1. Background
Acetaminophen (APAP) is a very safe over-the-counter (OTC) analgesic and antipyretic agent. However, intoxication from overdosing or the chronic use of APAP, nutritional deficiency, alcoholism, malabsorption syndromes, and CYP2E1 inducers may cause liver injury, which is characterized by elevated serum aminotransferases, hemorrhage, and central lobular necrosis, leading to death in a complicated situation (1, 2).
APAP hepatotoxicity begins with the metabolism of the N-acetyl-p benzoquinone imine (NAPQI) by cytochrome P (CYP)-450, which is very reactive. NAPQI depletes glutathione (GSH) and binds to proteins. Moreover, it causes oxidative stress and mitochondrial dysfunction (3, 4). However, the liver can regenerate hepatocytes. Due to the slow rate of regeneration, liver protectants such as antioxidants play an essential role in increasing survival rates by fighting against free radical species (5, 6).
Recently, evaluating the protective potentials of plants and phytochemicals has attracted much attention due to their natural origin, lower cost, higher effectiveness, and fewer side effects. These components can range in molecular weight from monomers to long-chain polymers (7, 8). Epicatechin (EPC) is one of the most potent antioxidants existing in the human diet and can be found predominantly in grapes, tea, apples, and cocoa (9). EPC is a significant member of the flavanol family with biological activities, including preventing LDL oxidation (10, 11), scavenging reactive oxygen species (ROS) (12), and anti-inflammatory activity (13).
Epidemiologic and animal studies have shown that EPC has beneficial health effects on cancer (14), inflammation, and diabetes (15, 16). In addition, EPC decreases infarct size and blood pressure (17). EPC has direct and indirect antioxidant properties. A direct antioxidant effect includes scavenging free radicals and chelating redox-active metals (18, 19). However, indirect antioxidant effects of EPC include modulating the NADPH oxidase (20), inhibiting the binding of tumor necrosis factor (TNF-α) to its receptor (15), interfering with NF-κB activation (21), and activating Nrf2 (22, 23). Moreover, EPC has a beneficial effect on reducing the steroid-induced fatty liver by activating AMPK, which increases fat oxidation and prevents hepatic steatosis (24).
2. Objectives
The present study aimed to evaluate the preventive and therapeutic effects of EPC against APAP-induced liver injury.
3. Methods
3.1. Chemicals
Epicatechin, thiobarbituric acid, trichloroacetic acid, and 5,5′-dithiobis (2-nitrobenzoic acid) were purchased from Sigma Aldrich. Ammonium molybdate, hydrogen peroxide (H2O2), APAP, and ethylenediaminetetraacetic acid (EDTA) were also obtained from Sigma Aldrich.
3.2. Animals and Ethical Statements
All animal experiments were approved by the Institutional Animal Care and Use Committee of Ahvaz Jundishapur University of Medical Sciences (AJUMS). Male BALB/c mice aged six to eight weeks, weighing around 25±2 g, were used. Mice were housed under a controlled light (6 AM)/dark (6 PM) cycle at 25 ± 2ºC and given free access to rodent chow and acidified (tap) water until experimentation. The animal studies were approved by the ethical committee of the AJUMS with the approval of code IR.AJUMS.REC.1394.730.
3.3. Experimental Design
This study was conducted using seven preventive and seven therapeutic groups to evaluate the effect of EPC on APAP-induced hepatotoxicity. The groups of six mice received intraperitoneal injections of saline, EPC, and APAP.
Group 1: Saline (10 mL/kg)
Group 2: EPC (100 mg/kg) + saline (10 mL/kg)
Group 3: Saline + APAP 400 mg/kg
Groups 4, 5, 6: EPC (25, 50, 100 mg/kg) + APAP 400 mg/kg
Group 7: N-acetyl cysteine (NAC) 50 mg/kg + APAP 400 mg/kg
In therapeutic groups, animals received EPC just before and 2 hours after the APAP injection. After receiving an APAP injection and fasting overnight, all animals were sacrificed under anesthesia. The serum was obtained through centrifugation of the blood collected via cardiac puncture at a rate of 3000×g 15 min. The liver was rapidly removed for histopathologic examination and measurement of total thiol (TT), TBARS (thiobarbituric acid reactive substances), and catalase activity (CAT). The level of protection was assessed utilizing biochemical markers, namely serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST).
3.4. Determination of Liver Markers in Serum
The activity of ALT and AST was determined according to the protocols provided with the commercial kits (Pars Azmoon Company).
3.5. Determination of Thiobarbituric Acid Reactive Substances
A standard protocol was used to estimate TBARS in homogenate liver samples (25). Briefly, the supernatant of liver samples was incubated with thiobarbituric acid at 95°C for 10 min, and the absorbance of the supernatant was measured at 532 nm. The amount of lipid peroxidation was determined using ɛ = 1.56 × 105 /M/cm and expressed as nmol/g tissue.
3.6. Determination of Total Thiol
Total thiol was determined according to the method reported by Ellman’s method (26). Liver supernatants were incubated with Ellman’s reagent, and the absorbance of the yellow-colored complex was read at 412 nm. The result was expressed as µmole/g tissue.
3.7. Determination of Catalase Activity
Catalase activity was investigated according to the method used by Goth (27). The sample and H2O2 were mixed and incubated for 10 minutes before adding ammonium molybdate. At 410 nm, the absorbance was measured, and the result was expressed as U/g tissue.
3.8. Histopathological Examination
The livers of mice were fixed in a 10% formalin solution and subsequently embedded in paraffin. The specimens were sliced into sections measuring 5 µm and subsequently subjected to staining via hematoxylin and eosin (H&E).
3.9. Statistical Analysis
One-way analysis of variance (ANOVA) with Tukey's post-hoc was used for statistical evaluation of biochemical factors. For statistical analysis, the PRISM 9 software was used, and P < 0.05 was considered significant.
4. Results
4.1. Preventive and Therapeutic Effects of EPC on Hepatic Histopathology in Mice Model of APAP Hepatotoxicity
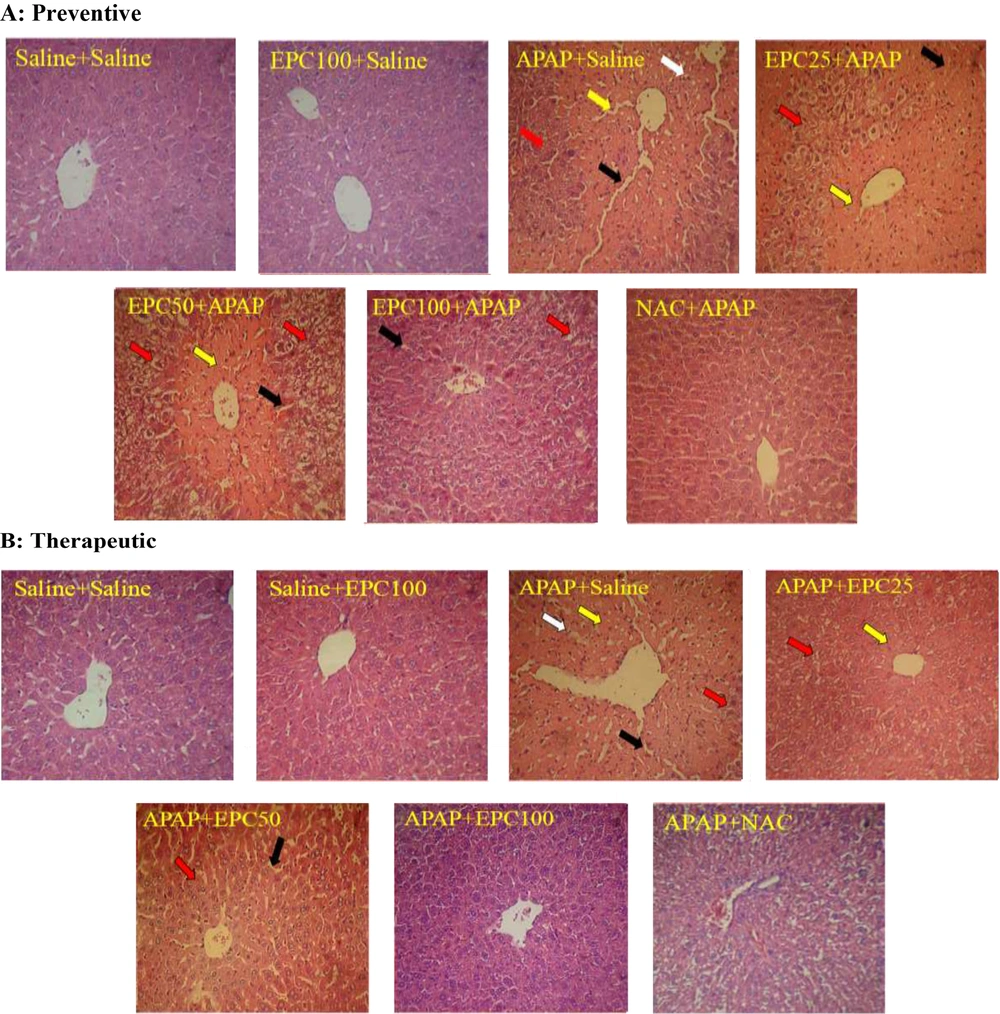
The representative histopathological examination of liver tissue is shown in Figure 1, and the descriptive histological scoring of liver damage is presented in Table 1. Liver sections showed normal hepatocytes in the saline group. Degeneration and coagulative necrosis in hepatocytes were observed in the APAP-treated group. Histopathological examination of the liver of the EPC (100 mg/kg)-treated mice in both preventive and therapeutic groups indicated similar results compared to their control groups. The histopathological analysis of the liver of mice co-treated with APAP and EPC resembled that of the control group in the therapeutic group. This indicates the protective effects of EPC in the APAP model of hepatotoxicity.
Preventive and therapeutic effects of epicatechin (EPC) and N-acetylcysteine (NAC) on APAP (acetaminophen)-induced hepatic histopathological injury in mice (n = 6 in each group). Preventive (A) and therapeutic (B) effects. Coagulative necrosis in hepatocytes (yellow arrow), fatty change in hepatocytes (red arrow), inflammatory cells (white arrow), and dilated sinusoids (black arrow). Saline, EPC 100 mg/kg (EPC100), APAP 400 mg/kg (APAP), EPC 25 mg/kg+ APAP 400 mg/kg (EPC25+ APAP), EPC 50 mg/kg + APAP 400 mg/kg (EPC50+APAP), EPC 100 mg/kg + APAP 400 mg/kg (EPC100+ APAP), NAC 50 mg/kg + APAP 400 mg/kg (NAC+APAP).
| Preventive Groups | Saline | EPC 100 | APAP | EPC 25 + APAP | EPC 50 + APAP | EPC 100 + APAP | NAC + APAP |
|---|---|---|---|---|---|---|---|
| Semi-quantitative Scoring of Damage on Histopathological Examination of the Liver | |||||||
| Degeneration of hepatocytes | - | - | ++++ | +++ | +++ | + | + |
| Fatty change in hepatocytes | - | - | ++++ | +++ | ++++ | ++ | _ |
| Coagulative necrosis in hepatocytes | - | - | ++++ | ++++ | +++ | + | + |
| Infiltration of inflammatory cells | - | - | +++ | +++ | ++ | + | + |
| Dilated sinusoids | - | - | ++ | + | ++ | + | + |
| Therapeutic groups | Saline | EPC 100 | APAP | APAP+ EPC 25 | APAP+ EPC 50 | APAP+ EPC 100 | APAP+ NAC |
| Therapeutic Groups | |||||||
| Degeneration of hepatocytes | - | - | ++++ | ++ | + | - | +++ |
| Fatty change in hepatocytes | - | - | ++++ | ++ | + | + | ++ |
| Coagulative necrosis in hepatocytes | - | - | ++++ | + | + | - | + |
| Infiltration of inflammatory cells | - | - | +++ | + | + | + | + |
| Dilated sinusoids | - | - | ++ | - | ++ | - | _ |
a (-) Normal, (+) mild, (++) moderate, (+++) severe, and (++++) extremely severe.
Nonetheless, the liver sections in the group that received preventive treatment exhibited a lower degree of improvement compared to the control group. Compared to the APAP-treated group, the livers of the EPC- and NAC-treated groups exhibited a significant improvement in their histological structure, as indicated by a decrease in the severity of hepatic degeneration, fatty changes, infiltration of inflammation cells, coagulative necrosis, and dilation of sinusoids.
4.2. Preventive and Therapeutic Effects of EPC on Liver Function Enzymes in Mice Model of APAP Hepatotoxicity
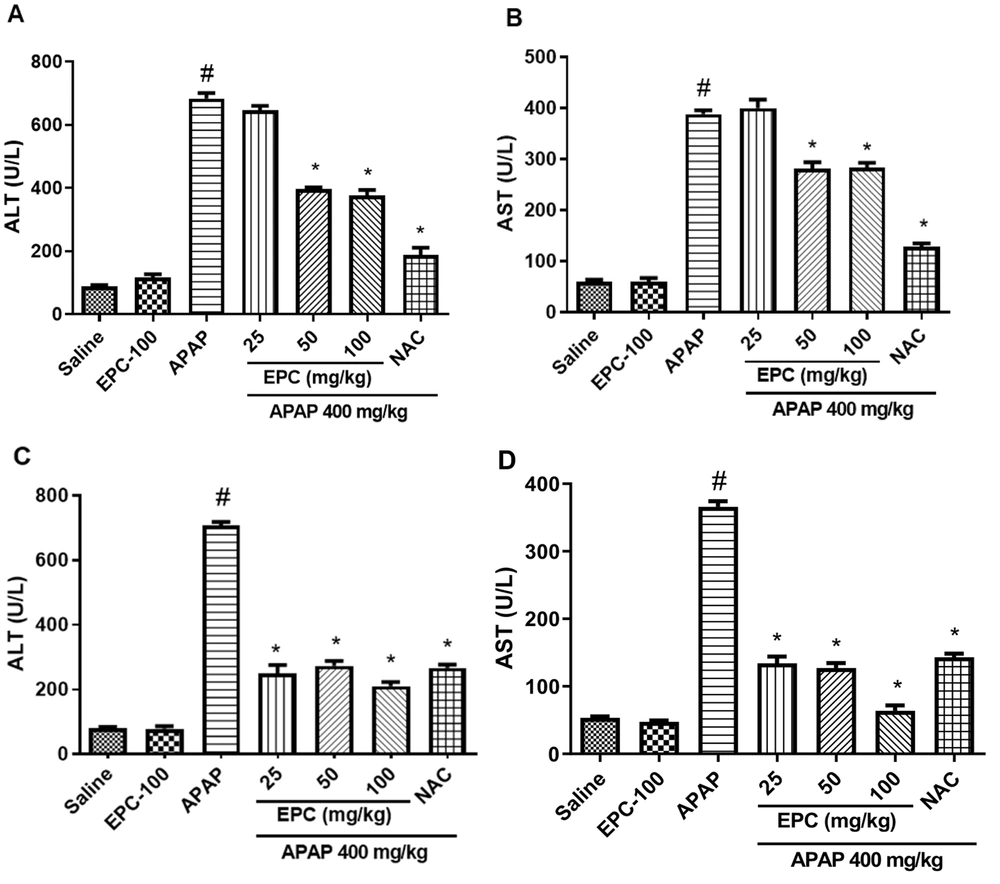
EPC did not show significant alterations in the biochemical parameters compared to the control group, but APAP significantly elevated the ALT and AST levels. Even so, the administration of EPC to the treated groups decreased the serum levels of ALT and AST compared to the group treated with APAP. The results are shown in Figure 2.
Preventive and therapeutic effects of epicatechin (EPC) and N-acetylcysteine (NAC) on liver function enzymes in APAP (acetaminophen)-induced hepatic injury in mice (n = 6 in each group). The effect of EPC on serum ALT and AST activity is shown in the preventive groups (A and B) and therapeutic groups (C and D). Values are mean with their standard error of the mean (SEM). ANOVA Tukey's post-hoc was used for statistical analysis by PRISM 9 software. *Indicates significant difference from the APAP group (*P < 0.05). #Indicates a significant difference in the APAP group from the saline-treated group (# P < 0.05).
4.3. Preventive and Therapeutic Effects of EPC on TBARS Level in Mice Model of APAP Hepatotoxicity
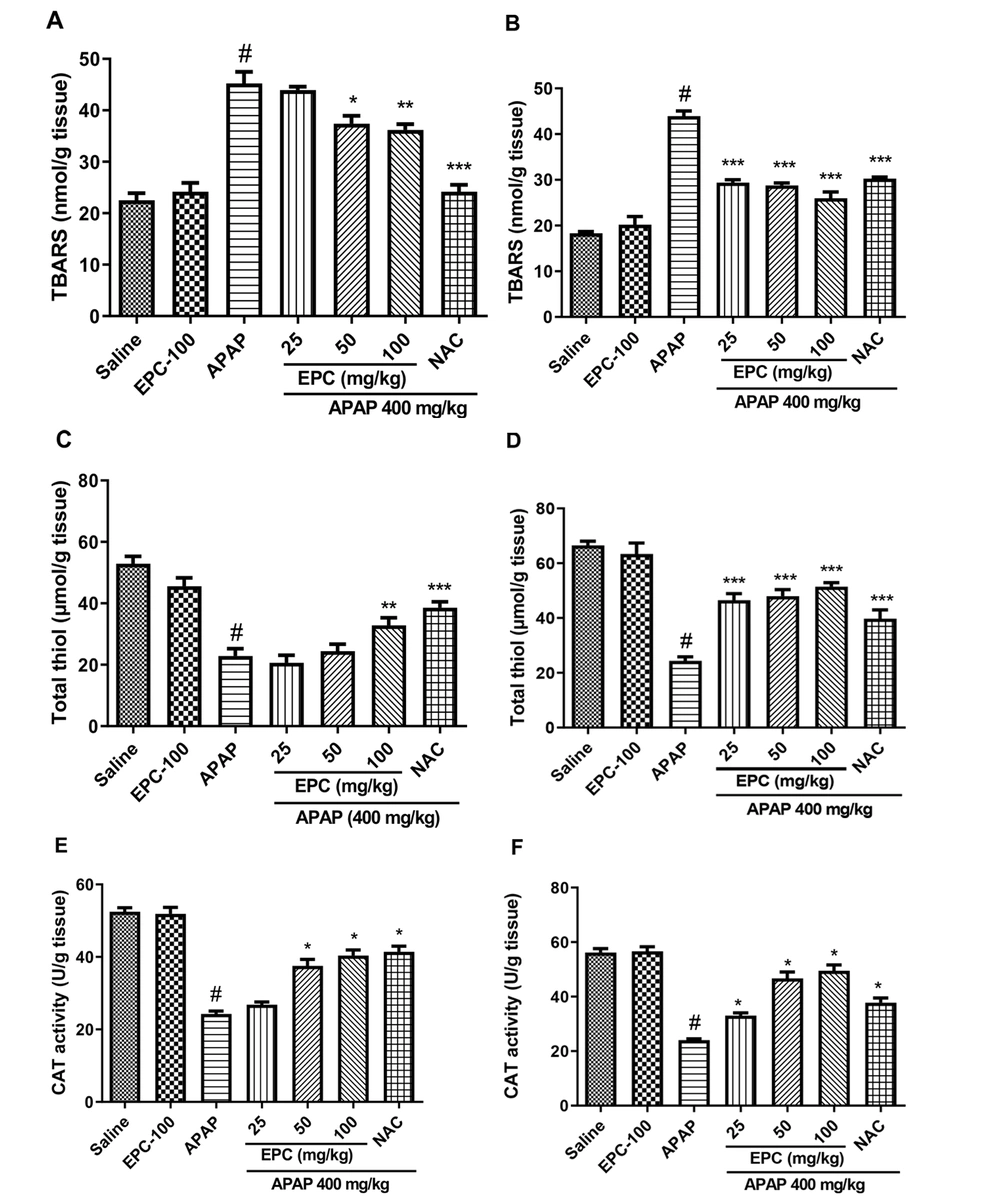
The administration of APAP significantly elevated lipid peroxidation compared to the control group. Despite this, the administration of EPC in the therapeutic groups significantly reduced the elevation in the TBARS level. Co-administration of EPC in a preventive group significantly reduced TBARS levels, but to a lesser extent than in the therapeutic groups (Figure 3).
Preventive and therapeutic effects of epicatechin (EPC) and N-acetylcysteine (NAC) on hepatic content of TBARS and total thiol and CAT activity in APAP (acetaminophen)-induced oxidative stress in mice (n = 6 in each group). Effects of EPC on liver TBARS level in preventive (A) and therapeutic (B) groups, hepatic total thiol level in preventive (C) and therapeutic (D) groups, liver CAT activity in preventive (E) and therapeutic (F) groups. Values are mean with their standard error of the mean (SEM). ANOVA with Tukey's post-hoc was used for statistical analysis by PRISM 9 software. * Indicates significantly different from APAP group (*P < 0.05, ** P < 0.01, and *** P < 0.001). # Indicates a significant difference between the APAP group from saline and the treated group (#P < 0.05).
4.4. Preventive and Therapeutic Effects of EPC on Total Thiol Content in Mice Model of APAP Hepatotoxicity
The APAP-treated group showed a significant decrease in hepatic total thiol levels compared to the control group. Nonetheless, the administration of EPC to the treated rodents mitigated the reduction of total thiol. No significant statistical differences were observed between the control group and the EPC-received group in both therapeutic and preventive groups. The levels of total thiol are illustrated in Figure 3.
4.5. Preventive and Therapeutic Effects of EPC on Catalase Activity in Mice Model of APAP Hepatotoxicity
The activity of catalase (CAT) is shown in Figure 3. CAT activity in the liver was considerably lower in the APAP-treated groups compared to the saline-treated groups. IP injection of EPC (100 mg/kg) had no significant effect compared to the saline-treated group. The combined administration of APAP and EPC elevated CAT activity. The improvement in CAT activity of the therapeutic groups was greater than that of the preventive groups. EPC and NAC-treated groups showed a significant improvement in CAT activity compared to the APAP-treated groups.
5. Discussion
Severe liver injury is a current serious clinical problem that develops due to overdose or chronic use of APAP. Therefore, finding an effective and affordable therapy for liver injury is a critical priority for many researchers (28). The extent of hepatic damage is directly linked to the activity of ALT and AST enzymes in the serum, which are considered the most sensitive biomarkers (29). According to the results, administering APAP to the mice led to a significant rise in the activities of serum AST and ALT, indicating that the hepatocellular system was damaged. This elevation is attributable to the release of these enzymes from the cytoplasm into the bloodstream, which indicates necrosis or inflammatory responses (30, 31).
Oxidative damage is caused by the generation of reactive oxygen species, such as hydroxyl radicals and hydrogen peroxide, which interact with biological molecules, including proteins, lipids, and nucleic acids, leading to the impairment of membranes and other tissues. The TBARS assay has been employed as a suitable method for detecting oxidative damage (32). GSH is an essential antioxidant that assists detoxication by conjugating with NAPQI and preventing free radical damage (33). The significant increase in TBARS and a decrease in GSH in rodents treated with APAP can lead to the leakage of ALT and AST into the circulation due to liver damage.
Oxidative stress typically modifies the antioxidant status of a cell, which determines its susceptibility to oxidative injury. Moreover, hepatotoxicity can be a result of the liver's decreased antioxidant enzyme activity (34). Indeed, the antioxidant enzyme CAT restricts the action of oxidant molecules in tissues and acts as a free radical scavenger in the defense against oxidative cell damage (35). Our results indicated that APAP administration altered CAT activity in the livers of mice. These findings are consistent with prior research, which found that APAP exposure causes lipid peroxidation and changes the antioxidant state (36). This change could be due to decreased enzyme production or oxidative inactivation of enzymes.
The hepatoprotective efficacy of medicinal plants against APAP-induced hepatotoxicity is still under investigation. The current investigation found that EPC reversed the increase in lipid peroxidation. As a result, the mechanism of EPC hepatoprotection may be linked to polyphenolic chemicals, which scavenge a wide variety of free radicals, including the most active hydroxyl radical, which causes lipid peroxidation (37). It has previously been shown that phenolic substances chelate metal ions, particularly iron and copper, can inhibit the production of hydroxyl radicals and the degradation of lipid hydroperoxides (38).
In this study, the histological abnormalities can be described as degeneration, coagulative necrosis, fatty change, inflammation, and dilated sinusoids in APAP-treated groups, which were improved by EPC administration. The alleviation of inflammation in EPC-treated groups cannot be merely attributed to EPC antioxidant activity; it can also be due to its anti-inflammatory activity through inhibition of TNF-α and IL-1 (37). In a similar study, but with a different method, EPC ameliorated the inflammatory cytokines, histopathological changes, and apoptotic markers in the liver after administration of APAP (39). However, the preventive and therapeutic effects of EPC in routine doses have not been determined.
Antioxidant properties of EPC decreased lipid peroxidation and increased the antioxidant enzyme capacity in the liver, thereby repairing membrane damage and normalizing cell permeability (40, 41). According to our results, therapeutic groups had a better outcome than preventive groups, which emphasizes the importance of designing different study protocols. Based on these findings and the effects seen in other antioxidants like quercetin, hesperidin (42), diacerein (43), 6-shogaol (44), and others, more research is needed to find new compounds that can be used with acetaminophen to treat and prevent hepatotoxicity.
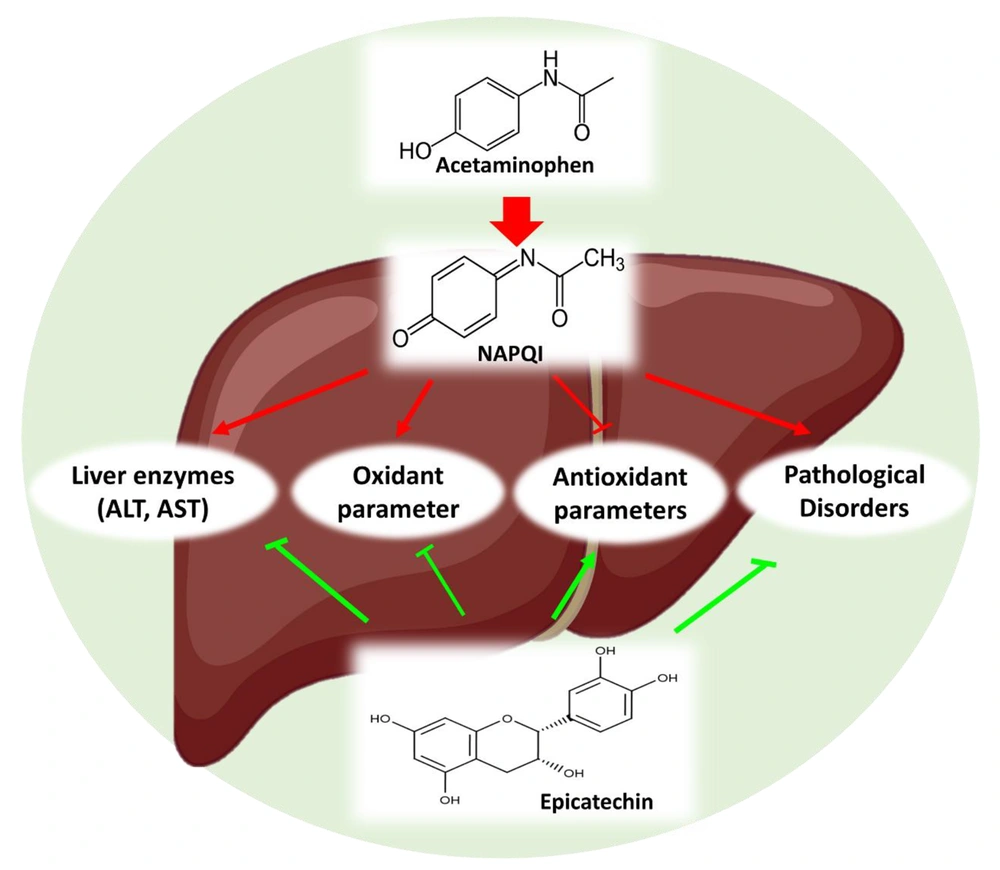
In conclusion, the current study found that APAP induced hepatotoxicity in mice. On the other hand, EPC decreased oxidative stress through its antioxidant capabilities, increased the structural integrity of the cell membrane, and subsequently alleviated histopathological and biochemical disturbances (Figure 4). Protective properties of EPC had the potential to mitigate APAP-induced hepatotoxicity by suppressing oxidative damage. One limitation of this research is that it investigated another mechanism and signaling pathway that EPC attenuates APAP hepatotoxicity. Based on our findings, it can be proposed that EPC can be used as an antioxidant for long-term therapeutic interventions against toxin-induced hepatotoxicity with minimal side effects.