1. Background
Acne vulgaris is one of the most prevalent dermal diseases (1), the eighth most common disease in the world, and 650 million people are estimated to suffer from it (2). Acne vulgaris affects about 80% of individuals between 12 and 14 years (the highest at-risk age group), 8% between 25 and 34 years, and 3% of those aged 35 to 44 years (3). Besides the financial burden of acne vulgaris treatment, recent studies revealed that some psychosocial problems are related to acne vulgaris as detrimental as conditions such as seizures and diabetes, and even higher than those of arthritis and asthma (4).
Several methods are used to treat acne depending on its severity, including topical treatment, oral antibiotics, oral anti-androgens, and even chemical peeling (5). The inefficacy of some available medicines, in addition to their side effects, as well as the scar resulting from acne and subsequent changes in the patient’s skin, necessitates the discovery of alternative medicines and methods for acne treatment (6). A global tendency to use natural treatments and traditional medicines is recently increased among patients and physicians, and scientific evidence has increased in this regard (7, 8). Studies have demonstrated that using medicinal plants with antimicrobial and anti-inflammatory properties could be effective against acne (9).
According to Persian medicines textbooks, Prunus domestica L., Tamarindus indica L., Terminalia chebula L., Ziziphus jujube L., and Cassia fistula L. may be effective in acne treatment (10). In addition, in recent studies, these medicinal plants have been demonstrated to be effective in treating acne lesions due to their antibacterial and anti-inflammatory effects (11-15).
2. Objectives
Hence, these medicinal plants were selected in this study to evaluate their effectiveness in reducing the symptoms of mild-to-moderate acne vulgaris.
3. Methods
3.1. Study Design
The current study was a triple-blinded randomized clinical trial on mild to moderate acne vulgaris. This trial was conducted at the Skin Clinic’s Ahmadieh clinic of the Persian Medicine School of Tehran University of Medical Sciences from 2017 to 2018. Ethical approval with the code IR.TUMS.VCR.REC.1397.543 was obtained from the Tehran University of Medical Sciences Ethics Commission. Before commencing this study, each participant signed a written informed consent form and was informed of the intervention's benefits and drawbacks. This study was registered at the Iranian registry of clinical trials (IRCT) (registration number: IRCT20191103045318N1).
3.2. Participants
Adult (≥ 18 years old) patients with mild to moderate acne based on the global acne grading system (GAGS) (16) from either sex were eligible to enter the study. Those with the following characteristics were excluded: Severe acne, pregnancy or breastfeeding, use of medications that influenced acne within the two last weeks, any infection or injury to the face, any facial procedure over the last 6 months, systemic diseases, and consumption of related drugs, stomachache and heartburn, inflammatory and peptic ulcer diseases, and defecation frequency more than 3 times a day.
3.3. Sample Size
The sample size was calculated by following the formula using the PASS 11 software. According to previous studies (17) and considering μ1 = 12, μ2 = 15.6, σ = 6, α = 0.05, β = 10%, the sample size was computed to be approximately 44. The significant level of the tests was also considered as P ≤ 0.05. Finally, assuming a 20% loss, the sample size was considered to be 57 patients in each group.
3.4. Randomization and Intervention
The simple random sampling method was used for this trial, and to create the random sequence in the selection of two arms, the coin-tossing method was used. Based on the sample size, the coin was tossed in the same number, and the subjects were assigned to one of the groups randomly. Participants in each group consumed 10 mL of either the herbal product or placebo syrup 3 times (every 8 hours) daily for 12 weeks. After that, they were followed to assess the symptoms’ recurrence for 4 weeks.
3.5. Drug Preparation
The adequate amount of ingredients, including plum (Prunus domestica L.), yellow myrobalan (Terminalia chebula L.), jujube (Ziziphus jujube L.), golden shower (Cassia fistula L.), tamarind (Tamarindus indica), honey, brown sugar, and vinegar, were provided from traditional herbal medicine vendors to prepare the herbal syrup. Plants were identified and confirmed by a botanist at the Herbarium Center of the School of Pharmacy, Tehran University of Medical Sciences, with the following voucher numbers: Tamarindus indica L: PMP-2684, Ziziphus jujube Mill: PMP-2680, Prunus domestica L: PMP-2683, Cassia fistula L: PMP-2681, Terminalia chebula Retz: PMP-2682.
Initially, plum, myrobalan, jujube, golden shower, and tamarind were soaked in water at normal temperature for 24 hours, separately. Plum, jujube, and tamarind were first heated in 50°C water and blended. Then, the extract of myrobalan, vinegar, sugar, and honey was added gradually, and finally, the golden shower extract was dissolved in the mixture. The overall process of syrup production took approximately an hour, and the syrup was stored in 240 cc bottles. All measurements for determining pH, density, viscosity, microbial, and phenolic content were conducted to standardize the syrup. These parameters are crucial in ensuring the consistent quality and safety of the syrup.
On the other hand, to reach the same appearance, taste, color, texture, and odor, the placebo syrup consisted of sugar, a brown color additive, and little tamarind essential oil. There was no detectable difference in any feature of these two syrups.
3.6. Outcomes
Initially, a socio-demographic questionnaire, which contained age, gender, job, marital status, duration of disease, consumed medications, and comorbidities, was completed by all participants. Patients were visited by an expert researcher who was blinded to groups at the baseline of the study (t0), after 6 weeks (t6), after 12 weeks (t12) which was the end of the intervention, and after 16 weeks (t16), as a follow-up. At each visit, the number of inflammatory (papule, pustule, nodule) and non-inflammatory (comedones) lesions were counted in any clinical observation. The acne severity index (ASI) score was calculated by this formula: 0.25 × comedone number + 2 × pustule number + papule number (18). Furthermore, a checklist was used to assess potential side effects related to the intervention.
Patients’ psychological status and quality of life were assessed by a validated Persian version of the Cardiff acne disability index (CADI) (19). In CADI, a 5-item questionnaire, each question is given a score between 0 - 3; therefore, the overall score ranges from 0 to 15. A higher score indicates a more severe impact of acne.
3.7. Statistical Analyses
The obtained data were analyzed using SPSS software (SPSS Inc. version 18.0. Chicago, IL, USA). To assess the demographic data, the quantitative variables were expressed as mean ± standard deviation (SD) and analyzed by the one-way analysis of variance (ANOVA) test. In contrast, qualitative variables were represented as a percentage (%) and analyzed by the chi-square test. To compare the mean scores of the variables between two groups of intervention and placebo, including papules, pustules, nodules, comedone, and ASI in four-time points, the independent sample t-test was applied. Cardiff acne disability index score was analyzed by independent t-test and paired t-test for between- and within-group analysis, respectively. The significant level of the tests was also considered as P ≤ 0.05.
4. Results
4.1. Quality Control of Herb
The pH of the syrup was measured to be 3.17, indicating an acidic nature. The viscosity of the syrup was found to be 6.8, which suggests a relatively thick consistency. The total phenolics content was determined to be 151 ± 2.1 (mg/100 mL), indicating the presence of bioactive compounds in the syrup.
4.2. Antimicrobial Test
The number of fungi and bacteria was 0 (CFU/g), indicating no detectable microbial growth. Furthermore, the absence of Escherichia coli and Salmonella species was confirmed in the syrup sample, indicating that it is free from pathogenic bacteria.
4.3. Baseline Characteristic
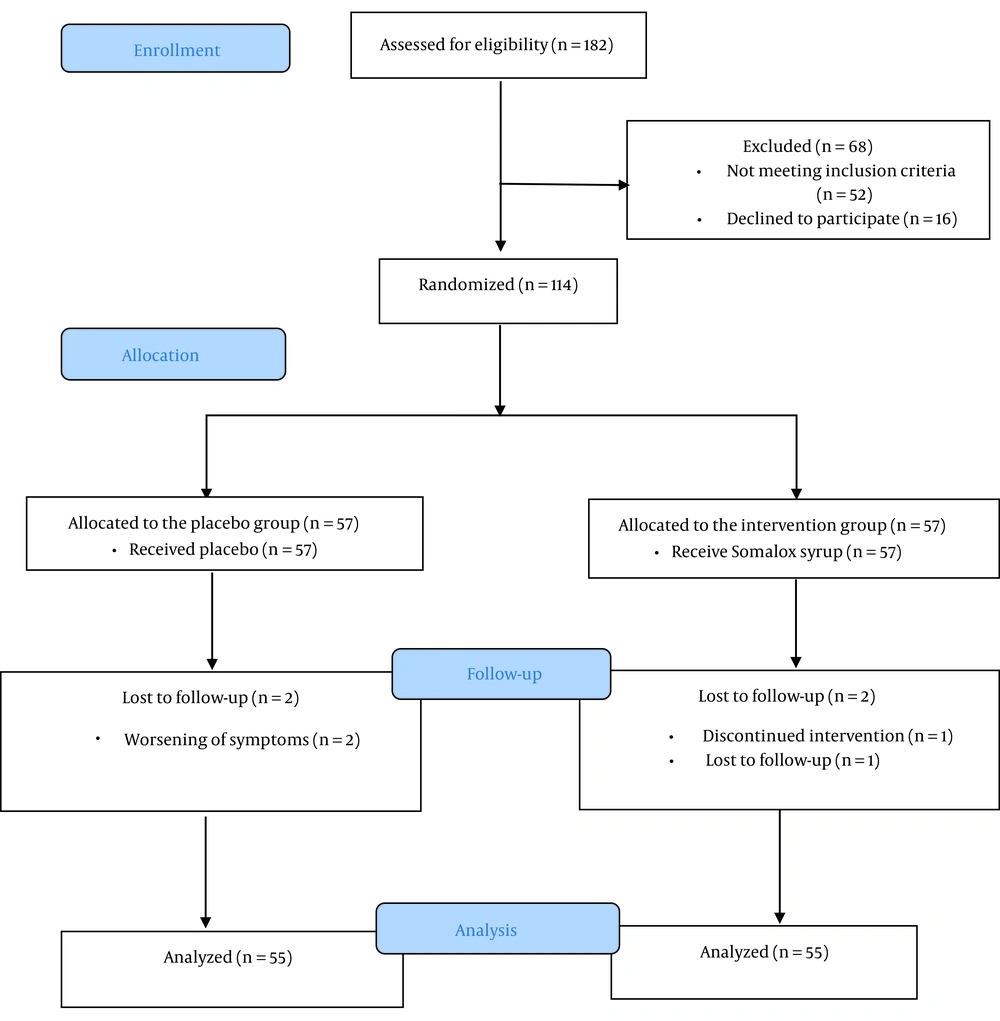
A total of 182 patients were initially screened; 68 did not enter the study, 52 did not meet the inclusion criteria, and 16 refused to participate. Thus, 114 participants were randomized into two groups (57 patients in each group). Finally, 2 patients from the intervention group and 2 from the placebo group were excluded, and data from 55 patients in each group were analyzed (Figure 1). The mean age was 22.73 ± 3.68 years in the intervention group and 22.11 ± 3.89 years in the control group, with no statistically significant difference (P = 0.390). Twenty percent of the participants in the intervention group and 16 % of the control group were male (P = 0.621). No significant difference was shown considering demographic characteristics between groups (Table 1).
4.4. Efficacy Evaluation
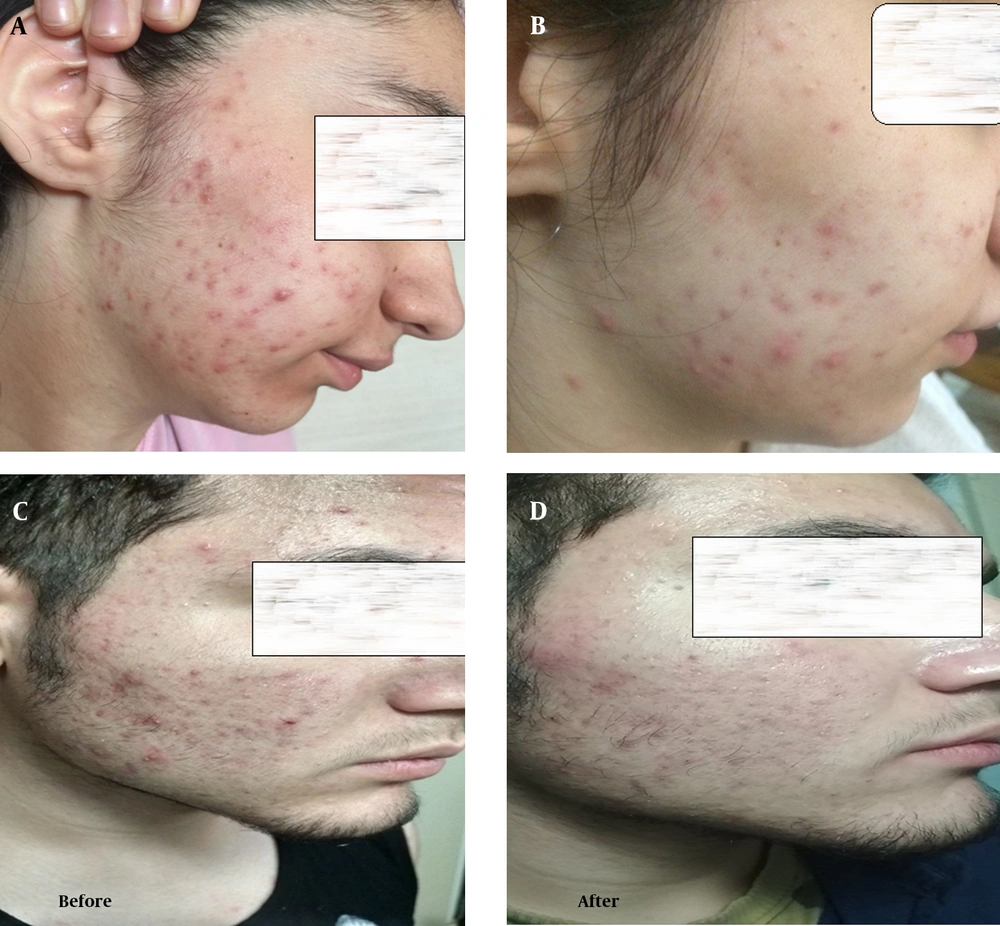
Comparison of the comedone, papule, pustule, nodule, and ASI in different time points in the two experimental and control groups showed that there was no significant mean difference of scores in the baseline and week 6 (P ≥ 0.05). However, the results demonstrated a statistically significant difference between the herbal product and placebo groups in the week 12, and patients in the intervention group experienced a significant reduction in all four types of lesions and ASI (P < 0.05). After four weeks of follow-up, symptoms related to acne recurred, and the two groups did not have any significant differences in the 16th week (Table 2). Photographs of two patients (a male and a female) who received the herbal syrup at the baseline and end of the treatment (at the 12th week) are shown in Figure 2.
| Symptoms | Mean ± SD | Mean Difference (95% CI) | t (df) | P-Value a | |
|---|---|---|---|---|---|
| Intervention | Placebo | ||||
| Comedo | |||||
| Baseline | 40.93 ± 9.43 | 39.75 ± 9.92 | 1.18 (-2.48, 4.84) | 0.64 (108) | 0.523 |
| Week 6 | 39.76 ± 10.30 | 39.53 ± 11.16 | 0.24 (-3.82, 4.29) | 0.11 (108) | 0.908 |
| Week 12 | 35.58 ± 11.69 | 40.13 ± 9.95 | -4.54 (-8.65, -0.44) | -2.19 (108) | 0.030 b |
| Week 16 | 40.37 ± 11.44 | 39.02 ± 7.78 | 1.71 (-1.99, 5.41) | 0.916 (108) | 0.362 |
| Papule | |||||
| Baseline | 15.78 ± 7.54 | 16.64 ± 6.46 | -0.85 (-3.51, 1.80) | -0.64 | 0.525 |
| Week 6 | 15.36 ± 6.73 | 16.35 ± 8.23 | -0.98 (-3.82, 1.86) | -0.68 | 0.495 |
| Week 12 | 12.60 ± 6.75 | 15.13 ± 6.30 | -2.58 (-4.99, -0.06) | -2.03 | 0.045 b |
| Week 16 | 14.82 ± 5.77 | 15.07 ± 6.14 | -0.25 (-2.51, 2.00) | -0.22 | 0.823 |
| Pustule | |||||
| Baseline | 7.22 ± 4.20 | 6.29 ± 4.47 | 0.93 (-0.71, 2.56) | 1.12 | 0.264 |
| Week 6 | 6.85 ± 5.33 | 5.78 ± 4.08 | 1.07 (-0.72, 2.87) | 1.18 | 0.238 |
| Week 12 | 4.64 ± 4.14 | 6.27 ± 3.44 | -1.63 (-3.08, -0.20) | -2.25 | 0.026 b |
| Week 16 | 7.16 ± 5.39 | 5.80 ± 4.20 | 1.36 (-0.46, 3.19) | 1.48 | 0.142 |
| Nodule | |||||
| Baseline | 1.67 ± 1.40 | 1.76 ± 1.35 | -0.09 (-0.61, 0.42) | -0.35 | 0.729 |
| Week 6 | 1.51 ± 1.44 | 1.58 ± 1.34 | -0.07 (-0.60, 0.45) | -0.27 | 0.785 |
| Week 12 | 1.05 ± 0.99 | 1.44 ± 0.98 | -0.38 (-0.75, -0.01) | -2.04 | 0.044 b |
| Week 16 | 1.44 ± 0.81 | 1.47 ± 0.86 | -0.04 (-0.35, 0.28) | -0.23 | 0.820 |
| ASI | |||||
| Baseline | 40.45 ± 12.51 | 39.15 ± 11.18 | 1.29 (-3.18, 5.78) | 0.57 | 0.568 |
| Week 6 | 39.01 ± 12.43 | 37.79 ± 11.54 | 1.22 (-3.31, 5.75) | 0.53 | 0.594 |
| Week 12 | 30.77 ± 9.01 | 37.70 ± 7.80 | -6.94 (-10.12, -3.75) | -4.31 | < 0.001 b |
| Week 16 | 39.33 ± 12.20 | 36.42 ± 11.33 | 2.90 (-1.55, 7.35) | 1.29 | 0.199 |
The Trend of Changes in the Acne Severity Index and the Total Number of Lesions in the Three Months of Treatment in Two Experimental and Control Groups
The between-group comparison of CADI score results indicated a significant difference at the study’s baseline (P = 0.006). Between-group analysis showed that the score of CADI in the intervention group decreased at the end of the study, while it increased in the placebo group. However, these changes were not significant, neither in the intervention group (P = 0.372) nor the placebo group (P = 0.460) (Table 3).
Nine patients reported adverse reactions, 8 from the intervention group (diarrhea, stomachache, and heartburn) and 1 from the placebo group (stomachache); furthermore, only 2 patients from the control group reported worsening of acne lesions.
5. Discussion
This clinical trial investigated herbal syrup's effect on treating patients with mild to moderate acne vulgaris. The results showed a significant decrease in the mean number of comedones, papules, pustules, and nodules and a reduction in ASI after 12 weeks of herbal syrup consumption. However, the results did not sustain after 4 weeks from discontinuing intervention.
There is a wide variety of methods for treatments of acne, such as anti-androgen, hormonal, or anti-seborrheic medicines and medications like retinoids, isotretinoids, azelaic acid, and salicylic acid, but many have some side effects (9). Herbal medicine has been used since antiquity in different conditions such as cardiovascular diseases, diabetes, hypertension, and cancer (9, 20). Many patients with acne or other infectious skin diseases use complementary and alternative medicine interventions, particularly medicinal plants (21). A systematic review by Fisk et al. demonstrated that approximately 23 studies indicated the positive effects of herbal products on acne vulgaris (22).
Several medicinal plants have demonstrated suppressive effects on the growth of bacteria, fungi, and viruses under ex vivo conditions, and some of them have been proven to have anti-inflammatory and lipid-lowering properties (23). Thus, herbs can be used along with other therapeutic measures or topical or orally in the case of acne. Traditional Persian medicine recommends some specific medicinal plants, such as plants used in the present study, that can be helpful in combination with conventional medications for the treatment of acne vulgaris (24).
Plum contains many bioactive compounds such as chlorogenic acids, anthocyanins, flavanols, flavonols, and coumarins with antimicrobial and antioxidant properties (25), which can alleviate inflammation and skin dryness from acne (26). In Persian medicine, the plum as cold-tempered food has laxative and moisturizer properties, which help to decrease inflammation and irritation (26). Plums' antimicrobial, anti-inflammatory, and anti-allergic effects were observed in recent studies (27).
According to Persian medicine, tamarind contains tannin, carbolic acid, and resin substances with cold-dry nature (28). A study by Waqas et al. demonstrated skin lightening and sebum reduction properties of tamarind seed extract, which could be useful in acne treatment (29). One of its important therapeutic properties is detoxification of the body to adjust the temperaments, particularly hot temperaments such as eczema and bile heat. For this reason, regular use of the tamarind extract considerably helps improve dermal diseases such as acne and rashes (30).
Jujube is a plant with a high medicinal value used traditionally in different conditions such as swollen joints, constipation, diarrhea, burns, and acne (31). About 79 bioactive compounds in this herb indicate various pharmacological effects of jujube, like anti-inflammatory, antifungal, antiulcer, anti-stress, and sedative properties (32). Several studies demonstrated a strong association between psychological stress and acne severity (33, 34). An animal study by Al-Reza and colleagues demonstrated that jujube essential oil could improve the inflammatory level in the skin (35).
Golden shower (Cassia fistula) is a popular medicinal plant that has been traditionally used alone or in combination with other herbs in Unani and Ayurvedic medicines for the treatment of different conditions such as liver disorders, diabetes, or skin diseases (36). It is widely used in dermatological problems, such as pruritus, leucoderma, impetigo, eczema, and ringworm (37). Due to this plant's antioxidant, anti-inflammatory, antimicrobial, and antifungal activities, traditional Persian medicine recommends it as an effective agent in treating acne. A trial by Khan and collaborators on healthy adult men concluded that C. fistula extract could improve skin hydration and help rejuvenate skin aging (38). Another study approved the efficacy and safety of this herb as a purgation therapy for skin diseases (39). In addition, creams that contain T. chebula demonstrated beneficial effects on rejuvenating human skin (40). An experimental study by Swindell et al. indicated that the positive effects of Terminalia chebula are not limited to the dermal layer and result from a change in the epidermis (41).
Psychological disorders should be considered when treating acne because they may lead to depression and anxiety (42). In the current study, there was a significant difference between groups regarding CADI scores at the beginning. This difference became more significant by an increase in score in the placebo group and a decrease in score in the intervention group after 12 weeks of study, but these changes were not significant. Some studies have demonstrated that it's possible that the improvement in acne may be attributed to psychological factors, leading to an overall improvement in one's quality of life (43, 44).
In the present study, although the number of adverse drug reactions in the intervention group was higher than in the control group, this difference was not significant.
Although this is the first study with a sufficient and appropriate sample size, triple-blinded design, and follow-up after 12 weeks of intervention, there were some limitations in the study. Firstly, no biochemical markers were analyzed during the study, which was due to limited resources. Secondly, a questionnaire was used to measure the number of comedones, papules, pustules, and nodules, as well as the ASI.
To sum up, the findings of the current trial showed that the herbal syrup containing plum, jujube, yellow myrobalan, golden shower, tamarind, and honey could effectively ameliorate inflammatory and non-inflammatory lesions in acne vulgaris. Therefore, this syrup can be considered a complementary medicine compound, but more studies are required to obtain more power and assess the long-term effects of this herbal product.
.jpg)