1. Background
Diabetes mellitus, the most common public health problem, is associated with many chronic disorders, including cardiovascular and peripheral vascular diseases. Over the last few decades, the prevalence of diabetes has been increasing, and it is estimated that the number of people with diabetes will double within 15 years (1).
There were about 194 million adults with diabetes in 2003, and it is estimated to increase to more than 333 million adults in 2030. According to statistics, the prevalence of diabetes in Iran is 7.7% (2). Impaired insulin secretion and high blood sugar levels are characteristics of patients with diabetes (3). There is a relationship between insulin resistance and lipid abnormalities in people with diabetes (4, 5). Although diabetes is defined as a multifactorial disorder, diet plays a key role in the progression and prevention of the disease (6). Considering the side effects of drugs, especially in long-term use, using complementary medicine and traditional herbs can effectively reduce complications and prevent the occurrence of abnormalities in patients with diabetes (7, 8).
The use of traditional herbs as medicine has spread worldwide in the past 20 to 25 years (9). Turmeric is a plant that grows in countries with favorable climates, such as India and China. It is a perennial plant from the ginger family (10, 11). As an effective substance, curcumin constitutes about 2 - 8% of turmeric and is extracted using ethanol (11). Several studies have shown curcumin's anti-inflammatory and antioxidant properties (12). Also, this substance affects angiogenesis and cell enzymes (13, 14). Oxidative stress and renal insufficiency in diabetic rats showed a significant reduction with the consumption of curcumin, which may indicate the role of curcumin in improving diabetic nephropathy (15). Ginger is one of the most commonly used spices worldwide, especially in the Middle East (16). However, in traditional medicine, ginger has been used to treat digestive disorders (anorexia, nausea, and vomiting) (17). The effective ingredients of this plant include gingerol, paradol, shogavel, and beta bisabolene (18). Vitamins B and C, magnesium, calcium, phosphorus, and potassium are also present in this plant (19). In addition, anti-cancer, anti-inflammatory, anti-arthritic, anti-thrombosis, blood lipid–lowering, and pain-relieving effects have been observed in this plant (20-23).
Recent studies on animals have shown that ginger has hypoglycemic effects (24, 25). Phenolic compounds of ginger (gingerol and shogaol) can inhibit enzymes related to hyperglycemia and carbohydrate metabolism, such as α-amylase and α-glucosidase (26, 27). Ginger can increase insulin secretion and cell sensitivity and decrease the number of reactive oxygen species in pancreatic beta cells (27). Black pepper belongs to the Piperaceae family, which contains 1% volatile oil, resin, wax alkaloids, terpenoids, and piperine (28). There are no reports on the antinociceptive activity of black pepper roots. It has been reported that medicinal plants of this genus (black piper, piper betle, and piper sarmentosum) have anti-diabetic activity (29, 30). It also has anticoagulant effects. The root extract has antioxidant activity by reducing lipid peroxide levels and maintaining glutathione levels, and it also has cardioprotective effects in a state of myocardial ischemia (31).
2. Objectives
The present study determined the effect of curcumex (a mixture of turmeric, ginger, and black pepper) on fasting blood sugar levels, insulin resistance, HbA1c levels, and serum lipid profile in type 2 diabetic patients.
3. Methods
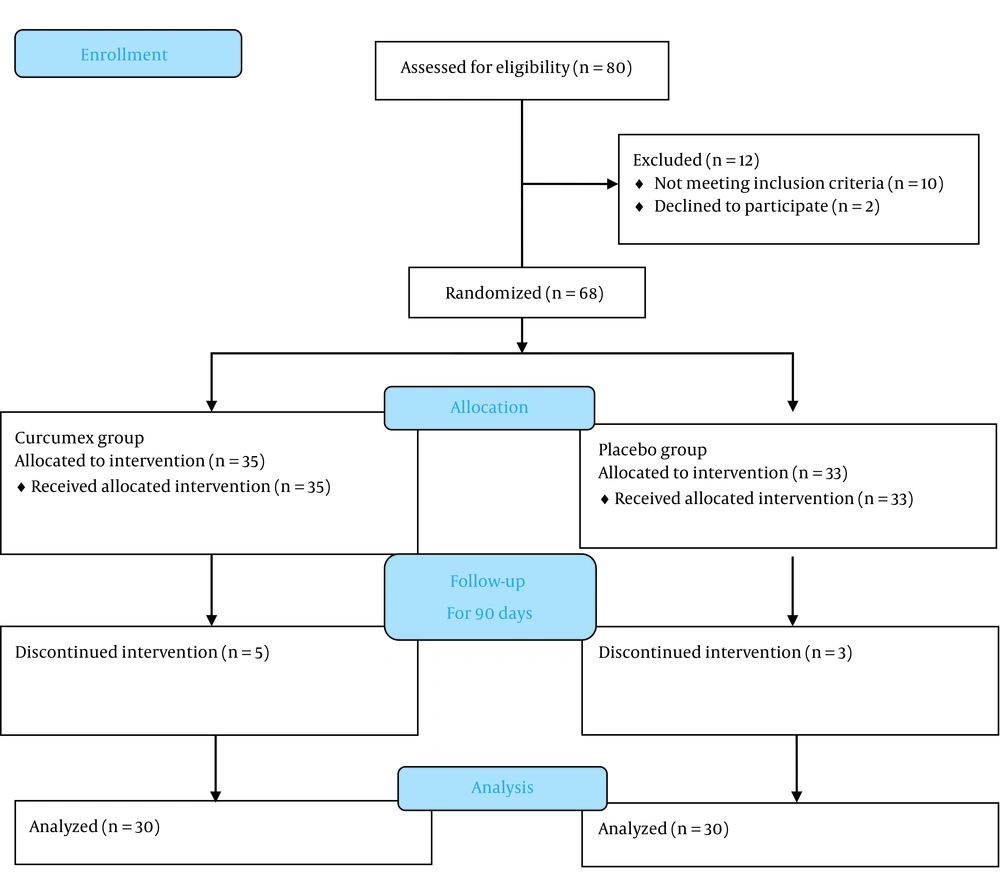
The present study is a double-blind, randomized controlled trial conducted in Golestan Hospital, Ahvaz, Iran, in 2019. A block randomization approach was used to randomize participants at a 1:1 ratio to receive the supplement or placebo (Figure 1). The study population included type 2 diabetic patients using oral anti-hyperglycemic drugs, such as metformin, sulfonylureas, sitagliptin, etc. These patients had poor blood sugar control and had been referred to the outpatient diabetes clinic of Ahvaz Golestan Hospital. Patients continued their previous anti-diabetic medications and were prohibited from taking other vitamin or mineral supplements during the treatment.
Inclusion criteria were the age between 30 and 80 years, hemoglobin A1C (HbA1c) levels of 7% to 9%, fasting blood sugar levels of between 126 and 200 mg/dL, the diagnosis made less than 10 years, and patients using only oral anti-diabetic drugs. Exclusion criteria included pregnancy and lactation, insulin therapy, allergy and sensitivity to black pepper or other ingredients in the combination, and significant kidney and liver failure.
Participants were randomly assigned to the supplement or placebo groups and were treated for 90 days in addition to their medications for type 2 diabetes.
3.1. Randomization and Masking
Placebo was prepared at the Faculty of Pharmacy, Ahvaz Jundishapur University of Medical Sciences. None of the subjects had access to the drug and placebo codes during the study.
3.2. Procedure
The project assistant carried out the initial visit. Based on the inclusion criteria, 60 patients were included in the study. They were asked to fast for 8-12 hours before sampling. The coded samples were prepared and then delivered to the project assistant. The project assistant delivered the drug or placebo based on a random sequence previously provided by the statistical consultant. Patients were given one of the coded options (A, B). Group A received curcumex supplement capsules (Tolou Gostar Bokhara Pharmaceutical Co., Iran. Each capsule contained the active ingredients of medicinal herbs, including turmeric (Curuma longa, 320 mg) and ginger (Zingiber officinale, 150 mg), and black pepper (Piper nigrum, 4 mg). The product was formulated as pellets using extruder-spheronization techniques. Finally, pellets were inserted into the hard gelatin capsules and orally administrated 2 times a day every 12 hours, and group B received the placebo two times a day every 12 hours. The drugs were given to the patients for 90 days. At the beginning of the study, the weight and height of all patients were checked by a physician and body mass index (BMI) was calculated, and blood samples were taken to assess fasting blood sugar levels (HbA1C levels, lipid profile (high-density lipoprotein (HDL), low-density lipoprotein (LDL), cholesterol (Chol), triglyceride (TG), and very low-density lipoprotein (VLDL)), and blood fasting insulin levels, and sent to the Diabetes Research Laboratory. Then, HOMA-B% and HOMA-IR indices were calculated based on fasting blood sugar levels and fasting serum insulin levels. At the end of 90 days, all baseline tests were again measured.
3.3. Analysis
The Shapiro-Wilk test was used to check the normality of the data. If the data was normal, an independent two-sample t-test was used; otherwise, a non-parametric Mann-Whitney test was used to compare the average of the variables. To compare the mean scores before and after the intervention, if the data was normal, a paired t-test was used; otherwise, the Wilcoxon nonparametric test was used. Finally, the chi-square nonparametric test was also used to compare the frequency distribution of qualitative variables. The significance level was considered less than 0.05. SPSS 22 was used for data analysis. In this study, according to similar research and considering the 80% test power and 95% confidence level, the sample size was 30 people in each group.
3.4. Ethical Guidelines
After obtaining the code of ethics from Ahvaz Jundishapur University of Medical Sciences (IR.AJUMS.REC.1397.522), the trial protocol was registered at the Iranian Registry of Clinical Trials (IRCT20181028041483N1), and written consent was obtained from all patients.
4. Results
The mean age in the intervention and placebo groups was 58.27 ± 9.83 and 52.07 ± 8.84 years, respectively. The intervention group included 22 women and 8 men, and the placebo group included 18 women and 12 men. Comparison of the gender between the two groups by the chi-square test showed that the two groups were not different in sex.
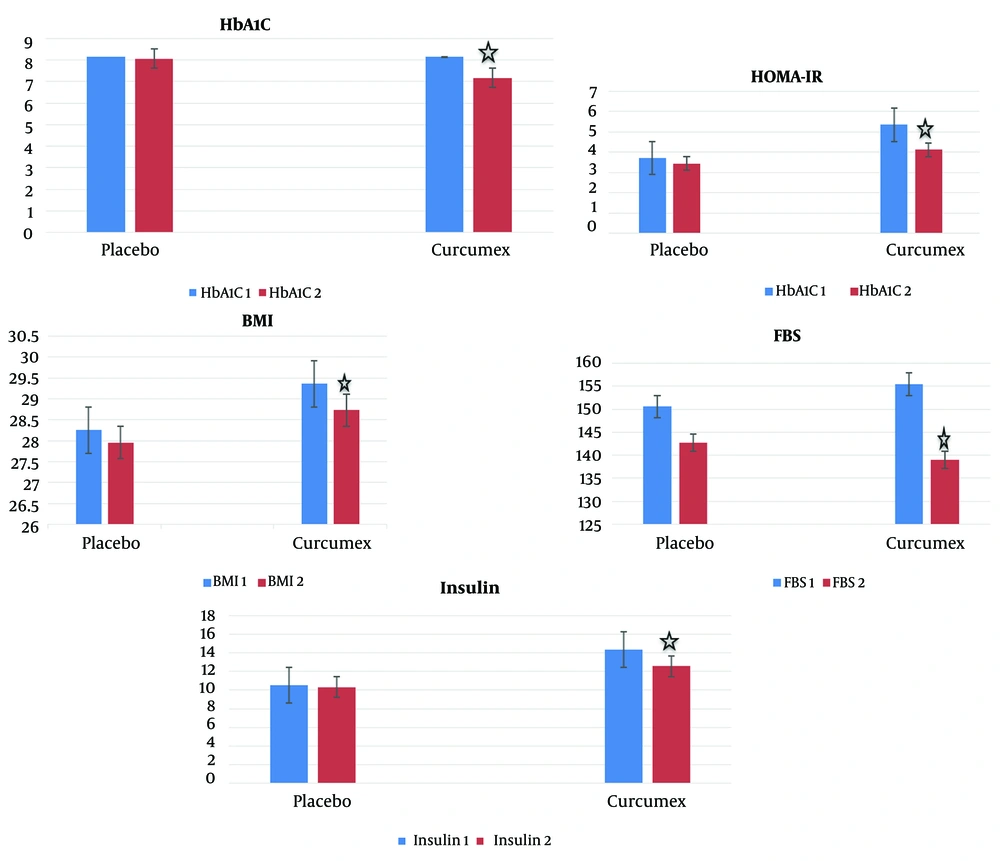
The results of paired t-test and Wilcoxon test to assess intra-group changes showed that mean BMI, fasting blood glucose, plasma insulin, HbA1C, and HOMA-IR levels significantly decreased in the group receiving curcumex supplement capsule for 90 days compared to before the intervention (P-value < 0.05) (Table 1 and Figure 2). But the mean HOMA-B, total Chol, LDL, VLDL, HDL, and TG levels did not change significantly in the group receiving curcumex supplement capsule after 90 days (P-value > 0.05) (Tables 1 and 2). Also, there was no statistically significant difference between the mean BMI, blood sugar indices, and lipid profile before and after the intervention in the placebo group (P-value > 0.05) (Tables 1 and 2).
| Variables and Time | Intervention Group | Placebo Group | P-Value a |
|---|---|---|---|
| BMI | |||
| Before intervention | 29.37 ± 5.28 | 28.25 ± 4.22 | 0.520 |
| After intervention | 28.73 ± 5.74 | 27.95 ± 4.14 | 0.564 |
| P-value b | 0.016 | 0.409 | --- |
| FBS | |||
| Before intervention | 153.00 ± 27.73 | 150.57 ± 26.65 | 0.657 |
| After intervention | 139.00 ± 27.09 | 142.73 ± 31.43 | 0.624 |
| P-value b | 0.006 | 0.120 | --- |
| Plasma insulin | |||
| Before intervention | 14.36 ± 10.03 | 10.51 ± 6.08 | 0.193 |
| After intervention | 12.54 ± 7.00 | 10.30 ± 5.74 | 0.311 |
| P-value b | 0.015 | 0.934 | --- |
| HbA1C | |||
| Before intervention | 8.14 ± 0.76 | 8.15 ± 0.59 | 0.900 |
| After intervention | 7.60 ± 1.20 | 8.05 ± 1.04 | 0.068 |
| P-value b | 0.003 | 0.138 | --- |
| HOMA-IR | |||
| Before intervention | 5.34 ± 3.99 | 3.68 ± 2.22 | 0.153 |
| After intervention | 4.13 ± 2.67 | 3.45 ± 1.97 | 0.554 |
| P-value b | 0.001 | 0.082 | --- |
| HOMA-B | |||
| Before intervention | 78.52 ± 86.61 | 47.69 ± 39.55 | 0.124 |
| After intervention | 78.20 ± 71.51 | 55.20 ± 71.03 | 0.024 |
| P-value b | 0.147 | 0.165 | --- |
a Comparison of the mean scores between the intervention and placebo groups (t-test or Mann-Whitney test).
b Comparison of the mean scores after the intervention compared to before the intervention in each group (paired t-test or Wilcoxon test).
| Variables and Time | Intervention Group | Placebo Group | P-Value a |
|---|---|---|---|
| Total cholesterol | |||
| Before intervention | 165.93 ± 51.89 | 157.10 ± 39.75 | 0.574 |
| After intervention | 157.60 ± 51.00 | 148.40 ± 34.86 | 0.723 |
| P-value b | 0.053 | 0.056 | --- |
| LDL | |||
| Before intervention | 95.27 ± 48.22 | 96.94 ± 41.93 | 0.712 |
| After intervention | 89.43 ± 41.67 | 93.33 ± 43.16 | 0.610 |
| P-value b | 0.119 | 0.262 | --- |
| VLDL | |||
| Before intervention | 33.03 ± 16.02 | 31.81 ± 11.25 | 0.767 |
| After intervention | 32.40 ± 16.28 | 30.77 ± 11.20 | 0.988 |
| P-value b | 0.551 | 0.558 | --- |
| HDL | |||
| Before intervention | 45.41 ± 11.24 | 42.74 ± 12.13 | 0.380 |
| After intervention | 47.09 ± 12.66 | 42.21 ± 11.48 | 0.123 |
| P-value b | 0.251 | 0.633 | --- |
| Triglyceride | |||
| Before intervention | 160.93 ± 95.63 | 140.87 ± 46.01 | 0.859 |
| After intervention | 150.67 ± 104.20 | 136.47 ± 52.79 | 0.894 |
| P-value b | 0.185 | 0.525 | --- |
Abbreviations: LDL, low-density lipoprotein; HDL, high-density lipoprotein; VLDL, very low-density lipoprotein.
a Comparison of mean scores between intervention and placebo groups (t-test or Mann-Whitney tests).
b Comparison of mean scores after the intervention compared to before intervention in each group (Paired t-test or Wilcoxon tests)
The results of the t-test and the Mann-Whitney test to evaluate the changes between groups showed that the average BMI and fasting blood glucose, plasma insulin, HbA1C, and HOMA-IR levels, before and after the intervention had no statistically significant difference between the two groups. Also, no statistically significant difference was observed in total LDL Chol, VLDL, HDL, and TG before and after the intervention between the two groups (P-value > 0.05) (Tables 1 and 2).
5. Discussion
Despite numerous reports on the beneficial effects of turmeric, ginger, and black pepper in glucose metabolism and blood fat reduction, no study has been conducted on the simultaneous effect of the mixture of these three supplements on glycemic status and lipid profile in type 2 diabetes. Also, more studies have been conducted on curcumin and animal models (32). Therefore, this study aimed to investigate the effect of curcumex (including the above three supplements) on fasting blood glucose, Hb A1C, insulin resistance, and serum lipid profile in type 2 diabetic patients.
In a study done by Sukandar et al. on type 2 diabetic patients in Indonesia, the results showed that ethanol extract of garlic and turmeric for 12 weeks significantly decreased BMI and plasma TG, total Chol, LDL, fasting blood glucose, and HbA1C levels. There was also a significant increase in serum HDL levels (33). Although in this study, the decrease in fasting blood glucose and HbA1C levels and BMI is consistent with our study, it is not consistent with our results regarding lipid profile. The reason can be the presence of garlic in the study.
Alwi et al. assessed 75 patients with acute coronary syndrome who consumed curcumin at 3 different doses for 2 months. The results showed that low-dose curcumin decreased Chol and LDL and increased HDL levels (34).
In another study done by Jang et al. on rats with a high-fat diet, curcumin supplementation significantly decreased plasma insulin levels and insulin resistance index (HOMA). It also decreased the concentrations of free fatty acids, TG, and Chol compared to the control group and increased plasma HDL levels (35).
Finally, aligned with the results of our study, in a study done by Baum et al. on healthy people, consumption of 1 to 4 g of curcumin once a day for 6 months showed no significant change in blood lipid profile (36).
In the present study, administering curcumex supplement capsules twice daily for 90 days to patients with type 2 diabetes significantly reduced BMI and HbA1C, insulin serum, HOMA-IR, and fasting blood glucose levels.
According to the literature, tetrahydro curcumin is one of the most important metabolites of curcumin as a biologically active component of turmeric, which has been identified in the cytosol of intestinal and liver cells of rats (37). This compound has antioxidant properties in both in vivo and in vitro conditions and improves insulin secretion and thereby reducing blood glucose levels by increasing glycolysis and inhibiting gluconeogenesis in the liver.
Also, several studies have been done on the effect of ginger on lipids profile and blood sugar with different results (38-40). In this regard, in a double-blind, randomized controlled trial by Talaei et al. on 81 patients with type 2 diabetes, daily intake of 3 capsules containing 1 g of ginger powder for 8 weeks in the intervention group significantly reduced the mean LDL levels after the intervention. Also, the mean fasting blood glucose after the intervention decreased by 10.5% in the intervention group and increased by 21% in the placebo group. Changes in mean HbA1C levels were similar to fasting blood glucose. However, mean HDL, TG, and Chol levels were not significantly different (39).
In another double-blind, randomized controlled trial by Aryaeian et al. on patients with type 2 diabetes, daily consumption of 1600 mg of ginger in 2 capsules of 800 mg ginger for 12 weeks significantly reduced FBS, serum insulin, HBA1C, and HOMA levels in the intervention group compared to the placebo group (41). The results of this study were consistent with our results.
Treatment with antioxidants improves glycemic control in patients with type 2 diabetes and animals. Ginger contains many antioxidants, including gingerol, shogaol, and paradol. The precise mechanism of action of these compounds needs to be better understood. These compounds may increase the GLUT protein of insulin receptors and improve pancreatic β-cell function (27).
Also, little research has been done on the effect of black pepper on lipid profiles and blood sugar indices. In this regard, Garmkhany et al. examined the effect of red and black pepper oral powder on serum levels of blood Chol in rats. Contrary to the results of our study, black pepper significantly decreased serum total Chol and LDL levels. This study also showed that the mean weight of animals in the red and black pepper groups significantly decreased compared to the control groups (42). Capsaicin has anti-obesity effects by limiting the production of white adipose cells and activating lipoprotein lipase (LPL) to reduce body fat levels (43, 44).
Since there are sesquiterpenoid terpene compounds, the antioxidant effects of black pepper are significant in the composition of the black pepper plant (45). Therefore, it can be used as a natural antioxidant and nutritional supplement in people susceptible to diabetes (46).
Accordingly, it can be concluded that the mixture of the three mentioned supplements should have significant beneficial effects in controlling glycemic indices and lipid profiles in type 2 diabetic patients. A subject that was proven at least in part during the present study. Fasting blood glucose, HBA1C, insulin resistance, and BMI are among the indicators that showed a significant decrease in the group receiving the supplement at the end of the study compared to baseline, which was not observed in the placebo group.
One of the limitations of this study was its length and the number of patients. In future studies, better effectiveness and the profile of possible side effects can be investigated by increasing the duration of the study and the number of patients, as well as the use of higher doses. An issue that is associated with the better effect of this supplement on glycemic indices and lipid profile with the least side effects.
5.1. Conclusions
Generally, the consumption of curcumex supplement capsule two times a day every 12 hours for 90 days in patients with type 2 diabetes decreases BMI and blood sugar indicators, including serum levels of HbA1C, insulin, HOMA-IR, and fasting blood sugar, but had no effect on the lipid profile of these patients. Considering the effectiveness of this supplement and its low complication rate (in this study, no specific side effect was reported by its users), it can be used in newly diagnosed type 2 diabetic patients (for whom lifestyle modification alone cannot lead to proper disease control). It can also be recommended for patients who use one or two oral antihyperglycemic drugs and still have not achieved proper control of glycemic indices, and especially patients who have a high BMI can benefit more.