1. Context
Ziziphora is a genus of the Lamiaceae family, consisting of 17 aromatic herbs or subshrubs, which can be annual or perennial. These plants are found across Southern and Eastern Europe, North-West Africa, and Asia, extending to the Himalayas. Ziziphora clinopodioides Lam., known as “kakooti-koohi” in Persian, is one of the most well-known aromatic and edible species within this genus. It is widely distributed in Asia and Europe, especially in Turkey and the western regions of Iran (1).
Ziziphora clinopodioides is a highly branched perennial herb with stems that can reach a height of up to 50 cm. It features white to red-colored flowers. In Iran, there are nine subspecies of Z. clinopodioides (2). In traditional Iranian folk medicine, it has been used in the form of a decoction for various purposes, such as a stomach tonic, sedative, carminative, and antipyretic agent (3). Additionally, Z. clinopodioides is considered an effective remedy for various conditions, including cough, the common cold, bronchitis, headache, viral diseases, diarrhea, nausea, typhus, inflammation, edema, cardiovascular diseases, insomnia, diabetes, and asthma (4, 5). Furthermore, in Iran, the dried aerial parts of this plant are used as a spice to flavor food and beverages (6).
Essential oils are composed of volatile components found in aromatic plants and can be viewed as natural preservatives due to their high content of bioactive natural products, such as monoterpenoids. These essential oils also possess significant biological properties, including antimicrobial, antifungal, and antioxidant activity (7).
2. Evidence Acquisition
A review of the literature revealed that the chemical composition and biological properties of the essential oil of the aforementioned species have been investigated in various studies. This article compiles the major chemical constituents and categorizes the biological properties of Z. clinopodioides essential oil. The literature survey was conducted using search engines such as Google Scholar, Scopus, and PubMed. The search spanned within 1990 to 2022, and the inclusion criteria encompassed phytochemical components and reported biological activities of Z. clinopodioides essential oil.
3. Results
3.1. Essential Oil Composition
Table 1 provides a summary of detailed information regarding the essential oil composition of Z. clinopodioides. Essential oil yields ranged from 0.1% to 4.5% based on the weight of dry plant material. Most of the plants studied were collected from various regions of Iran. Additionally, there are reports of plant species collected from China, Turkey, and other Asian countries listed in the table. The primary method employed for the extraction of Z. clinopodioides essential oil was hydrodistillation using a Clevenger-type apparatus. Although most researchers selected the aerial parts of Z. clinopodioides for essential oil extraction, a few studies also investigated the essential oil composition of leaves or the entire plant.
| Species Name | Origin | Part Used | Extraction Method | Major Components (> 10%) | % Yield | References |
|---|---|---|---|---|---|---|
| Z. clinopodioides | Kermanshah, Iran | Leaves | HD | Carvacrol (65.2), thymol (19.5) | 0.65 a | (8) |
| Z. clinopodioides subsp. Bungeana (Juz.) Rech. f. | Khorasan, Iran | Aerial parts | HD | Pulegone (65.2), isomenthone (11.9) | 1 | (6) |
| Z. clinopodioides subsp. rigida (BOISS.) RECH. f. | Tabriz, Iran | Aerial parts | HD | Pulegone (45.8),k pipertenone (17.4), p-Menth-3-en-8-ol (12.5) | 1 | (3) |
| Z. clinopodioides Lam. | Lorestan, Iran | Aerial parts | HD | Pulegone (30.1), thymol (21.3), p-Menth-3-en-8-ol (12.9) | 1.4 | (9) |
| Z. clinopodioides Lam. | Khorasan Razavi, Iran | Aerial parts | HD | Pulegone (44.5), terpineol (14.5), methyl acetate (10.9) | 0.75 | (10) |
| Z. clinopodioides Lam. | Ankara, Turkey | Aerial parts | HD | Pulegone (21.9), 1,8-Cineole (14.0) | 0.33 | (11) |
| Z. clinopodioides | Yovon, Tajikistan | Aerial parts | HD | Pulegone (35.0 - 72.8), neomenthol (23.1), menthone (13.3) | 0.7 – 0.8 | (12) |
| Z. clinopodioides Lam. | Lorestan, Iran | Aerial parts | HD | Pulegone (30.1 - 44.6), thymol (up to 21.3), p-Menth-3-en-8-ol (10.5 - 12.9), 1,8-Cineole (up to 10.4), isomenthone (up to 11.6) | 0.65 - 1.1 | (13) |
| Z. clinopodioides | Erzurum-Palandoken Mountain, Turkey | Aerial parts | HD | Pulegone (31.9), 1,8-Cineole (12.2), limonene (10.5) | 0.44 | (14) |
| Z. clinopodioidesssp. rigida | Hamedan, Iran | Aerial parts | HD | Pulegone (0.7 - 44.5), 1,8-Cineole (2.1 - 26), neomenthol (2.5 - 22.5) | -- | (15) |
| Z. clinopodioides | Tacheng,Terks of the Yili region, China | Whole plant | HD | Pulegone (32.5 - 86.4), p-Menthanone (3.2 - 43.7) | -- | (16) |
| Z. clinopodioides Lam. | Kerman, Iran | Aerial parts | HD | Pulegone (43.3), piperitenone (15.1) | 0.96 | (17) |
| Z. clinopodioides | East-Azerbaijan, Iran | Aerial parts | HD | Pulegone (25.9), 1,8-Cineole (19.7) | -- | (18) |
| Z. clinopodioides | Coruh Valley, Turkey | Aerial parts | HD | Pulegone (41.1 - 51.1) | -- | (19) |
| Z. clinopodioides | North Khorasan, Iran | Aerial parts | HD | Pulegone (40.1), menthone (13.8), isomenthone (12.3) | 1 | (20) |
| Z. clinopodioides | Kashan, Iran | Leaves | HD | Thymol (45.5) | -- | (21) |
| Z. clinopodioides Lam. | Xinjiang Uyghur Autonomous, China | Aerial parts | HD | Pulegone (53.5), isomenthone (10.4) | 1.2 | (22) |
| Z. clinopodioides | Mazandaran, Iran | Aerial parts | HD | 1,8-Cineole (10.4) | 0.98 | (23) |
| Z. clinopodioides Lam. | North of Iran | Aerial parts | HD | Pulegone (36.4), piperitenone (19.1) | 0.9 | (24) |
| Z. clinopodioides Lam. | R. Chubai-Nura, Kazakhstan | -- | HD | Pulegone (62.4) | -- | (25) |
| Z. clinopodioides Lam. | Iran | Aerial parts | HD | Pulegone (49.4), piperitenone (10.7) | 2.3 - 2.5 | (26) |
| Z. clinopodioides | Alborz, Iran | Aerial parts | HD | Pulegone (58.8) | 1 | (27) |
| Z. clinopodioides Lam. | Ilam, Lorestan, Kermanshah, Kurdestan, Iran | Leaf, flower, stem | HD | Carvacrol (65.1 - 74.3), thymol (14.4 - 55.6), γ-Terpinene (24.4 - 24.6), p-Cymene (10.2 - 10.2) | 0.1 - 4.5 | (28) |
| Z. clinopodioides Lam. | Urumq, China | Entire plant | HD | Pulegone (77.5 - 87.3), p-Menthanone (2.8 - 12.4) | 1.1 - 1.8 | (29) |
| Z. clinopodioides Lam. | Charmahal va Bakhtiyari, Isfahan, Iran | Aerial parts | HD | Pulegone (17.5 - 57.8), p-Menth-3-en-8-ol (15 - 15.1), 1,8-Cineole (up to 27.4), limonene (up to 12.8) | 0.12 - 0.98 | (30) |
| Z. clinopodioides Lam. | Baluchestan, Iran | Aerial parts | HD | Pulegone (61.7), cis-Caran-trans-2-ol (12.7), 1,8-Cineole (10.2) | 1.86 | (31) |
| Z. clinopodioides | North Khorasan, Iran | Aerial parts | HD | Pulegone (41.5), piperitenone (18.5) | 1.1 | (32) |
| Z. clinopodioides | Golestan, Iran | Aerial parts | HD | Pulegone (7.3 - 16) | 0.44 - 0.9 | (33) |
| Z. clinopodioides | Erzurum, Turkey | Leaves | HD | Pulegone (60.2), menthone (13.6), menthol (10.9) | (34) | |
| Z. clinopodioides | Tehran- Qazvin- Azarbaijan- Fars, Iran | Aerial parts | HD | Pulegone (23.0 - 52.0), 1,8-Cineol (up to 32.4), limonene (up to 29.0), neomenthone (up to 26.0), p-Menth-3-en-8-ol (up to 24.3), piperitenone (up to 16.8), γ-Terpinene (up to 16.1) | 0.13 - 1.44 | (35) |
| Z. clinopodioides | Hamedan- Kurdestan, Iran | Aerial parts | HD | Pulegone (22.3 - 60.4), 1,8-cineol (up to 29.9), p-Menth-3-en-8-ol (up to 14.0), piperitenone (up to 10.9), neomenthol (up to 10.8), isomenthone (up to 10.3), terpinen-4-ol (up to 10.2) | 0.37 - 1 | (36) |
| Z. clinopodioides Lam. | Iran | Aerial parts | Perculation (n-hexane fraction) | Pulegone (24.4), menthol (14.0) | -- | (37) |
| Z. clinopodioides | Iran | -- | HD | Geraniol (20.6), carvacrol (18.2) | -- | (38) |
| Z. clinopodioides | Khorasan Razavi, Iran | -- | SD | Pulegone (37), p-Piperitone (19.6) | -- | (39) |
| Z. clinopodioides | Ermenek, Turkey | -- | HD | α-Terpineol (up to 33.3), camphor (10.9 - 12.4), borneol (up to 24.4), α-Pinen (10.8 - 17.6), isoborneol (up to 21.2) | 0.12 - 0.5 | (40) |
| Z. clinopodioides Lam. | Kashan, Iran | Aerial parts | SDE, HD, SD, ultrasonic | Pulegone (25.9 - 35.2), piperitenone (10.1 - 27.9), menthol (11.4 - 17.5) | 0.3 - 1.3 | (41) |
| Z. clinopodioides Lam. | Fars, Iran | Aerial parts | HD | Pulegone (27.4), isomenthone (10.1) | 0.15 | (42) |
| Z. clinopodioides Lam. | Iran | Leaves | HD | Pulegone (51.8) | -- | (43) |
| Z. clinopodioides | Kerman, Iran | -- | HD | Pulegone (31.2), menth-3-en-8-ol (23.8) | -- | (44) |
| Z. clinopodioides subsp. Elbursensis; Z. clinopodioides subsp. Filicaulis | Mazandaran, Tehran, Iran | Aerial parts | HD | Pulegone (21.5 - 38.9), menthone (up to 10.4), carvacrol (up to 16.6), thymol (up to 10.1) | 0.5 - 1 | (45) |
| Z. clinopodioides | Van, Turkey | Aerial parts | HD | Pulegone (29.3), menthone (21.8), 1,8-Cineole (15.3) | -- | (46) |
| Z. clinopodioides subsp. bungeana (Juz.) | Khorasan, Iran | Aerial parts | HD | Pulegone (20.2 - 34.2), iso-menthone (9.1 - 18.3) | 0.23 - 0.61 | (2) |
| Z. clinopodioides Lam. | Fars, Iran | Aerial parts | HD | Pulegone (26.3 - 50.9), p-Menth-3-en-8-ol (11.3 - 14.7), menthone (10 - 10.4) | 0.2 - 1.2 | (47) |
| Z. clinopodioides Lam. | Iran | Aerial parts | HD | Pulegone (23.1), menthone (19.5), p-Menth-3-en-8-ol (10.4) | -- | (48) |
| Z. clinopodioides | Tehran, Iran | Aerial parts | HD | Thymol (41.7) | -- | (49) |
| Z. clinopodioides Lam. | North Khorasan, Iran | Aerial parts | HD | Pulegone (46.1), menthol (10.7) | (50) | |
| Z. clinopodioides | Bojnurd, Iran | Aerial parts | HD | Pulegone (26.6), iso-menthone (17.5), limonene (10.5) | 1.5 | (51) |
| Z. clinopodioides Lam. | Hamedan, Iran | Aerial parts | HD | Pulegone (59.3), β-caryophyllene (10.4) | -- | (52) |
| Z. clinopodioides | Mazandaran, Iran | -- | HD | Pulegone (48.2), 1,8-cineol (11.2) | -- | (53) |
| Z. clinopodioides | Alborz, Iran | Aerial parts | HD | Carvacrol (54.3), thymol (12.5) | 2.5 | (54) |
| Z. clinopodioides | Iran | -- | -- | Pulegone (79.3) | -- | (55) |
| Z. clinopodioides Var. rigida | Kerman, Iran | Aerial parts | HD | Pulegone (52.4), dihydrocarvyl acetate (14.1), 1,8-cineole (13.0) | -- | (56) |
| Z. clinopodioides | Urmia, Iran | Aerial parts | HD | Thymol (54.4) | -- | (57) |
| Z. clinopodioides | Kordestan, Iran | -- | HD | Thymol (34.2), pulegone (14.4), carvacrol (10.9) | 0.8 | (58) |
Characteristics of Different Ziziphora clinopodioides Essential Oils
The compounds that comprised more than 10% of the total essential oil were listed in the table as major components. A general view apparently revealed that the essential oil of Z. clinopodioides could be considered a pulegone-rich essential oil. It is interesting to note that 45 out of 54 studies mentioned in Table 1 have identified more than 10% pulegone in Z. clinopodioides essential oil, and 37 studies reported more than 30% pulegone content. Two studies reported more than 80% pulegone content in Z. clinopodioides essential oil (16, 29). Various Biological activities have been reported for pulegone, antimicrobial, antifungal, insecticidal, anti-inflammatory, and gastrointestinal-related activity, to name a few (59). Therefore, one can reasonably predict that pulegone-rich essential oils possess outstanding biological effects.
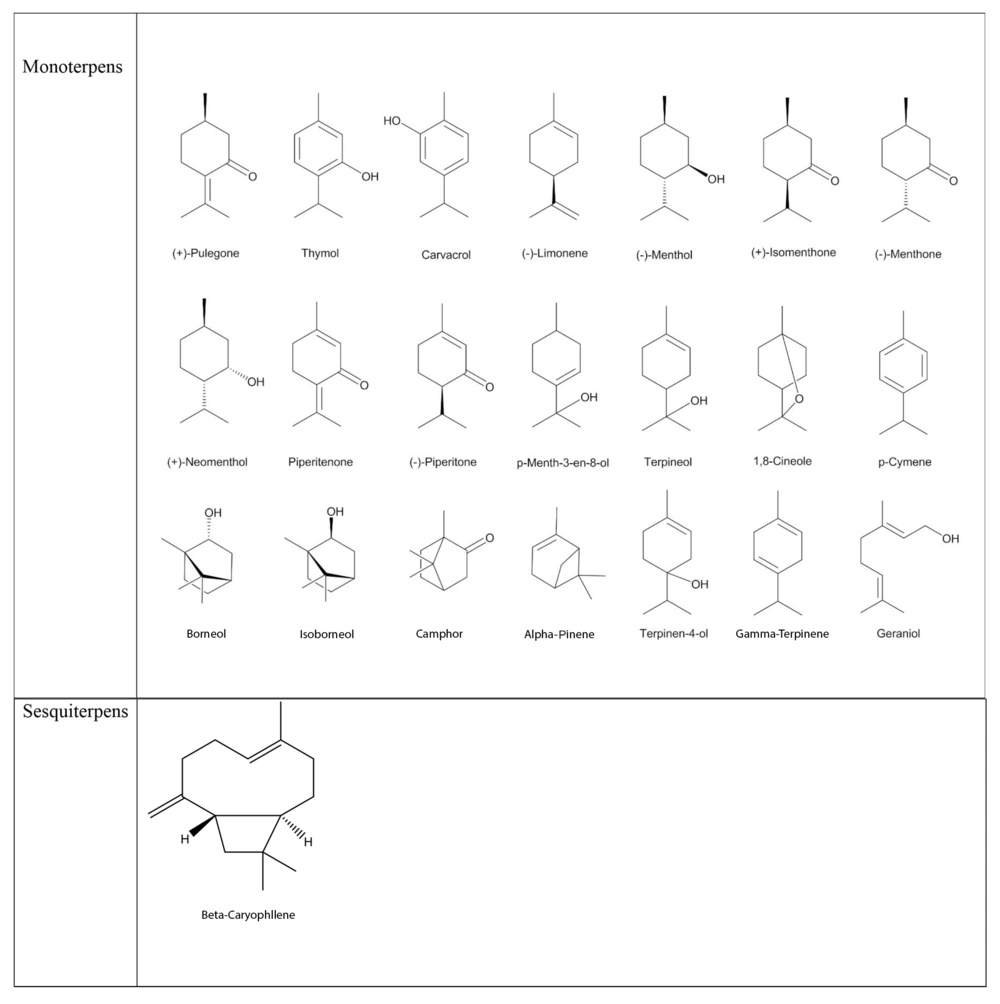
Several studies reported relatively high amounts of aromatic monoterpenoids thymol (41.7 - 55.6%) and carvacrol (54.3 - 74.3%) in the Z. clinopodioides essential oil as the main components. These two monoterpenoids have been known for their prominent antimicrobial properties. The structures of frequently identified compounds in the essential oil of Z. clinopodioides are represented in Figure 1. Most structures have menthane type skeleton, and due to their interesting structural similarity, it has been obviously understandable that the monoterpenoids biosynthetic pathway activated in the Z. clinopodioides. Oxygenated monoterpenes are more abundant than hydrocarbon monoterpenes in the Z. clinopodioides essential oil.
3.2. Biological Activities
A review of the literature regarding the biological properties of Z. clinopodioides essential oil revealed that most studies focused on its antibacterial effects (3, 6, 8, 10, 14, 17, 18, 28, 32, 49, 60-65), antifungal activity (10, 22, 44, 57, 66-73), and antioxidant capacity (9, 19, 28, 56, 64, 74, 75). A few other biological effects were assessed in some studies, which will be discussed later in this review. It is important to note that studies investigating the biological activity of formulations containing Z. clinopodioides essential oil have also been reviewed in this article to the extent that they have also examined the effect of pure essential oil.
3.2.1. Antibacterial Activity
The reported antibacterial effects of Z. clinopodioides essential oil are summarized in Table 2. An overview revealed that the most frequently studied gram-positive bacterial strains include S. aureus, B. subtilis, L. monocytogenes, B. cereus, and S. epidermidis. Additionally, the most studied gram-negative bacterial strains include E. coli, K. pneumoniae, and S. typhimurium. Most researchers evaluated the antibacterial effects of Z. clinopodioides essential oil using microdilution and disk-diffusion methods. It is evident that Z. clinopodioides essential oil exhibits significant antibacterial activity against both gram-positive and gram-negative bacteria, in addition to certain antibiotic-resistant bacterial strains.
| Species | Target Organism | Method | Result | References |
|---|---|---|---|---|
| Z. clinopodioides | S. aureus, B. subtilis, B. cereus, L. monocytogenes, S. typhimurium, E. coli O157:H7 | Microdilution | MIC (μL/mL) = MBC = 0.0025, 0.0012, 0.0012, 0.0012, 0.0025, 0.0025 | (8) |
| S. aureus, B. subtilis, B. cereus, L. monocytogenes, S. typhimurium, E. coli O157:H7 | Agar disk diffusion (20 μL/disk) | IZ (mm) = 30, 23, 23, 28, 22, 26 | ||
| Z. clinopodioides subsp. bungeana (Juz.) Rech. f. | B. subtilis, S. epidermidis, S. aureus, E. faecalis, K. pnemoniae, E. coli | Microdilution | MIC (mg/mL) = 3.75, 3.75, 3.75 , > 15, > 15, 3.75 | (6) |
| B. subtilis, S. epidermidis, S. aureus, E. faecalis, K. pnemoniae, E. coli | Disk diffusion (10 μL/disk) | IZ (mm) = 20, 22, 19, 14, 11, 20 | ||
| Z. clinopodioides subsp. rigida (BOISS.) RECH. f. | B. subtilis, S. epidermidis, S. aureus, E. faecalis, K. pnemoniae, E. coli | Microdilution | MIC (mg/mL) = 3.8, 7.5, 7.5, > 15, 15, 15 | (3) |
| B. subtilis, S. epidermidis, S. aureus, E. faecalis, K. pnemoniae, E. coli | Disk diffusion (15 μL/disk) | IZ (mm) = 18, 16, 15, 12, 11, 15 | ||
| Z. clinopodioides Lam. | E. coli, K. pnemoniae, S. aureus, P. aeruginosa, S. typhi | Microdilution | MIC (%V/V dilution) = 0.003, 0.067, 0.033, 0.033, 0.067 | (10) |
| Z. clinopodioides | (total 52 bacterial strains) B. sphaericus, B. flexus, C. ammoniagenes, E. sakazakii, X. arboricola corylina | Disk diffusion (10 μg/disk) | IZ (mm) = 27, 25, 25, 24, 24 | (14) |
| (total 35 bacteria) E. faecalis, M. catarrhalis, E. hormaechei, E. acetylicum, C. ammoniagenes, B. subtilis, B. marinus, | Microdilution | MIC (μg/mL) = 7.81, 7.81, 15.60, 15.60, 15.60, 15.60, 15.60 | ||
| Z. clinopodioides Lam. | S. enterica, B. cereus, E. aerogenes, S. aureus, L. monocytogenes | Disk diffusion (10 μg/disk) | IZ (mm)= 19.3, 20.3, 19, 14, 32.1 | (17) |
| S. enterica, B. cereus, E. aerogenes, S. aureus, L. monocytogenes | Microdilution | MIC (μg/mL) = 0.25, 1, 0.25, 0.5, 0.125 | ||
| S. enterica, E. aerogenes, S. aureus, L. monocytogenes | MBC (μg/mL) = 2, 2, 0.5, 0.125 | |||
| Z. clinopodioides | Antibiotic resistant S. aureus | Disk diffusion (50, 100 μL) | IZ (mm) = 0.81, 0.18 | (18) |
| Z. clinopodioides | S. aureus, B. subtilis, B. cereus,L. monocytogenes, S. typhimurium, E. coli O157:H7 | Microdilution | MIC (μL/mL) = 0.0003, 0.0003, 0.0004, 0.0003, 0.0004, 0.0004 | (63) |
| Z. clinopodioides Lam. | S. aureus | Microdilution (%V/V dilution) | MIC = 0.03 - 0.04 ; MBC = 0.04 - 0.05 | (28) |
| B. subtilis | MIC = 0.03 - 0.04; MBC = 0.03 - 0.05 | |||
| B. cereus | MIC = 0.04 - 0.05; MBC = 0.05 | |||
| L. monocytogenes | MIC = 0.03 - 0.04; MBC = 0.03 - 0.04 | |||
| S. typhimurium | MIC = 0.04 - 0.05; MBC = 0.05 - 0.06 | |||
| E. coli O157:H7 | MIC = 0.04 - 0.05; MBC = 0.05 | |||
| S. aureus | Disk diffusion (20 μL/disk) | IZ (mm) = 28.4 - 33.3 | ||
| B. subtilis | IZ (mm) = 20.2 - 28.4 | |||
| B. cereus | IZ (mm) = 21.4 - 28.1 | |||
| L. monocytogenes | IZ (mm) = 26.2 - 32.4 | |||
| S. typhimurium | IZ (mm) = 18.3 - 23.1 | |||
| E. coli O157:H7 | IZ (mm) = 23.1 - 27.4 | |||
| Z. clinopodioides | S. aureus, B. cereus, K. pnemoniae, E. coli | Microdilution | MIC (μg/mL) = 4.25, 1.25, 7.25, 5.75; MBC (μg/mL) = 90, 4.50, 7.25, 5.75 | (32) |
| Z. clinopodioides | L. acidophilus, B. bifidum | Lethal dose (ppm) = 1750, 1500 | (49) | |
| Z. clinopodioides L. | S. aureus, S. epidermidis, S. saprophyticus, E. coli, K. pnemoniae, P. vulgaris, E. aerogenes, C. frundii, P. areuginosa | Disk diffusion (30 μL/disk) | IZ (mm) = 22, 20, 21, 21, 29, 19, 19, 20, 5 | (60) |
| Z. clinopodioides Lam. | E. coli, MRSA | Microdilution | MIC (mg/mL) = 5, 10; MBC (mg/mL) = 5, 10 | (64) |
Antibacterial Activity of Different Ziziphora clinopodioides Essential Oils
However, considering the variations in subspecies, origins, and compositions of the essential oils, it can be expected that some differences might exist in the reported antibacterial activities. Additionally, variations in antibacterial treatment methods, such as the concentration of essential oil used in the disk-diffusion method, should be taken into account when comparing the data presented in Figure 1.
Although information regarding the composition of essential oil components is not available in a few reference articles, a general review indicates that essential oils containing high amounts of carvacrol (65 - 74%), thymol (41 - 55%), and pulegone (25 - 65%) have exhibited strong antibacterial effects. The carvacrol-rich (65.2%) Z. clinopodioides essential oil demonstrated prominent antibacterial activity against both gram-positive and gram-negative bacteria (8, 63). Considerable antibacterial effects were reported for Z. clinopodioides essential oil, containing 44.5% and 43.3% pulegone content, respectively (10, 17). Moreover, significant antibacterial activities were observed in carvacrol- and thymol-rich Z. clinopodioides essential oils (28). It has been proven that the antibacterial action of carvacrol and thymol is based on targeting the cytoplasmic membrane, resulting in bacterial lysis and the leakage of intracellular contents (61, 62, 65). Considering that the antibacterial properties of the above-mentioned monoterpenoids have been widely studied and proven, it is not surprising that essential oils containing high amounts of these compounds exhibit strong antibacterial activity.
3.2.2. Antifungal Activity
The reported antifungal effects of Z. clinopodioides essential oil have been summarized in Table 3. A general overview revealed that the most studied fungi include several species of Aspergillus and Trichophyton. Most researchers assessed the antifungal activity of Z. clinopodioides essential oil using broth macrodilution, broth microdilution, and mycelium inhibition methods. Predictable variations in the antifungal activity results of the represented studies have been observed due to differences in subspecies, origins, and essential oil compositions. Nevertheless, it is apparent that Z. clinopodioides essential oil exhibits significant antifungal activity. An evaluation of the composition of essential oils possessing antifungal activity listed in Table 2 revealed some extent of similarity to those with antibacterial effects. Distinguished antifungal effects have been reported for Z. clinopodioides essential oil, containing 54.4% Thymol content (57). In addition, notable antifungal activities were observed for Z. clinopodioides essential oils containing 53.5%, 44.5%, 37%, and 31.2% pulegone content (10, 22, 44, 72). Therefore, although not mentioned in these studies, the antifungal effects of carvacrol have been proven (71). Moreover, as supported by various published research based on the antifungal mechanism of action of thymol and pulegone (66-68), one would reasonably predict that the presence of high amounts of these monoterpenoids in Z. clinopodioides essential oil could also lead to strong antifungal effects.
| Species | Target Organism | Method | Result | References |
|---|---|---|---|---|
| Z. clinopodioides Lam. | A. niger, T. rubrum, T. reesei, M. gypseum | Mycelium inhibition | Inhibition conc. (μL/mL) = 1, 0.5, 0.5, 0.5 | (10) |
| Z. clinopodioides Lam. | S. sclerotiorum | Mycelium inhibition | Contact condition: Inhibition conc. (μL/mL) = 1.25; vapor phase condition: Inhibition conc. (μL/mL) = 0.15 | (22) |
| Germination inhibition | Contact condition: Inhibition conc. (μL/mL) = 1.00; vapor phase condition: Inhibition conc. (μL/mL) = 0.15 | |||
| Z. clinopodioides | A. flavus, A. parasiticus | Broth macrodilution | MIC (μg/mL) = 48.82, 48.82; MFC (μg/mL) = 781.25, 390.625 | (73) |
| Z. clinopodioides | A. fumigatus, A. flavus | Broth macrodilution | MIC 90 (mg/mL) = 0.5, 0.25; MFC (mg/mL) = 1, 0.5 | (70) |
| Broth microdilution | MIC 90 (mg/mL) = 1.5, 1.5; MFC (mg/mL) = 3, 3 | |||
| Z. clinopodioides L. | B. cinerea | Mycelium inhibition | Inhibition conc. (mL/L) = 1.00, 2.00 | (69) |
| Z. clinopodioides | A. flavus, A. parasiticus | Mycelium inhibition | Inhibition conc. (μL) = 9.00 | (44) |
| Z. clinopodioides | A. parasiticus, A. fumigatus | Broth macrodilution | MIC 90 (mg/mL) = 0.37, 0.43; MFC (mg/mL) = 1.2, 1.37 | (72) |
| Broth microdilution | MIC 90 (mg/mL) = 2.1, 1.5; MFC (mg/mL) = 5.5, 3.2 | |||
| Z. clinopodioides | M. canis, M. gypseum, T. mentagrophytes, T. rubrum, T. schoenleinii | Broth microdilution | MIC (μL/mL) = 0.01, 0.01, 0.03, 0.06, 0.01; MFC (μL/mL) = 0.03, 0.01, 0.03, 0.06, 0.01 | (57) |
| Mycelium inhibition | (150 ppm) inhibition percentage = 59.1, 48.8, 28.2, 60.9, 100 |
Antifungal Activity of Different Ziziphora clinopodioids Essential Oils
3.2.3. Antioxidant Capacity
The reported antioxidant capacity of Z. clinopodioides essential oil has been summarized in Table 4. Most researchers assessed the antioxidant capacity of Z. clinopodioides essential oil using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging method and β-carotene-linoleic acid bleaching assay. Examining the antioxidant results represented in Table 3 reveals that the total essential oil compounds should be considered in addition to the major components. As mentioned before, oxygenated monoterpenes make up the majority of Z. clinopodioides essential oil compounds. It has been proven that the presence of oxygenated monoterpenes and monoterpen hydrocarbons in the composition of essential oils leads to antioxidant activity (74, 75). Therefore, it is not unexpected that various Z. clinopodioides essential oils show different antioxidant activities due to the large variation in the percentage composition of the components. Therefore, the inhibition rate in the DPPH radical scavenging assay varies between 34 μg/mL and 5.12 mg/mL. However, due to the presence of oxygenated monoterpenes that comprise the majority of the essential oil components, Z. clinopodioides essential oil definitely possesses notable antioxidant activity.
| Species | Method | Result | References |
|---|---|---|---|
| Z. clinopodioides Lam. | DPPH a | IC50b = 55.3 μg/mL | (9) |
| β-catotene-linoleic acid | 61.6% inhibition | ||
| Z. clinopodioides | DPPH | IC50 = 3.6 - 4.2 mg/mL | (19) |
| Z. clinopodioides Lam. | DPPH | IC50 (mg/mL) = 0.30 - 0.56 | (28) |
| Ferric reducing power | EC50 (mg/mL) = 0.40 - 0.91 | ||
| β-catotene-linoleic acid | EC50 (mg/mL) = 0.09 - 0.23 | ||
| Z. clinopodioides var. rigida | DPPH | IC50 = 33.94 - 61.48 μg/mL | (56) |
| β-catotene-linoleic acid | 108.69 % inhibition | ||
| Z. clinopodioides Lam. | DPPH | IC50 = 5.12 mg/mL | (64) |
| ABTS c | IC50 = 0.79 mg/mL | ||
| FRAP d | 66.9 μM Fe(II)/mg |
Antioxidant Capacity of Different Ziziphora clinopodioids Essential Oils
3.2.4. Insecticidal Activity
The potential of Z. clinopodioides essential oil as an insecticidal agent has been discussed in a few studies. The lethal concentration 50 (LC50) value of pulegone-rich Z. clinopodioides Lam. essential oil was reported to be equivalent to 68.3 μL L-1 of air against Tribolium castaneum (43). In addition, the LC50 value of Z. clinopodioides essential oil against Ephestia kuehniella was reported as 47.95 μL L-1 of air and 0.94 mL.m-2 for fumigant and contact bioassay, respectively (76). The insecticidal activity of pulegone-rich Z. clinopodioides essential oil was assessed against Ephestia kuehniella Zeller, and the LC50 values of 54.61 and 1.39 μL L-1 of air were obtained for larva and adult insects (26). In another study, the pesticidal activity of Z. clinopodioides essential oil was assessed against Plodia interpunctella, and the LC50 values of 10.12 and 25.77 μL L-1 of air were obtained for larva and adult pests (77). The LC50 value of Z. clinopodioides Lam. essential oil against Anopheles stephensi and Culex pipiens was reported as 14.9 and 16.5 μg/mL, respectively (24). Therefore, Z. clinopodioides essential oil could be considered a natural agent possessing potential insecticidal properties.
3.2.5. Other Biological Properties
The antinociceptive activity of carvacrol-rich (65.2%) Z. clinopodioides essential oil was evaluated, and the obtained results demonstrated that this essential oil possesses antinociceptive effects, with the mechanism of action potentially involving an opioidergic pathway (78). In another study, the effect of using Z. clinopodioides essential oil and nisin as preservatives was assessed in relation to the sensory properties of fish burgers (79). The results revealed that adding Z. clinopodioides essential oil improved the odor, taste, and overall acceptability of the fish burgers.
Furthermore, the anti-inflammatory activity of Z. clinopodioides essential oil was assessed, and the half maximal inhibitory concentration (IC50) values for soybean 5-lipoxygenase (5-LOX) inhibition were calculated as 33.12 μg/mL, confirming the moderate anti-inflammatory activity of the essential oil (64).
4. Conclusions
Ziziphora clinopodioides is a well-known aromatic and medicinal herb of the genus Ziziphora. Extensive research has been undertaken on the chemical composition and biological properties of its essential oil. A comprehensive review of all published literature on the essential oil composition of this species reveals that oxygenated monoterpenoids, such as pulegone and other menthane-type structures, in addition to aromatic monoterpenoids, such as carvacrol and thymol, comprise the majority of Z. clinopodioides essential oil components. Due to the presence of high amounts of bioactive monoterpenoids in the essential oil, it has shown outstanding antibacterial properties against both gram-positive and gram-negative bacteria and several drug-resistant bacterial strains. Similarly, it seems that the bioactive monoterpenoids are responsible for the significant antifungal activity of Z. clinopodioides essential oil. The antioxidant capacity and insecticidal activity of Z. clinopodioides essential oil have been proven in several studies. Therefore, Z. clinopodioides essential oil could be considered a natural bioactive product possessing various biological properties. However, due to the lack of studies concerning its safety, it is suggested that further research is needed for future industrial usage of Z. clinopodioides essential oil.