1. Background
Reactive oxygen species (ROS) are produced in the human body and are believed to be a cause of chronic disease progression (1). Under normal physiological conditions, ROS level is low enough to be efficiently eliminated by antioxidative defense mechanisms, including superoxide dismutase, glutathione reductase, glutathione peroxidase, and catalase. The function of ROS, as defending enzymes, is well established and has been widely investigated (2, 3). Nevertheless, an imbalance in this mechanism in favor of ROS overproduction leads to oxidative stress, which plays an influential role in the etiology of many chronic diseases, including cardiovascular diseases, neurodegenerative diseases, diabetes, and cancer (4).
Human hepatoma cell lines (HepG2), which are distinctively transformed, well differentiated cell lines from hepatic origins, have been utilized for the enhancement of cell-oriented bioassays in the analysis of drug antioxidant activity (5). Also, A549, a human lung adenocarcinoma cell line, has been introduced in an in vitro model of alveolar epithelium type II by Foster and colleagues (6). Hydrogen peroxide (H2O2), as a major ROS, has been widely used as an activator of oxidative stress in such models (7). Therefore, treating HepG2 and non-small-cell lung cancer A549 cells with H2O2 can act as a model for assessing the antioxidant activity of novel compounds.
Furthermore, a range of natural products have been shown to have potential protective effects against chronic diseases (8, 9). Generally, the main objective is to highlight the role of some natural products which ameliorate oxidative stress and reduce pathological complications, related to chronic diseases. In this regard, H. Kalantari et al. reported that Morus alba L. and Ficus carica leaf extracts can improve the protective capacity against CCl4-induced oxidative damage in mice (10, 11).
Angipars™ is a registered drug, extracted from Melilotus officinalis through electromagnetic processes. It contains compounds, including 7-hydroxycoumarin and flavonoids and is available in markets in form of a suspension in 9.2% ethanol. Angipars™ has accepted safety and has been assessed in clinical trials; in fact, it has been registered as the first-line treatment for human diabetic foot ulcer (12-15). The major compound of this drug, coumarin, is recognized for its anti-inflammatory and antioxidant properties and has the capacity to inhibit superoxide and nitric oxide production in leukocytes, thereby, reducing the phagocyte activity (16).
2. Objectives
With this background in mind, in this study, we sought to assess the in vitro antioxidant capacity of Angipars™ and its protective role against H2O2-induced oxidative stress in HepG2 cells through evaluating cytotoxicity, cell viability, and the related mechanisms.
3. Methods
3.1. Cells and Chemicals
HepG2 and A549 cells were purchased from Pasteur Institute of Iran (Tehran, Iran). Fetal bovine serum (FBS) and RPMI were obtained from GibCo, while methylthiazole diphenyl tetrazolium bromide, trypan blue, and DTNB were purchased from Merck Co. (Germany). Also, Na2HPO4 and thiobarbituric acid (TBA) were obtained from Sigma Company. Other chemicals were of the highest quality accessible in the market. Angipars™, which contains compounds such as 7-hydroxycoumarin and flavonoids and is soluble in distilled water, was purchased from Pars Rooz Company (Tehran, Iran).
3.2. Phenolic Content Measurement
Total polyphenol content was determined by modifying the Folin-Ciocalteu method, using gallic acid as the standard (17). To prepare the calibration curve, 0.5 mL aliquots of 0.024 - 0.3 mg/mL gallic acid solution, 2 mL (75 g/L) of sodium carbonate, and 2.5 mL of Folin-Ciocalteu reagent were used. The final solution was mixed and absorption was calculated at 765 nm by a spectrophotometer after 30 min. Then, 0.5 mL of Angipars™ was added to the same reagents as described above, and absorption was calculated after 1 hour. All the experiments were run in triplicate. Gallic acid equivalent was calculated for determining the total content of phenolic compounds.
3.3. Antioxidant Assays
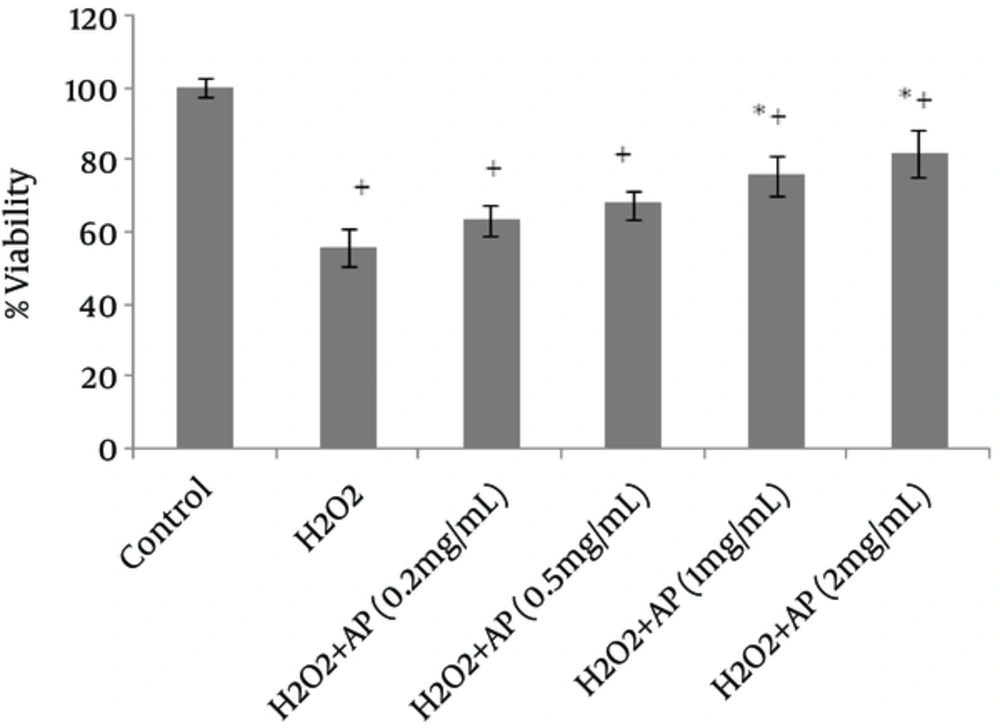
The antioxidant activity of Angipars™ was measured by evaluating the radical-scavenging ability, using DPPH radical (18). A range of concentrations (50 - 800 μg/mL; 20 μL) were mixed with DPPH ethanol solution (100 mM; 200 μL) (7). The plates were incubated for 30 minutes at 25°C, and absorbance was calculated at 492 nm, using a microplate reader. The gathered data were used to establish the half maximal inhibitory concentration (IC50) or 50% of the free-radical-scavenging activity. The results are presented as mean ± SD of three replicates (Figure 1).
3.4. Ferric-Reducing Antioxidant Potential (FRAP)
FRAP was determined according to the method described by Benzie with FeSO4.7H2O as the standard compound (19). Reagent A including 3.1 g of sodium acetate trihydrate and 16 mL of glacial acetic acid, reagent B consisting of 40 mM of HCl and 10 mM of TPTZ, and reagent C including 20 mM of FeCl3.6H2O were prepared. Before use, FRAP was prepared by mixing reagents A, B, and C (10: 1: 1). Afterwards, 30 μL of FRAP reagent was mixed in 10 μL of the standard compound and the samples. The absorbance rate was read at 593 nm with 1-minute intervals for 6 minutes.
3.5. Cell Culture
HepG2 and A549 cell lines were cultivated in RPMI, containing 37% NaHCo3, enriched with 10% FBS, streptomycin, and 1% penicillin (pH: 7.3) in moistened 5% CO2-95% air combination at 37°C. A549 human non-small-cell lung cancer cells were grown and cultured in Dulbecco's Modified Eagle Medium (DMEM) at 37°C under the abovementioned conditions. Then, 96-well microplates were used for cell seeding (30000 cells/well/90 μL) and were routinely cultured in an incubator with optimal humidity for 24 hours.
Oxidant exposure was initiated at 80% confluence, following antioxidant treatment. Different concentrations of Angipars™, ranging from 1 μg/mL to 1000 μg/mL (10 μL/well) were used to treat the cells at 1 hour prior to exposure to H2O2 solution (4 mM). The appropriate, safe, and non-toxic dose of Angipars™ was determined for further use in other experiments on cells.
Following the initial incubation for 24 hours, 10 μL of MTT was introduced into the wells, and re-incubation was performed for 4 hours. MTT solution and the culture medium were displaced and HepG2 cells were placed in the bed of the plates. Afterwards, dimethyl sulfoxide (DMSO; 100 μL) was added to the wells to liquefy the formed crystals of formazan; absorbance was assessed at 570 nm. For each concentration of Angipars™, five wells were utilized, and three independent experiments were performed in triplicate for each case.
3.6. Lipid Peroxidation
To determine lipid peroxidation in cells, 1 ml of the cell aliquot was treated with 70% trichloroacetic acid (TCA) and then boiled for 20 minutes with 0.8% TBA. Then, the level of TBA reactive substances (TBARS) was measured during the decomposition of lipid hydroperoxides, using a spectrophotometer at 532 nm (20).
3.7. Glutathione (GSH) and Glutathione Disulfide (GSSG) Content Measurement
Enzymatic recycling method was used to determine the reduced (GSH) and oxidized (GSSG) glutathione states. For determining the GSH content, the aliquot of cell suspension (1 mL) was prepared, mixed with 5% TCA (2 mL), and centrifuged. Afterwards, 3.0 mL of phosphate buffer (pH: 8.0) and 0.5 mL of Ellman’s reagent were added to it. Absorbance of the produced dye was set by the spectrophotometer at 412 nm. For evaluating the GSSG content, the cellular GSH was covalently bonded to 2-vinylpyridine. Then, triethanolamine was neutralized with 2-vinylpyridine, and GSSG was decreased to GSH by nicotinamide adenine dinucleotide phosphate (NADPH) and GSH reductase enzyme (21).
3.8. Statistical Analysis
The data are presented as mean ± SD of a minimum of three distinct tests. For statistical analysis, Tukey’s post-hoc test and ANOVA were performed, using SPSS version 11.5 (SPSS Inc., Chicago, IL, USA). Significant difference among the experimental and control groups was set at P < 0.05.
4. Results
The analysis results of DPPH radical-scavenging activity, total phenolic content, and FRAP assay of Angipars™ are presented in Table 1. The phenolic content (mg/g dry weight) of Angipars™ was measured as gallic acid equivalent. The phenolic content was measured to be 17 ± 6 mg/g. The percent deterrence of DPPH radical by Angipars™ (117 ± 25) was compared with a known antioxidant, quercetin. Angipars™ demonstrated significant antioxidant capacity, based on FRAP test results. It showed 53 ± 12 mM Fe2+/mg sample value, which is somehow similar to the diminishing power of ascorbic acid (60.75 mM Fe2+/mg sample) (Table 1).
| Total Phenolic Content, Expressed as mg (Gallic Acid)/g (Dry Weight) | IC50 for DPPH Assay, μg/mL | FRAP |
|---|---|---|
| 17 ± 6 | 97 ± 25 | 53 ± 12 |
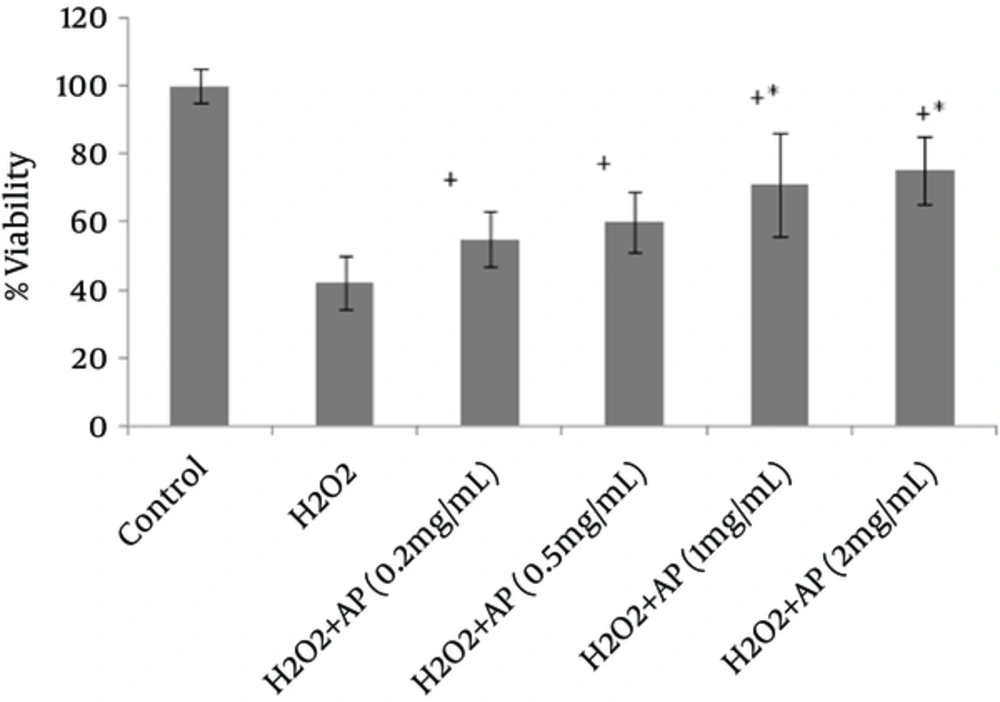
Viability of the cells, as assessed by MTT assay, remarkably declined following HepG2 and A549 cell exposure to H2O2 (Figures 1 and 2). However, once the cells were incubated beforehand with Angipars™ (0.2 - 2 mg/mL), H2O2-induced cell toxicity was significantly attenuated. The protective effect of Angipars™ was dose-oriented and the greatest effects were reported at the highest concentration (2 mg/mL).
Incubation of the cells with 300 µM of H2O2 for 4 hours diminished the GSH content and raised the GSSG level; also, TBARS level of HepG2 and A549 cells markedly reduced (Tables 2 and 3). Overall, the protective role of Angipars™ cannot be attributed to its effects on cell GSH content. Exposure to H2O2 caused a marked increase in TBARS level, a rise more than the control level (untreated), whereas pretreatment with 0.2 - 2 mg/mL of Angipars™ significantly prevented H2O2-induced lipid peroxidation.
| GSH, µmol/ 3 × 103 Cell | GSSG, µmol/ 3 × 103 Cell | TBARS, ng/ 5 × 105 Cell | |
|---|---|---|---|
| Control | 24.96 ± 2.66 | 3.83 ± 0.103 | 0.889 ± 0.043 |
| H2O2, 300 μM | 12.52 ± 3.38b | 17.02 ± 3.47c | 1.99 ± 0.032b |
| +Angipars, 0.5 mg/mL | 17.101 ± 3.81 | 11.17 ± 4.71 | 0.638 ± 0.047d |
| +Angipars, 1 mg/mL | 28.65 ± 2.52d | 15.26 ± 1.79 | 0.836 ± 0.064d |
| +Angipars, 2 mg/mL | 15.431 ± 2.81 | 18.87 ± 3.65 | 0.651 ± 0.151d |
aValues are expressed as mean ± SD.
bSignificant difference with the control group (P < 0.05).
cSignificant difference with the control group (P < 0.001).
dSignificant difference with the H2O2 group (P < 0.001).
| GSH, µmol/ 3 × 103 Cell | GSSG, µmol/ 3 × 103 Cell | TBARS, ng/5 × 105 Cell | |
|---|---|---|---|
| Control | 26.96 ± 3.11 | 3.32 ± 0.2 | 0.929 ± 0.052 |
| H2O2, 300 μM | 14.52 ± 2.98b | 15.82 ± 4.22c | 2 ± 0.066b |
| +Angipars, 0.5 mg/mL | 19.101 ± 2.1 | 11.17 ± 4.71 | 0.638 ± 0.047d |
| +Angipars, 1 mg/mL | 25.45 ± 2.58d | 18.26 ± 1.79 | 1.336 ± 0.24d |
| +Angipars, 2 mg/mL | 15.431 ± 2.81 | 12.87 ± 3.22 | 0.651 ± 0.151d |
aValues are expressed as mean ± SD.
bSignificant difference with the control group (P < 0.05).
cSignificant difference with the control group (P < 0.001).
dSignificant difference with the H2O2 group (P < 0.001).
5. Discussion
Oxidative stress plays an important role in the etiology of an array of human diseases (6). Some in vitro investigations have confirmed that oxidative stress caused by chemical oxidants, including H2O2, gives rise to cell death. H2O2 has been also demonstrated to cause apoptotic variations, which consequently result in the death of a number of cell structures (18), including human liver cells (HepG2) (17, 20) and non-small-cell lung cancer A549 cells (21-23). In the present study, H2O2 was selected to bring about oxidative stress-associated cytotoxicity in HepG2 and A549 cells.
As oxidative stress seems to be influential in many disorders, antioxidant management may be helpful in controlling and managing these diseases. Today, there is a growing interest in bioactive, naturally derived compounds, with significant cytoprotective effects against cell death caused by oxidative stress (24-27). Angipars™, as a new herbal drug, consists of Melilotus officinalis extracts, 7-hydroxycoumarin, oleanene glucuronide, urea, selenium, flavonoids, and fructose. According to the literature, Melilotus officinalis can reduce the activity of circulating phagocytes and exhibit anti-inflammatory, anti-edematous, and antioxidant activities (28).
In this regard, Larijani et al. (29) in their study on the antioxidant effects of Angipars™ concluded that this agent contains substances such as hydroxycoumarin and flavonoids. In the present study, the effects of Angipars™ (0.2 - 2 mg/mL concentrations) on H2O2-induced cytotoxicity were investigated in HepG2 and A549 cell lines through MTT assay, GSH content analysis, and lipid peroxidation evaluation. According to the obtained results, preincubation of 2 mg/mL of Angipars™ before H2O2 exposure had the greatest positive effects on cell viability and lipid peroxidation products in HepG2 and A549 cells. In conclusion, the present findings can highlight the benefits of Angipars™ on cytoprotection, resulting from its anti-oxidative properties.