1. Background
Polycystic ovary syndrome (PCOS), which has a prevalence of 5 - 21%, is the most prevalent cause of sterility, irregular menstrual periods, or no periods at all (1). Symptoms of this disease include irregular periods or no periods at all, difficulty getting pregnant (because of irregular ovulation or failure to ovulate), hirsutism or hairiness, increased LH hormone, obesity, increased insulin resistance, formation of multiple cysts in the ovaries, hyperandrogenism, and infertility (2-4). Insulin resistance can lead to type II diabetes (5). Hyperglycemia occurs in PCOS patients because of insufficient pancreatic insulin production or pancreatic beta cell problems (5). According to numerous studies, low vitamin D and calcium levels can also lead to higher oxidative stress and an increased risk of PCOS (6-8). Several studies indicate that oxidative stress caused by hormonal and metabolic problems significantly contributes to the development of the disease (9, 10). According to ovarian histological research, some PCOS patients have ovarian tissues larger than average, increasing the ovary's volume to more than 10 cm (11, 12).
This illness is multifactorial, and no single cause has been identified hitherto. One of the major causes of PCOS is a dysfunction of the hypothalamus-pituitary-ovary axis. In actuality, PCOS is linked to abnormal gonadotropin secretions and elevated steroid synthesis in the ovary (13). The production of androgens in the ovaries rises when LH content is higher than FSH concentration (14, 15). Theca cells in PCOS patients are much more active than normal at converting androgenic precursors to testosterone, according to research conducted in vitro and in vivo (16). In actuality, theca cells create androgens in reaction to luteinizing hormone, and patients' blood levels of androgens rise as a result. Additionally, there is an increase in insulin and insulin-like growth factors (IGFs), which boost androgen synthesis in theca cells and improve LH function (17).
Metabolic abnormalities are caused by various environmental and genetic factors in individuals with PCOS (18). Free radicals, such as reactive nitrogen species (RNS) and reactive oxygen species (ROS), are among the very potent environmental variables (19). The antioxidant defense system is one of the body's defenses against the impacts of ROS, and by lowering ROS levels, it can mitigate some of the harm and complications caused by ROS and oxidative stress (20).
Polycystic ovary syndrome may result from altered ovary steroidogenesis caused by oxidative stress (21). Oxidative stress results from a mismatch between the body's free radicals, such as ROS and RNSs, and the antioxidant system's effectiveness (22, 23). Inflammation may result from an increase in reactive stress brought on by the buildup of free radicals or a decrease in the antioxidant defense system (24). Even though ovulation and fertility are both regarded as inflammatory processes and the ovary naturally expresses certain cytokines or inflammatory factors (25), the reduction of antioxidant factors or the increase of oxidative stress causes the inflammation to increase. The expression level of the factors that promote inflammation, such as interleukin 6 (IL-6), interleukin 18 (IL-18), TNF-α, and C-reactive protein (CRP) in polycystic ovarian tissue are observed compared to healthy tissue (26).
Therapeutic supplements that reduce oxidative stress to treat complications in patients with PCOS are considered effective (27, 28). Propolis is a natural substance that is made by honey bees. Hitherto, its many biological effects, including anti-inflammatory (29), antibacterial (30), antifungal (31), antioxidant, anti-cancer (32), and immune effects (33), have been reported. The basic propolis ingredients responsible for its biological properties include phenols, flavonoids, and aromatic compounds (34). It has recently been discovered that propolis can boost the activity of antioxidant enzymes like glutathione peroxidase, catalase, and superoxide dismutase in animal models of diabetes mellitus (35, 36). One study reported that Iranian propolis can halt oxidative stress and histopathological alterations in the ovaries of newborn stress-exposed rats (37).
Bees make propolis from a variety of plant gums (38). Propolis has been linked to several biological and pharmacological effects, including immune, anti-inflammatory, antibacterial, antifungal, and antioxidant benefits (39, 40). Therefore, this natural substance's antioxidant and anti-inflammatory properties are being extensively researched (41-43). According to research by Rivera-Yanez et al. on an animal model of diabetes mellitus in 2018, propolis can boost the activity of antioxidant enzymes (36). Propolis significantly reduces the oxidative stress that various xenobiotics cause in newborn rats' ovaries (37).
2. Objectives
The current study aims to assess the protective and therapeutic effects of the natural substance propolis on PCOS in animals induced by estradiol valerate. The possible protective mechanisms of this natural substance were examined by analyzing oxidative stress biomarkers and anti-inflammatory functional indices. Also, considering the importance of oxidative stress in the development of PCOS and the necessity of using antioxidant compounds to reduce the complications caused by oxidative stress in women with PCOS, as well as the importance of metabolic disorders related to glucose metabolism and insulin hormone function, in this research, the impacts of the natural substances propolis and chitosan in nanoparticle form on the levels of estrogen, progesterone, vitamin D, calcium, and the insulin resistance index in mouse models of PCOS induced by estradiol valerate are examined.
3. Methods
3.1. Chemicals
Iranian propolis (Chenaran in Khorasan-Razavi Province, Juniperus polycarpos; 36.64908 N) was purchased directly from bee farms (Ahota Company, Mashhad, Iran). Metformin was purchased from Pursina Company (CAS 1691-5G; Tehran, Iran) and chitosan (CAS 448877, 17β-Oestradiol-17-valerat (CAS E1631) from Sigma-Aldrich. Kits for estrogen (CAS 2844-96), progesterone (CAS P-069), and vitamin D (CAS 6744-96) were supplied by Diaplus Company (Seoul, Korea), and the insulin kit (CAS 1391038) was purchased from Mercodia Company (Northern Sweden). All salts used to prepare buffer solutions were obtained from Merck (Merck KGaA, 64271, Darmstadt, Germany). Easy Gluco was used to assess the glucose serum levels.
3.2. Preparation of Propolis Extracts
Propolis was extracted from wax-free bee hives. 50 g of propolis was dissolved in 500 mL of 80% alcohol and shaken for 48 hours at room temperature (44). Then, it was filtered, and the alcohol was rotary evaporated. After weighing the purified extract, a 10% solution in 80% alcohol was made and kept at 4°C. One mg/mL stock solutions were prepared and utilized in subsequent studies.
3.3. Preparation of Chitosan-Propolis Nanoparticles
The ionic gelation technique was modified to create chitosan nanoparticles enclosed in alcohol extract. Chitosan solutions at 0.2 w/v concentrations were made in glacial acetic acid at 0.1% v/v and then filtered. In deionized water, sodium tripolyphosphate solution (TPP) (0.2% w/v) was made. 0.4 ± 1.6 mg/mL of alcohol extract was added to a chitosan solution that contained 0.4% w/v tween 80. The TPP solution was then gradually added while stirring continuously after the mixture had been sonicated for five minutes. Throughout the trial, the chitosan/TPP ratio was held constant at 2:1. The chitosan-propolis conjugated nanoparticles were separated from the obtained supernatant by ultracentrifugation at 25000 rpm for 20 minutes. These particles were then submitted for further characterization (44).
3.4. Physical Quality Control of Chitosan-Propolis Nanoparticles
After preparing the suspensions, the size distributions of the chitosan-propolis nanoparticles were determined using a laser diffraction particle size analyzer (Japan) at room temperature.
3.5. Experimental Protocol
3.5.1. Animals
Mature female Wistar rats (age 10 - 12 weeks and weighing 140 - 160 g) were obtained from Birjand University of Medical Sciences' Comparative and Experimental Medicine Center, Birjand, Iran. Animals had free access to water and food in the cage. They were kept in a controlled environment with regulated light (12:12 h), temperature (23 - 25°C), and relative humidity (40%). The rats were managed according to the animal management procedure approved by the Ethical Committee of Research at Birjand University of Medical Sciences (ethical number: IR.BUMS.REC.1400.163).
3.5.2. Estradiol Valerate Induced-Polycystic Ovarian Syndrome
This research used the hormonal induction method with estradiol valerate (Abu Reyhan Pharmaceuticals, Tehran). The selected animals underwent three consecutive estrus cycles following fifteen days of daily vaginal smear tests (each cycle has four stages: Proestrus, estrus, metestrus, and diaestrus). Throughout the estrus period of the reproductive cycle, 40 mg/kg of estradiol valerate was subcutaneously injected into each animal. To achieve complete syndrome induction, the vaginal smear test was continued after the daily injection until the alteration in the estrous cycle and its abnormality and achieving the phase of persistent vaginal certification and persistent vaginal cornification as the most significant symptoms of the syndrome.
3.5.3. Groups
Animals (six animals per group) were randomly allocated into (1) the control group (30 µL DMSO, gavages); (2) PCOS group (by a single intramuscular injection of estradiol valerate-induced (EV) at a dose of 4 mg/kg/day (dissolved in 0.4 mL of sesame oil) for 28 days); (3) PCOS (4 mg/kg EV BW) + chitosan-propolis nanoparticles (500 mg/kg; 2 times per weeks for 42 consecutive days, gavages); (4) PCOS (4 mg/kg EV) + metformin (150 mg/kg, dissolved in normal saline (NS), daily for 42 consecutive days, gavages). Metformin (45) and EV (46) doses were determined according to previous studies. Duration of metformin and chitosan-propolis nanoparticles treatment were set according to vaginal smear alteration in the estrous cycle and its normality in the metformin group.
3.5.4. Serum Hormone Analysis
All animals were anesthetized with 10 mg/kg of xylazine and 70 mg/kg of ketamine (IP) 48 hours after the final therapeutic dosage of metformin and chitosan-propolis nanoparticles. Serum samples were kept at -70°C for later analysis after blood samples (5 mL) from the right atrium were taken and centrifuged for 5 minutes at 3500 rpm. The animals’ estrogen, progesterone, vitamin D, calcium, and insulin serum levels were measured using an ELISA reader following the manufacturer's recommendations.
3.5.5. Biochemical Assays
After the intervention, blood was drawn from the rats’ tails, and the Easy Gluco device was used to test the blood sugar levels to determine serum glucose levels. The HOMA-IR (homeostasis model evaluation of insulin resistance) index was calculated as (fasting serum glucose × fasting serum insulin/22.5) to measure insulin resistance.
3.5.6. Histological Analysis
After the course of therapy, the tissue of the ovaries was removed and fixed for 24 hours in a buffered formalin solution (0.64% NaH2PO4, 0.4% Na2HPO4, and 10% formaldehyde in distilled water; pH = 7.4). Then, tissue samples were mounted on glass plates, sectioned at a thickness of 5 μm, stained with H&E or Trichrome Masson, and examined under a light microscope (Olympus CX21®, Japan) (23). According to Erickson's categorization, antral follicles, secondary and primary follicles were counted on all slides (24). Only visible nuclei in follicles were counted, and follicular cysts were quantified.
3.5.7. Determination of Superoxide Dismutase
According to the kit's instructions, the commercial rat ELISA kit (Eastbiopharm, China) was used to find superoxide dismutase (SOD) activity in the blood of the control and experimental groups. The superoxide anion was converted to oxygen and hydrogen peroxide at 420 nm, where the SOD activity was determined. Superoxide dismutase activity (inhibition rate) was calculated using the formula below:
SOD activity (inhibition rate %) = (AB1-AB3) - (AS-AB2)/AB1-AB2
A = Absorbance, B = Blank, S = Sample.
3.5.8. Measurement of Malondialdehyde Assay
Using a commercial rat ELISA kit, malondialdehyde (MDA) levels were used to assess the amounts of free radicals (lipid peroxidation) in serum samples. (Eastbiopharm, China). At 535 nm, the LPO content was determined spectrophotometrically.
3.5.9. Quantitative Real‑time PCR Analysis
The ovaries of all animals were removed. An RNA extraction kit (Pars Toos, IRN) was used to obtain total RNA from the samples. The RNA extraction's measurements and purity were confirmed using a NanoDrop spectrophotometer (Epoch Biotech). A cDNA synthesis reagent was used to reverse-transcribe the RNAs (Pars Toos, Iran). Using the Oligo 7 primer tool, particular primers for the monocyte chemoattractant protein (MCP), IL-18, and CRP genes were created (Table 1). For the quantitative RT-PCR study, the SYBR Green PCR Master Mix (Amplicon, Denmark) was used. cDNA was amplified using the ABI Step One Plus real-time PCR apparatus. (Applied Biosystems). To normalize the results on gene expression, GAPDH was used. The 2-ΔΔCt technique was used to determine the expression levels of the target genes.
| Name | Forward | Reverse |
|---|---|---|
| CRP | GCAGTAGGTGGGCCTGAAAT | CCCGTCAAGCCAAAGCTCTA |
| MCP1 | TGCAGTTAATGCCCCACTCA | AGTTCTCCAGCCGACTCATTG |
| IL-18 | CAGCCAACGAATCCCAGACC | AGATAGGGTCACAGCCAGTCC |
| GAPDH | CGAACCTCTCTGCTCCTCCTGTTCG | CATGGTGTCTGAGCGATGTGG |
Sequences of Primers Used for Real-time PCR
3.6. Statistical Analysis
Graph Pad Prism 6 software (Graph Pad Software Inc., San Diego, CA, USA) was used for the statistical analysis. The threshold for statistical significance was set at a P-value of less than 0.05. Data were presented as the mean ± SEM, and data comparison was performed by the one-way analysis of variance with the Dunnett post-test.
4. Result
4.1. Physical Quality Control of Chitosan-Propolis Nanoparticles
The average size of chitosan-propolis nanoparticles was measured. The findings showed that the chitosan-propolis nanoparticles had an average particle size of approximately 251.18 ± 17.70 mm (V).
4.2. Effects of Chitosan-Propolis Nanoparticles on the Body Weight
All rats in all groups finished the experiment. First, since the rise in body weight is one of the most significant clinical characteristics of PCOS, the body weights of all animals were measured on the first and last days of the experiment. According to our findings, estradiol valerate administration increased the end body weight of the PCOS group compared to the control group (P < 0.01, Table 2). Compared to the PCOS group, rats treated with metformin or chitosan-propolis nanoparticles (500 mg/kg) had lower body weights, but the difference was not statistically significant.
| Control | PCOS | Metformin | Chitosan-Propolis Nanoparticles | |
|---|---|---|---|---|
| Before the experimental period | 151 ± 9.42 | 149 ± 7.8 | 153 ± 7.5 | 151 ± 14.1 |
| After the experimental period | 180 ± 5.5 | 207 ± 6.6 b | 199 ± 5.17 | 200 ± 13.8 |
The Animals' Body Weight Was Measured Daily for the 42-Day Experiment (42 Days After Estradiol Valerate Injection to Induce Polycystic Ovary Syndrome and Administration of Chitosan-Propolis Nanoparticles or Metformin) a
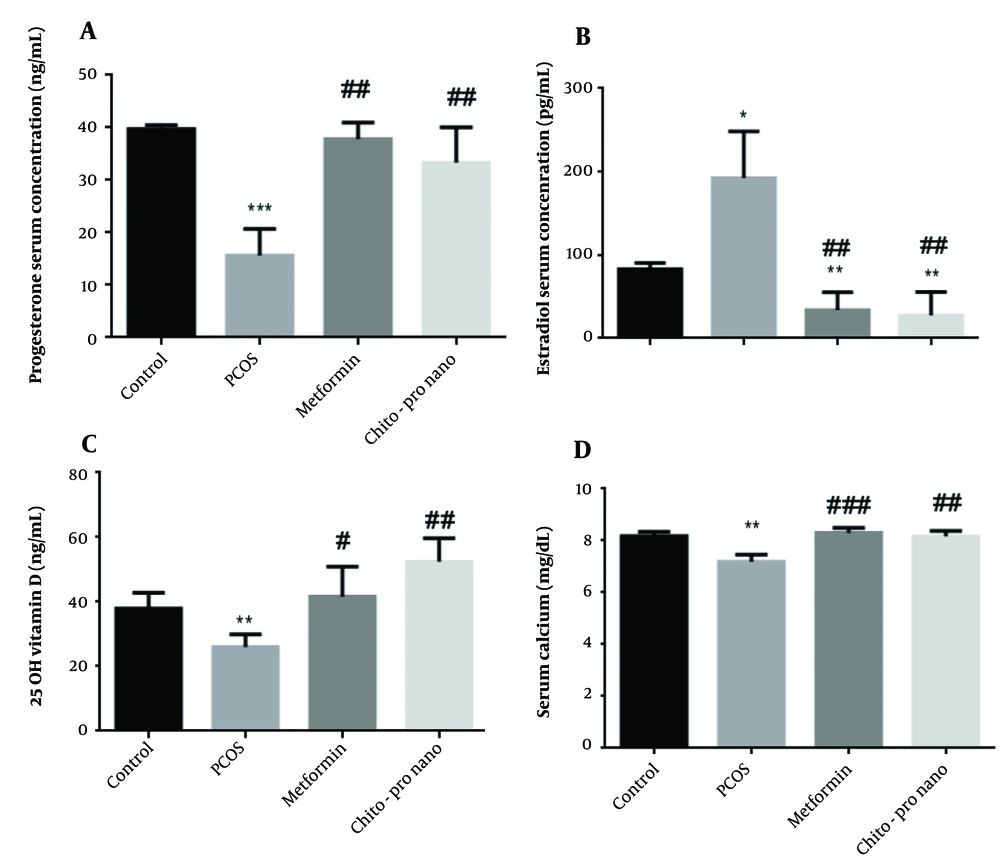
4.3. Effects of Chitosan-Propolis Nanoparticles on Hormone Levels
The study's findings demonstrated that the PCO group's progesterone serum levels were considerably lower than the control group's (P < 0.001). Additionally, compared to the PCOs group, a notable rise in progesterone serum level was seen in the experimental groups treated with metformin and chitosan-propolis nanoparticles (P < 0.01) (Figure 1, F (2.381, 11.90) = 32.26). However, compared to the control group, the PCO group's serum estrogen level rose significantly (P < 0.05). In contrast, a significant decline and an increase in estrogen levels were observed in the experimental groups treated with metformin and chitosan-propolis nanoparticles, respectively, compared to the control and PCOs groups (P < 0.01) (Figure 1, F (1.762, 8.810)).
Serum progesterone, estradiol, vitamin D, and calcium levels in control, polycystic ovary syndrome (PCOS), metformin, and chitosan-propolis nanoparticle rats after six weeks of the experimental period. Values are presented as mean ± SE (n = 6/each group). * Statistically different from the control rats (P ≤ 0.05), ** statistically different from the control rats (P ≤ 0.01), *** statistically different from the control rats (P ≤ 0.001); # statistically different from the PCOS rats (P ≤ 0.05), ## statistically different from the PCOS rats (P ≤ 0.01), ### statistically different from the PCOS group. Chito-pro nano: Chitosan-propolis nanoparticle; PCOS: Polycystic ovary syndrome.
4.4. Effects of Chitosan-Propolis Nanoparticles on Vitamin D Levels
Compared to the control group, the administration of EV substantially reduced vitamin D serum levels (P < 0.01). The PCOS-induced reduction in vitamin D was exacerbated by the delivery of metformin and chitosan-propolis nanoparticles (P < 0.05 and P < 0.01, respectively) (Figure 1, F (2.578, 12.89)).
4.5. Effects of Chitosan-Propolis Nanoparticles on Calcium Level
According to Figure 1, the administration of EV significantly reduced calcium serum levels compared to the control group (P < 0.01), and the treatment with metformin significantly increased calcium levels after EV administration (P < 0.001). Additionally, compared to the PCOs group, the chitosan-propolis nanoparticle group's calcium serum level was considerably (P < 0.01) higher (F (2.680, 10.72)).
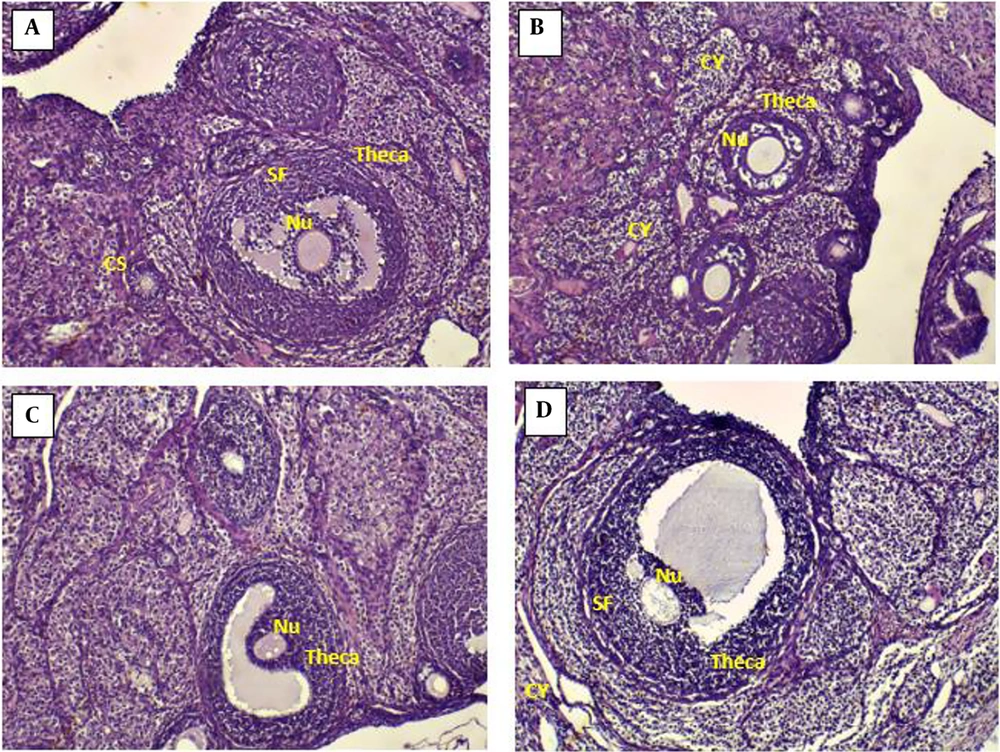
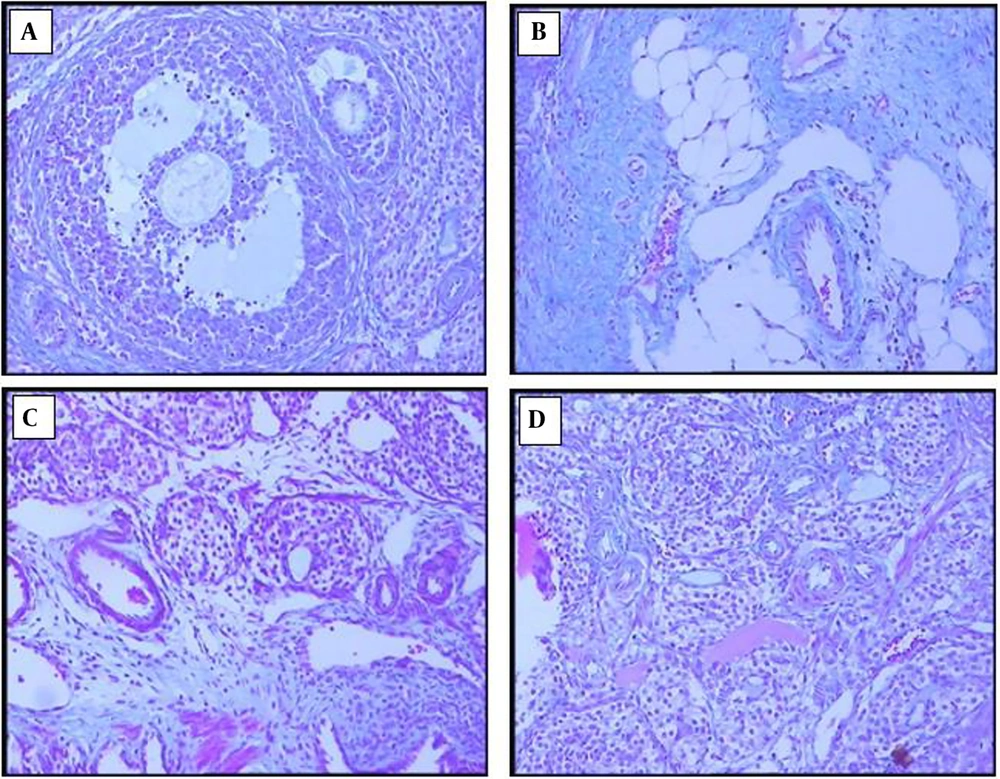
4.6. Effects of Chitosan-Propolis Nanoparticles on the Histology of Ovaries
The ovarian sections taken from the control animals showed a natural structure with follicles at different stages of growth and normal granulosa cell layers. In contrast, numerous follicular cysts were present in PCOS ovaries with degrading granulosa cells in the thin layer of granulosa cells. Compared with control animals, the numbers of primary and antral follicles significantly decreased in the PCOS group. In comparison with the ovaries of the PCOS group, treatment with chitosan-propolis nanoparticles (500 mg/kg) or metformin for 42 days significantly decreased the number of follicular cysts (Figures 2 and 3).
4.7. Effects of Chitosan-Propolis Nanoparticles on Biochemical Markers
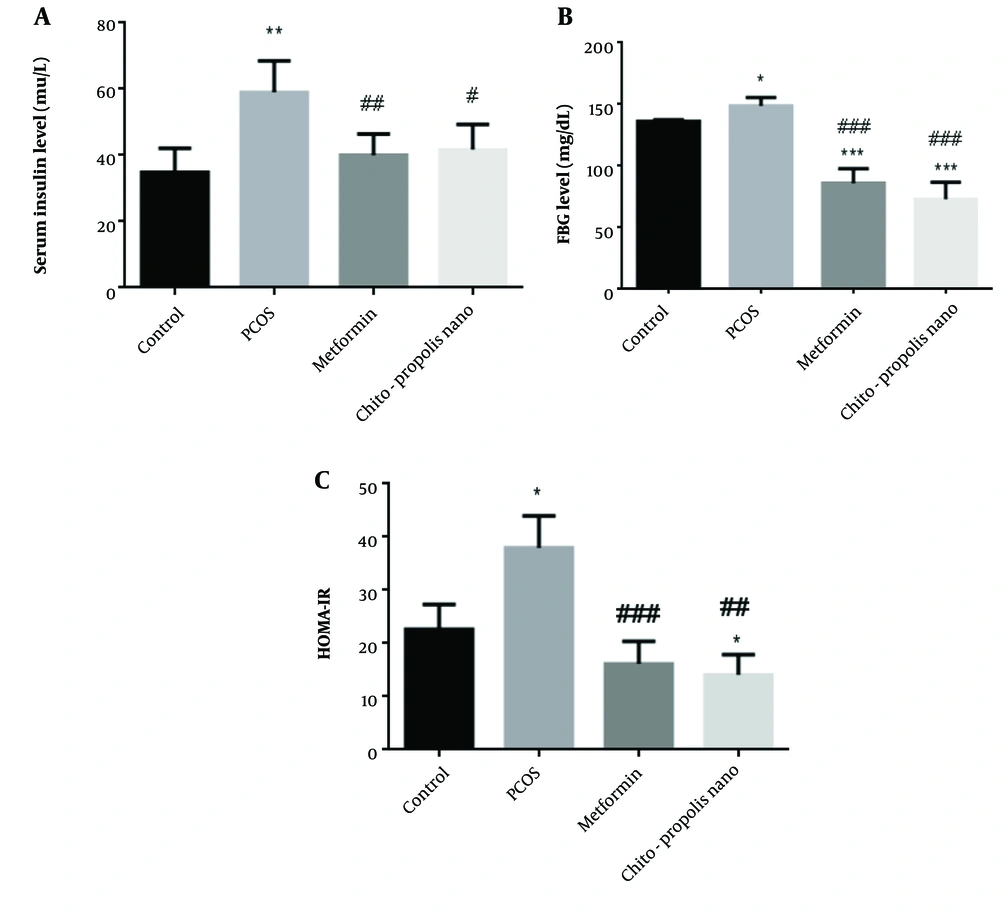
In individuals with PCOS, metabolic abnormalities are primarily characterized by insulin resistance and hyperinsulinemia. As anticipated, the fasting insulin levels in PCOS rats were higher than in control rats. Compared to the control rats, insulin levels in PCOS rats were substantially higher (P < 0.01). There was no difference between the metformin and chitosan-propolis nanoparticle groups compared to the control group, and insulin levels decreased in the metformin and chitosan-propolis nanoparticle groups compared to the pcos group (P < 0.01 and P < 0.05) (Figure 4, F (2.475, 12.38)). The administration of EV also considerably raised fasting blood glucose (FBG) levels compared to the control group, as shown in Figure 4 (P < 0.05). Additionally, compared to the control group, metformin and chitosan-propolis nanoparticles significantly reversed the FBG level increase caused by EV (P < 0.001). In addition, administration of metformin and chitosan-propolis nanoparticles to rats with PCOS resulted in a reduction of their FBG levels compared to the PCOS group (P < 0.001) (F (1.823, 9.117)). After six weeks of testing, the HOMA-IR score in control, PCOS, metformin, and chitosan-propolis nanoparticle rats was shown in Figure 4. When compared to the reference group, the administration of EV significantly raised the HOMA-IR index (P < 0.05), and the administration of chitosan-propolis nanoparticles significantly decreased the HOMA-IR index (P < 0.05). Additionally, the HOMA-IR index was substantially (P < 0.01) lower in the metformin (P < 0.001) and chitosan-propolis nanoparticle (P < 0.01) groups compared to the PCOs group (F (2.974, 14.87)).
Serum insulin concentration (mLU/L), FBG (mg/dL), and HOMA-IR levels in control, PCOS, metformin, and chitosan-propolis nanoparticle rats after six weeks of the experimental period. Values are presented as mean ± SE (n = 6/group). * statistically different from the control rats, ** statistically different from the control rats (P ≤ 0.01), *** statistically different from the control rats; # statistically different from the PCOS rats (P ≤ 0.05), ## statistically different from the PCOS rats (P ≤ 0.01), ### statistically different from the PCOS group. Chito-pro nano: Chitosan-propolis nanoparticle; FBG: Fasting blood glucose; PCOS: Polycystic ovary syndrome.
4.8. Effects of Chitosan-Propolis Nanoparticles on SOD
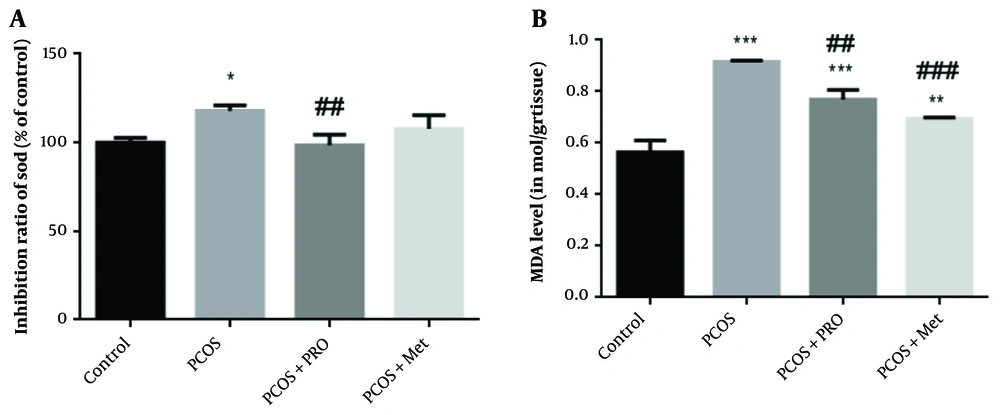
Several enzymes are important in the antioxidant defense system. One of these enzymes, SOD, catalyzes the dismutation of superoxide into hydrogen peroxide, which is subsequently oxidized by catalase or glutathione. As seen in Figure 4, PCOS significantly reduced SOD activity compared to the control group (P < 0.05), but PRO treatment considerably increased SOD activity compared to the PCOS group (P < 0.01) (Figure 5). Between the metformin (Met) therapy groups and the control groups, there was no statistically significant difference (P > 0.05), (F (3, 8) = 8.359).
Percentage inhibition of superoxide dismutase (SOD) and malondialdehyde levels in blood samples of different groups. * P < 0.05, ** P < 0.01, *** P < 0.001 for each group compared to the control group; ## P < 0.01, ### P < 0.001 for each group compared to the polycystic ovary syndrome (PCOS) group.
4.9. Effects of Chitosan-Propolis Nanoparticles on MDA Levels
Compared to the control groups, a significantly higher amount of MDA was found in the serum of the PCOS groups, as shown in Figure 5 (P < 0.001). Compared to the induced PCOS groups, the levels of MDA were considerably lower in the PCOS + PRO and PCOS + Met groups (P < 0.01 and P < 0.001, respectively) F (3, 8) = 75.99.
4.10. Quantitative Real‑time PCR Analysis
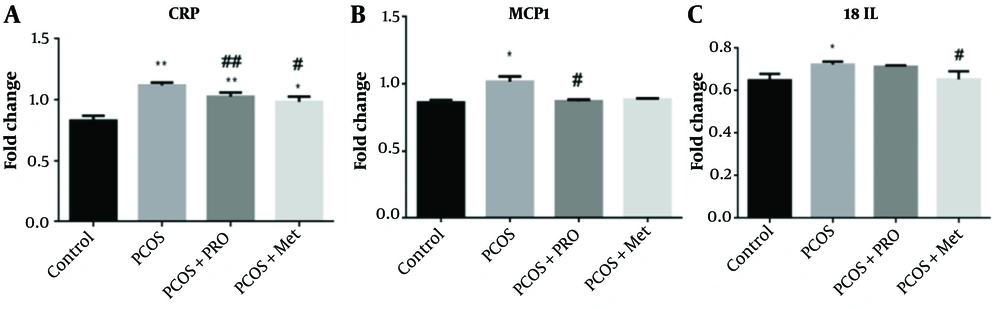
Data from qPCR showed that the CRP, MCP1, and IL-18 genes were all expressed at different levels (Figure 6). The PCOS group significantly outperformed the control group regarding CRP levels (P < 0.01). In contrast, the PRO and met-treated PCOS groups significantly outperformed the PCOS group in terms of CRP levels (P < 0.01 and P < 0.05, respectively), F (1.173, 2.345) = 161.0. The PCOS group's MCP1 gene expression increased (P < 0.05). Monocyte chemoattractant protein 1 gene expression was significantly downregulated in the PCOS + PRO group compared to the PCOS group (P < 0.05) but not significantly different from the control group in the PCOS + Met group (P > 0.05), F (1.359, 2.719) = 36.57. Compared to the control group, in the PCOS group, upregulation of the gene expression for IL-18 was substantially decreased (P < 0.05). Additionally, the PCOS + Met group significantly reduced the expression of this gene in comparison to the PCOS group (P < 0.05), while the PCOS + PRO group's comparison to the control group did not reach statistical significance (P > 0.05); F (3, 8) = 7.024.
Comparison of the gene’s expression levels: C-reactive protein (CRP), monocyte chemoattractant protein 1 (MCP1), and interleukin 18 (IL-18). * P < 0.05, ** P < 0.01 for each group compared to the control group; # P < 0.05, ## P < 0.01 for each group compared to the polycystic ovary syndrome (PCOS) group.
5. Discussion
The findings of the current research demonstrated that estradiol valerate treatment changed the histology of the ovarian tissue for 42 consecutive days as the number of primary follicles and antral follicles greatly declined and the number of large fluid-filled cystic follicles increased in the estradiol valerate group. In the estradiol valerate group, progesterone blood levels fell, and estradiol levels rose. Studies utilizing the estradiol valerate PCOS model have demonstrated a correlation between hyperandrogenism and sympathetic ovarian system hyperactivity.
A serious clinical complication that may result in polycystic ovary syndrome or increased estrogen levels is estradiol valerate-induced PCOS (47). Estradiol production is caused by the dominant follicle growing, and high estrogen levels indicate that FSH production discontinues through a negative feedback system. However, a high and sustained estrogen level causes a one-time LH surge, resulting in ovulation (48). Elevated androgen levels may cause follicle disintegration by accelerating the destruction of oocytes and pyknotic granulosa cells. Similar outcomes were observed in the present study's rats when PCOS was induced using estradiol valerate. Infertility has been linked to PCOS' abnormal ovarian tissue shape, function, and steroidogenesis regulation (49). Patients with PCOS have a severe rise in estradiol, insulin resistance, and glucose intolerance, all associated with ovarian tissue damage (50).
Our results also demonstrated the number of cysts in the estradiol valerate group by the HE stain method. Histopathology of ovarian tissue in people with PCOS has demonstrated that numerous cysts are formed in the central area of the ovary (47). In our study, when metformin or chitosan-propolis nanoparticles were administered to PCOS rats, the number of cystic ovaries decreased compared to the PCOS group.
Propolis nutritional supplements can be a therapy for women with polycystic ovaries by influencing the body's inflammatory index, testosterone, and metabolic rate. As demonstrated by Tatli Seven et al., propolis and nano-propolis (NP) administration can reduce oxidative stress by increasing GSH, CAT, and GPx activities (51) and has a function in PCOS. People have long used propolis as a remedy for different illnesses (52), and due to its numerous therapeutic effects, it is now extensively used in the food and pharmaceutical industries (52). The propolis nanoparticle's shape may contribute to the intensity of these effects. Additionally, chitosan alone has therapeutic benefits for several illnesses, and when combined with propolis, it can potentially reverse the negative impacts of estradiol valerate in PCOS.
Our findings demonstrated that estradiol valerate treatment for 42 days substantially raised the serum levels of estradiol in PCOS animals, and progesterone levels decreased. Additionally, administering metformin or chitosan-propolis substantially reversed the impairment induced by estradiol valerate. The effects of chitosan-propolis nanoparticles on hormones and insulin resistance index seem to be the fundamental mechanisms for these nanoparticle effects in the current model. This may be due to propolis reducing oxidative stress and improving bio-distribution when combined with chitosan nanoparticles.
According to research by Taghvaee Javanshir et al., rats can develop PCOS when given estradiol valerate for 25 days. It can cause morphological changes, anovulation, metabolic problems, hormonal changes, and follicular cysts in the ovaries (47). Polycystic ovary syndrome is often associated with being overweight, which increases the risk of other PCOS disorders. It has been established that elevated estradiol can contribute to obesity in PCOS patients with this condition. These findings support earlier findings published by Stener-Victorin et al. (53) and Taghvaee Javanshir et al. (47). According to these studies, estradiol valerate increases lipid metabolism, which results in body weight reduction. Additionally, PCOS increases sympathetic system function, which may also affect weight (47). However, not all PCOS sufferers exhibit obesity-related symptoms (54). Metformin administration and chitosan-propolis nanoparticles reduced body weight, but these variations were not statistically significant.
The lack of regulation of calcium (55) and vitamin D (56) serum levels can also play a role in PCOS, and this study demonstrated that the reduction of calcium and vitamin D serum levels plays a role in the antioxidant imbalance (55). In our study, in the estradiol valerate group, a significant decrease in the level of these two markers was observed, and the treatment with metformin and chitosan-propolis nanoparticles balanced their serum levels, which may be attributed to the antioxidant role and the activation of antioxidant enzyme pathways, including glutathione and superoxide dismutase.
Stress can alter the levels of hormones and corticosterone, which play a significant role in accelerating follicular atresia (37). Estradiol valerate increased the oxidative stress factor of the ovary, with the combined impact greater than the individual exposure. Malondialdehyde levels rose in the ovarian tissue of PCOS animal models, according to Tahmasebi et al. still, there was no discernible difference between the normal and PCOS groups regarding the ovarian tissue's overall antioxidant capacity (57). In experimental animals exposed to estradiol valerate, Kokabiyan et al. (58) found that the levels of MDA, AST, ALT, and ALP significantly rose. In contrast, the levels of SOD in liver tissue and serum decreased.
Inflammatory markers such as CRP, MCP1, and IL-18 regulate ovary functions, and upregulation or downregulation of these factors can lead to PCOS. Interleukin 18, one of the most important cytokines in cell proliferation, has a key role in interferon-gamma (IFN-γ) secretion and splenocyte proliferation. In addition, serum IL-18 levels appear to be closely linked to total testosterone levels and have emerged as a significant predictor of insulin resistance. C-reactive protein is a low-grade chronic inflammatory factor the liver generates in response to interleukin-6 stimulation. On the other hand, CRP was elevated not only compared to age and BMI (59, 60) but also similar between PCOS and control groups (61, 62). According to Wu et al., women with PCOS had substantially higher CRP levels (63). Monocyte chemoattractant protein 1, a chemokine that attracts monocytes to inflammatory sites, is another inflammatory factor closely linked to PCOS. In some research, elevated MCP1 mRNA expression has been seen in THP-1 human mononuclear macrophages, which suggests that inflammatory factors are present in PCOS patients' serum (64). As a result, other investigations have found, according to a meta-analysis study, that PCOS patients have substantially higher levels of MCP1, which are unrelated to people's BMI (65). In this research, CRP, MCP1, and IL-18 gene expression were significantly higher in the estradiol valerate group compared to the control group, indicating inflammation in mice with PCOS.
In this research, propolis-treated animals simultaneously showed decreased MDA levels, CRP, MCP1, and IL-18 gene expression, and increased SOD activity. Arabameri et al. (37) have demonstrated that Iranian propolis can reduce abnormal ovaries in newborn rats and the psychological stress caused by the separation of the mother and her offspring.
In the current research, we measured the MDA level and SOD activity using estradiol valerate alone and its co-administration with metformin or chitosan-propolis nanoparticles. According to the findings, it modulated inflammatory factors. According to other researchers, the chitosan nanoparticles mentioned in the paper help reduce toxicity (66). The use of nanoparticles in conjunction with natural products for the "green chemistry" of nanoparticle creation is appreciated today in nanotechnology, nanomedicine, and nano-based drug delivery systems. Consequently, utilizing green nanoparticles for drug transport can lessen adverse drug effects. The bioactivity of these nanomaterials can also be improved by modifying the nanostructures' size, form, hydrophobicity, and surface. Because of its ease of manipulation (67), quick solubility effects (68), biocompatibility (69), and low toxicity (70) without side effects, chitosan has been promising in the synthesis of nanoparticles. Chitosan nanoparticles are considered when creating novel drug delivery methods because they improve biodistribution and lower drug toxicity (66). Chitosan aids in the transport of drugs by enhancing absorption. On the other hand, chitosan, in conjunction with other compounds, including sodium tripolyphosphate (in nanoparticle form) (66) and fennel seed extract (71), plays a significant role in improving polycystic symptoms, including ovulatory activity. Considering that chitosan contributes to the treatment by improving drug delivery mechanisms, Tatli Seven and coworkers demonstrated that administering propolis and NP can reduce oxidative stress through increased GSH, CAT, and GPx activities (51) and also plays a role in PCOS. Propolis has been used for medicinal purposes for a significant period (52). Due to its numerous therapeutic effects, it is now extensively used in the food and pharmaceutical industries (52). The propolis nanoparticle's shape may contribute to the intensity of these effects. Additionally, chitosan alone has therapeutic benefits for several illnesses, and when combined with propolis, it can potentially reverse the negative impacts of estradiol valerate in PCOS.
5.1. Conclusions
The data from the present study suggest that chitosan-propolis nanoparticles have antidiabetic effects in PCOS animals. The defensive abilities of this nanoparticle appear to be significantly influenced by the control of insulin and the reduction of hormonal levels in the ovarian tissue. We can gain a better understanding of this nanoparticle's effects on PCOS and diabetes by examining its impact on key targets such as vitamin D, serum calcium, oxidative stress, and inflammation factors. As a result, PCOS patients may benefit from using chitosan-propolis nanoparticles as a preventative measure and an additional therapy.