1. Background
Caffeine is an edible chemical compound found in a variety of herbs, including the seed of the Coffea plant and Camellia sinensis, and is a stimulant that can prevent sleep deprivation. Caffeine is an alkaloid in the methyl xanthine tribe that has similar properties to theophylline (1). Pure caffeine powder is soft, odorless, white, shiny, and bitter. Caffeine is the most extensively used drug among people. Almost 90% of people use it daily. Increasing the body's metabolism, stimulating the central nervous system, and increasing awareness and perception of the environment are the main effects of caffeine (2).
Most drugs pass through human skin so slowly that they cannot exert a sufficient systemic effect (3). The stratum corneum is the main limiting layer for topical drug delivery. Therefore, the main challenge for topical formulation today is to achieve an increase in drug penetration into the skin (4). So far, many studies have been carried out to identify and investigate the mechanism of various factors affecting the increase in permeability and adsorbent through the skin. These agents are used in designing various drug formulations under the name of adsorbent and in various natural and synthetic forms. Chemical enhancers are materials that increase the adsorbent of drugs from the skin layers and accelerate absorption (5).
Caffeine is a compound that is highly hydrophilic and has limited skin permeability. The lipophilic nature of the stratum corneum is a major barrier to the passage of this substance through the skin, so it is important to develop topical drug delivery systems that can effectively transport caffeine into the skin (1).
2. Objectives
Topical use of this substance can increase the effectiveness of the drug in the local area about local treatment goals (such as hair loss) while reducing the side effects caused by oral use. Given all this, the effect of several chemical enhancers (Sodium Lauryl Sulfate [SLS], Sodium Lauryl Ethyl Sulfate [SLES], tynoline, nonoxinol, and lecithin) on the skin permeation of caffeine was investigated in this study. It is expected that the results may be effective in the skin permeability of caffeine and in designing a new topical formulation.
3. Methods
3.1. Materials
Caffeine powder, SLS, SLES, tynoline, nonoxinol, and lecithin were prepared from Behsa Daruo Pharmaceutical Company, Iran.
3.1.1. Animals
The present study was conducted on 10- to 12-week-old male Wistar rats (150 - 170 g). The Ethics Committee of Jundishapur University of Medical Sciences (IR.AJUMS.ABHC.REC.1398.053) provided ethical approval for this study. All rats involved in the study underwent anesthesia via ketamine/xylazine at a dose of 50/10 mg/kg before being relieved. The rats were humanely sacrificed. Hairs from the specimens' abdomens were then cut and collected, followed by skin removal. The skin samples were stored at a temperature of -20°C in a freezer until permeability tests (6).
3.2. Caffeine Determination
A valid measurement method is required to accurately calculate how much of an administered drug penetrates the skin. The protocol employed in the present study included UV spectroscopy. The wavelength chosen was 273 nm (per permeability and release studies), which corresponds to the caffeine's spectral absorption spectrum when integrated into a phosphate buffer solution with a pH of 7. During the first step, caffeine was added to the solution at an amount of 5 mg in 10 mL (0.5 mg/mL).
The linearity of the relationship between the concentration and the response of the device (in this study, a UV spectrophotometer) was used to measure caffeine. The response produced in UV spectroscopy is light absorption. At least four standard concentrations of 0.0025, 0.005, 0.015, and 0.05 mg/mL of the drug were prepared in phosphate buffer (pH 7) to investigate the relationship between concentration and response. Four concentrations of 0.0025, 0.005, 0.015, and 0.05 mg/mL of caffeine in phosphate buffer (pH 7) were prepared to establish a standard curve of stock solution with a concentration of 0.5 mg/mL. The absorbance of the samples was measured by UV spectrophotometry. This method was repeated for 3 consecutive days, then the calibration curve was drawn using different concentrations in relation to atomic absorption, and the permeability of the drug was obtained by each of the enhancers (6).
3.3. Investigation of Caffeine Permeability
Rat skin was cut into pieces and placed on the Franz diffusion cell. One gram of one of the chemical enhancers was taken and poured on the skin. Then, the enhancer was removed from the skin, and the receptor phase was filled with 35 mL of phosphate buffer solution (pH 7). This process was repeated separately for each time (5, 15, or 30 minutes) and chemical enhancer. Meanwhile, 5 mL of a 0.05% drug solution was added during the donor phase.
In the next step, all Franz diffusion cells were placed on a heater (37°C) and stirred by a magnet at 200 rpm. Then, 2 mL of the receiver phase was removed after the elapse of certain predetermined periods (0.5, 1, 2, 3, 4, 5, 6, 7, 8, and 24 hours). Next, 2 mL of phosphate buffer with pH 7 was withdrawn, and the absorption of the sample was read with UV spectrophotometry. As a negative control for permeability, the skin was placed on the bulb. Only distilled water was used in the donor phase, while phosphate buffer (pH 7) was used in the receptor chamber to set the instrument to zero. Then, the water on the donor phase was removed, and the drug was placed instead, and the permeability factors of the drug were measured for the negative control (7).
3.4. Statistical Analysis and Permeability Calculation
Each experiment was repeated three times, and the data were presented as a mean ± standard deviation (SD). The results were then analyzed statistically using a two-sample t-test and analysis of variance. Furthermore, a full factorial test was devised using Minitab 17 software (8).
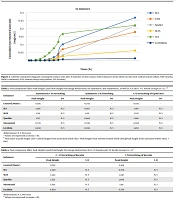
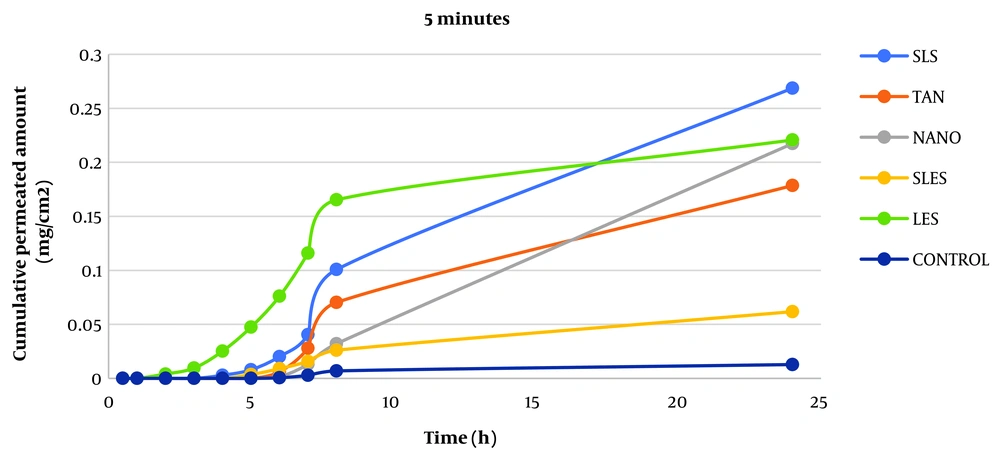
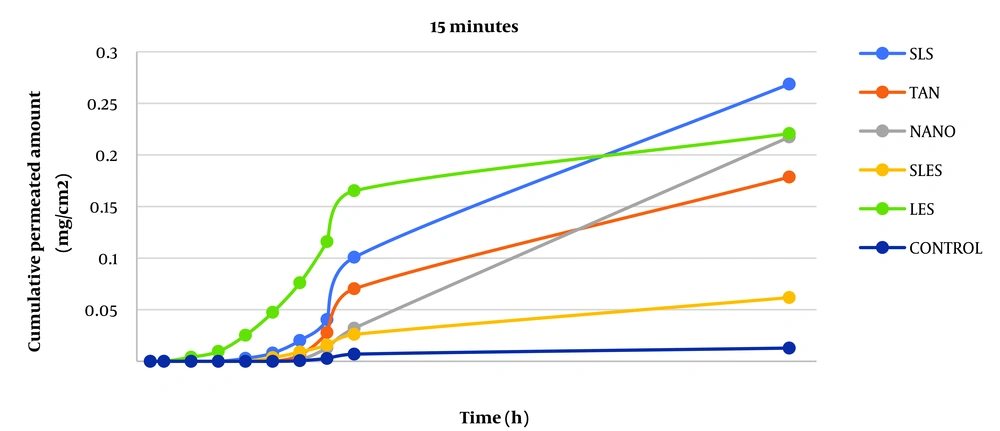
This study was designed to measure caffeine's permeability from rat skin. Additional parameters of interest included steady-state flux (Jss), permeability coefficient (P), incubation time (Tlag), and apparent diffusion coefficient (Dapp). Findings comparing the drug-containing microemulsions' ERflux, ERD, and ERp levels to those of the drug-saturated control samples are shown in Tables 1 and 2. The total amounts of the drug that passed through the skin surface were plotted against time to determine the values for the permeability parameters mentioned above.
Equation was employed to calculate the permeability coefficient (p) (7),
Where C represents the concentration of the drug during the donor phase.
| Parameter | Flux (mg.cm-2.h-1) | Tlag (h) | D (cm2.h-1) | P (cm/h) |
|---|---|---|---|---|
| After 5 Minutes of Skin Contact | ||||
| Control (water) | 0.001 ± 0.00015 | 5.71 ± 0.07 | 0.01865 ± 0.0002 | 0.00018 ± 0.00002 |
| SLS | 0.007 ± 0.00060 | 4.35 ± 0.09 | 0.02450 ± 0.0005 | 0.00076 ± 0.00006 |
| SLES | 0.001 ± 0.00006 | 3.03 ± 0.03 | 0.03514 ± 0.001 | 0.00017 ± 0.00001 |
| Tynolin | 0.018 ± 0.00046 | 5.71 ± 0.01 | 0.01868 ± 0.0004 | 0.00181 ± 0.00005 |
| Nanoxinol | 0.010 ± 0.00040 | 5.94 ± 0.11 | 0.01796 ± 0.00028 | 0.00103 ± 0.00004 |
| Lecithin | 0.006 ± 0.00021 | 1.57 ± 0.001 | 0.06796 ± 0.003 | 0.00065 ± 0.00002 |
| After 15 Minutes of Skin Contact | ||||
| Control (water) | 0.001 ± 0.00015 | 5.71 ± 0.07 | 0.01865 ± 0.0002 | 0.00018 ± 0.00002 |
| SLS | 0.007 ± 0.00060 | 4.35 ± 0.09 | 0.02450 ± 0.0005 | 0.00076 ± 0.00006 |
| SLES | 0.001 ± 0.00006 | 3.03 ± 0.03 | 0.03514 ± 0.001 | 0.00017 ± 0.00001 |
| Tynolin | 0.018 ± 0.00046 | 5.71 ± 0.01 | 0.01868 ± 0.0004 | 0.00181 ± 0.00005 |
| Nanoxinol | 0.010 ± 0.00040 | 5.94 ± 0.11 | 0.01796 ± 0.00028 | 0.00103 ± 0.00004 |
| Lecithin | 0.006 ± 0.00021 | 1.57 ± 0.001 | 0.06796 ± 0.003 | 0.00065 ± 0.00002 |
| After 30 Minutes of Skin Contact | ||||
| Control (water) | 0.001 ± 0.0001 | 5.71 ± 0.07 | 0.018 ± 0.0002 | 0.00018 ± 0.00002 |
| SLS | 0.009 ± 0.0006 | 2.005 ± 0.14 | 0.053 ± 0.0033 | 0.0009 ± 0.00006 |
| SLES | 0.003 ± 0.0002 | 0.05 ± 0.0008 | 1.843 ± 0.0849 | 0.0003 ± 0.00002 |
| Tynolin | 0.017 ± 0.0004 | 5.44 ± 0.05 | 0.019 ± 0.0002 | 0.0017 ± 0.00005 |
| Nanoxinol | 0.014 ± 0.001 | 4.70 ± 0.20 | 0.022 ± 0.001 | 0.0014 ± 0.00011 |
| Lecithin | 0.003 ± 0.0003 | 1.588 ± 0.19 | 0.068 ± 0.009 | 0.0003 ± 0.00003 |
Effects of Enhancers' Adsorption on Caffeine Permeability Parameters of Whole Rat Skin (n = 3) a
| Parameter | ERflux | ERD | ERP |
|---|---|---|---|
| After 5 Minutes of Skin Contact | |||
| SLS | 4.18 ± 0.18 | 1.24 ± 0.02 | 4.18 ± 0.18 |
| SLES | 1.52 ± 0.28 | 1.35 ± 0.02 | 1.52 ± 0.28 |
| Tynolin | 5.16 ± 0.27 | 2.86 ± 0.21 | 5.16 ± 0.27 |
| Nanoxinol | 5.48 ± 0.56 | 0.82 ± 0.007 | 5.48 ± 0.56 |
| Lecithin | 1.30 ± 0.03 | 1.07 ± 0.05 | 1.30 ± 0.03 |
| After 15 Minutes of Skin Contact | |||
| SLS | 4.31 ± 0.43 | 1.31 ± 0.04 | 4.31 ± 0.43 |
| SLES | 0.98 ± 0.08 | 1.88 ± 0.06 | 0.98 ± 0.08 |
| Tynolin | 10.31 ± 1.18 | 1.001 ± 0.01 | 10.31 ± 1.18 |
| Nanoxinol | 5.85 ± 0.70 | 0.964 ± 0.01 | 5.85 ± 0.70 |
| Lecithin | 3.67 ± 0.34 | 3.64 ± 0.18 | 3.67 ± 0.34 |
| After 30 Minutes of Skin Contact | |||
| SLS | 5.16 ± 0.27 | 2.86 ± 0.21 | 5.16 ± 0.27 |
| SLES | 1.78 ± 0.17 | 98.87 ± 5.37 | 1.78 ± 0.17 |
| Tynolin | 9.82 ± 0.89 | 1.05 ± 0.008 | 9.82 ± 0.89 |
| Nanoxinol | 8.24 ± 1.30 | 1.21 ± 0.08 | 8.24 ± 1.30 |
| Lecithin | 2.15 ± 0.06 | 3.65 ± 0.54 | 2.15 ± 0.06 |
Moreover, Equation was utilized to calculate D (diffusion coefficient) (2, 7):
In the above equation, Tlag denotes the commune time (per the drug curve) from the skin along the equilibrium line to the time axis, while his skin diameter.
Note that D, as determined by Equation equals the apparent D. This is because h is not representative of the true drug passage length. Because the calculations made using the above equations depend on the steady-state range of the cumulative drug passage chart, the parameters are not valid unless the appropriate synchronous conditions are met. During the receptor phase, concentrations did not reach 10% of the drug saturation solution.
4. Results
The permeability of caffeine was studied after skin contact with absorption enhancers for 5, 15, and 30 minutes to evaluate the effect of absorption enhancers on skin permeability. The parameters of caffeine permeability for each component are given in Tables 1 and 2. The cumulative graph of caffeine passing through the surface unit before contact for 5, 15, and 30 minutes is shown in Figures 1 - 3. Phosphate buffer was used as a control in the phase chamber.
The FT-IR spectrum analysis (before adsorption) evaluated chemical adsorbent-stratum corneum interactions in the skin by observing absorption bands at various wavelengths. The bands observed via this analysis indicate lipid and protein molecule vibrations within the stratum corneum (9). Tables 3 - 5 show the FT-IR data depicting how adsorption affected the rat skin samples.
| Enhancer | Asymmetric C-H Stretching | Symmetric C-H Stretching | C=O stretching of Lipid Ester | |||
|---|---|---|---|---|---|---|
| Peak Height | D% | Peak Height | D% | Peak Height | D% | |
| Control (Water) | 0.395 | - | 0.242 | - | 0.015 | - |
| SLS | 2.916 | N. S | 0.807 | N. S | 1.164 | N. S |
| SLES | 1.531 | N. S | 1.121 | N. S | 3.747 | N. S |
| Tynolin | 1.717 | N. S | 0.989 | N. S | 1.280 | N. S |
| Nanoxinol | 0.745 | N. S | 0.538 | N. S | 0.850 | N. S |
| Lecithin | 0.450 | N. S | 0.590 | N. S | 1.450 | N. S |
| Enhancer | C=O Stretching of Keratin | C-N Stretching of Keratin | ||
|---|---|---|---|---|
| Peak Height | % D | Peak Height | % D | |
| Control (Water) | 0.012 | - | 0.414 | - |
| SLS | 2.490 | N. S | 0.708 | N. S |
| SLES | 1.444 | N. S | 1.438 | N. S |
| Tynolin | 1.555 | N. S | 1.096 | N. S |
| Nanoxinol | 1.610 | N. S | 1.118 | N. S |
| Lecithin | 0.650 | N. S | 0.770 | N. S |
Post-component Effect Peak Heights (and Peak Height Percentage Reductions) for C=O Tensile and C-N Tensile Groups (n = 3) a
| Enhancer | C-H Stretching Asy | C-H Stretching Sym | C=O Stretching of Lipid Ester | Amid I | Amid II |
|---|---|---|---|---|---|
| Control (Water) | 2915.13 ± 0.2 | 2840.74 ± 0.4 | 1690.2 ± 1 | 1642.58 ± 0.9 | 1535.9 ± 0.2 |
| SLS | 2999.98 ± 0.15 | 2863.51 ± 0.11 | 1760.89 ± 0.24 | 1640.87 ± 0.21 | 1580.65 ± 0.17 |
| SLES | 2946.49 ± 0.1 | 2845.88 ± 0.1 | 1725.45 ± 0.1 | 1676.76 ± 0.9 | 1537.27 ± 0.2 |
| Tynolin | 2960.66 ± 0.15 | 2880.40 ± 0.11 | 1769.663 ± 0.24 | 1599.42 ± 0.21 | 1502.55 ± 0.17 |
| Nanoxinol | 2917 ± 0.382 | 2855.32 ± 0.229 | 1760.89 ± 0.19 | 1640.87 ± 0.404 | 1629.58 ± 0.258 |
| Lecithin | 2916.92 ± 0.15 | 2808.49 ± 0.11 | 1758.58 ± 0.24 | 1672.06 ± 0.21 | 1672.06 ± 0.17 |
Wavelengths for –CH and C=O groups in Hydrated Skin and Pre-contacted Skin (n = 3) a
5. Discussion
The impact of additive uptake on drug passage through rat skin compared to control was determined by calculating ERFlux, ERP, and ERD. The results showed that the adsorption of SLS, SLES, tynoline, nanoxinol, and lecithin caused a significant increase after ERflux, ERP, and ERD at 5-, 15- and 30-minutes' contact with skin (P < 0.05). While lecithin was not significantly different from the control at five-minute pre-exposure, 15- and 30-minute pre-exposure to this combination resulted in a significant increase in caffeine skin passage (P < 0.05).
The results similarly demonstrated a significant relationship between Jss and the adsorption of the sample after 5, 15, and 30 minutes compared to the control sample, indicating an increase in permeability with the adsorption studied (P < 0.05). According to a study by Tabosa et al., high Jss can lead to higher concentrations of the drug in the upper layers of the skin. It may also enhance the penetration of the components of the formulation into the bloodstream through the skin (10, 11) because, in any formulation, all components can act as penetration components with different mechanisms (12).
The results revealed that adsorption of all enhancers had a greater increase in drug delivery rate (flux) than in drug release (Dapp) (except for nanoxinol, there was no significant difference in the permeability increase at 5 and 15 minutes, but permeability increased at 30 minutes). Among them, tynolin and lecithin showed the greatest effect on increasing the permeability of rat skin at 5 and 15 minutes, respectively.
Also, the results showed that after 30 minutes of using all the enhancers, Jss had a significant increase compared to the control group, which indicates the increase in drug permeability by using the enhancers after 30 minutes (P < 0.05).
The highest and lowest absorption efficiencies of the drug were related to the increase in dermal absorption of the drug, respectively.
In order from the highest to the lowest increase in permeability belongs to the use of lecithin, SLES, SLS, nanoxinol, and tynolin (5 minutes); SLES, tynolin, nanoxinol, lecithin, and SLS (15 minutes); SLES, lecithin, tynolin, nanoxinol, and SLS (30 minutes).
According to the results of FT-IR from pre-contact skin, the absorption wavelength within the asymmetric CH region was improved after the application of sodium lauryl sulfate. Also, the absorption wavelength's position advanced in the symmetric CH. These findings signify that liquefaction (and subsequent impairments to barrier properties) occurred in the stratum corneum membrane. It is also possible that increasing the passage of the drug through the skin's surface caused the C=O wavelength of stress to increase, which indicates that the hydrogen bonds weakened between lipid molecules. It also reduces amide I's wavelength number while increasing that of amide II; as a result, the related dam effects are increased and decreased, respectively. These results indicate that this compound affects both the lipid bilayers and protein parts of horny tissues. Nevertheless, this adsorption process's effect on the lipid parts of the stratum corneum exceeds its effect on the protein part, and it seems that this compound is disturbed in the group. Also, it seems that the presence of horny lipids enhances the drug's permeability through the skin.
The results related to sodium lauryl ethyl sulfate's FT-IR skin adsorption (before contact) indicate an increase in the absorption wavelength within both the asymmetric and symmetrical CH region. Such a finding signifies that the bilayers of the stratum corneum undergo a blue shift in liquefaction. This finding also suggests an increase in the extent to which dam properties are influenced. The drug passes through. The adsorption of the drug as it permeates the stratum corneum causes the C=O stress wavelength to increase, which, in turn, causes the bonds between the hydrogen molecules located between the lipid molecules to weaken. Furthermore, the presence of sodium lauryl ethyl sulfate mitigates the barrier's effects by causing amide I's and amide II's wavelength numbers to increase.
The results of FT-IR of pre-contact skin with tynolin demonstrate that C-H is asymmetric and C-H is more symmetrical towards the absorption band, indicating an aqueous shift. Tynolin indicates the orientation of lipid bilayer groups, impairs barrier properties and possibly increases drug passage through the stratum corneum. The results also showed that tynolin shifted the absorption band in the amide regions I and II to a lower wavelength. Such a state shows the orientation of protein groups in the bilayer of the stratum corneum.
The FT-IR results from the skin of pre-contact rats with nanoxinol show that this compound caused a very small increase in the wavelength of asymmetric CH peak absorption and an increase in the absorption wavelength in the CH symmetric region, indicating liquefaction of bilayers in the stratum corneum membrane. Moreover, there is a disruption in the skin's barrier properties to this drug. This attraction decreased the absorption wavelength in the amide region 1 and increased the absorption wavelength in the amide region 2, which indicates the increase and decrease of the skin barrier effects, respectively.
5.1. Conclusions
Transdermal drug delivery never occurs without some problems. This study represents an attempt to overcome challenges in drug delivery caused by the barrier formed by the cutaneous stratum corneum. The utilized method was intended to aid transcutaneous drug delivery. The use of chemical and herbal additives changed the skin structure, thus leading to improvements in drug delivery and absorption into the skin. The future use of the adsorbents considered in this study could significantly improve the dermal delivery of caffeine.