tanideh et al. studied the healing effect of Pistacia atlantica fruit oil extract in acetic acid-induced colitis in rats and concluded that the oral and rectal administration of Pistacia atlantica fruit extract was effective in treating induced colitis in rats (29).
Minaiyan et al. studied the anti-inflammatory effect of Pistacia atlantica volatile oil and gum on acetic acid-induced acute colitis in rats and found that oral gum and volatile oil of Pistacia atlantica have anti-inflammatory effects in acetic acid-induced colitis in rats (30).
Ghaedi et al. studied the protective effect of Pistacia khinjuk on gentamycin (GM)-induced nephrotoxicity in rats and found out that the administration of Pistacia khinjuk extract has a protective effect against GM-induced nephrotoxicity. They concluded that this effect may be due to Pistacia khinjuk antioxidant properties (31).
In conclusion, the present study revealed that the gastroprotective effect of Pistacia atlantica in the experimental lesions induced by ethanol could be related to phenolic compounds that exist in Pistacia atlantica and produce antioxidant properties, which reduce the level of malondialdehyde and increase the activity of antioxidant agents such as nitric oxide. Therefore, we suggest that due to both antioxidant and antiulcer properties, Pistacia atlantica might be useful in the clinical treatment of gastric disorders.