1. Background
Wound healing represents a multifaceted biological process characterized by the intricate interplay of diverse cellular and molecular events. Critical determinants governing the progression of wound healing encompass inflammation, tissue regeneration, and infection prevention (1).
Within the realm of natural compounds, plants have the pharmaceutical potential for therapeutic applications (2). Allium sativum, for instance, is endowed with a broad spectrum of antibiotic properties, capable of obliterating and thwarting gram-positive and gram-negative bacterial and fungal infections (3). The roots of Arnebia euchroma manifest anti-inflammatory, antimicrobial, and antipyretic attributes, primarily attributable to the presence of shikonin, alkannin, and naphthoquinones iso-hexyl ester. These roots have traditionally been employed for treating ocular ailments and healing wounds (4). Echinacea, commonly known as the purple rose, is a member of the Asteraceae family (5). Within the alcoholic extract of Echinacea reside natural water-soluble constituents, including lipophilic alkamides and caffeic acid derivatives, exhibiting formidable antioxidant properties. Echinacea, a well-researched herbal remedy, has demonstrated its ability to fortify immune response to pathogenic infections. This immunomodulatory effect is achieved by activating pivotal immune cells, including neutrophils and macrophages, which subsequently contribute to generating essential inflammatory mediators (6).
When the integumentary barrier is compromised or the immune system becomes compromised, pathogenic bacteria have the potential to proliferate within the skin or wound (7, 8). Moreover, most wound infections stem from bacterial colonization, cutaneous flora, other bodily organs, or the surrounding environment. Staphylococcus aureus is the most prevalent culprit behind infection (9).
Nanoparticles (NPs), conversely, exhibit inherent antibacterial properties, offering an efficacious and safer alternative to conventional wound dressings (10). Polyvinyl alcohol (PVA) finds application in biomedicine owing to its cost-effectiveness and commendable attributes such as solubility, mechanical robustness, biocompatibility, resistance to chemical interactions, and antibacterial activity. Consequently, it is poised as a promising material for cutaneous dressings (11).
2. Objectives
The primary objective of this study was to assess the wound healing potential of PVA microfibers immersed in a combination of three herbal extracts on skin wounds infected by S. aureus in rats. The aim was to identify these herbal extracts as viable wound dressing agents.
3. Methods
3.1. Extract Preparation and GC-MS Analysis
The medicinal plants used in this study were acquired from the Medicinal Plants Research Center at Tehran University. Arnebia euchroma, A. sativum, and Echinacea purpurea root, cloves, and flowers were harvested, respectively.
Before the maceration extraction method is processed, the plant is appropriately washed, ground, and immersed in a solution prepared with 1000 mL of water and 1000 mL of ethanol (1: 1).
Forty grams of prepared powder from each plant were added to 200 mL of the prepared solvent, and each was placed individually on a magnetic stirrer for three days. The contents were periodically stirred to ensure complete extraction. The micelles were separated by evacuation and subsequently concentrated by rotational evaporation at 100°C for 30 minutes. The resulting dried extract was stored at temperatures ranging from 2 to 8°C for both in vitro and in vivo studies.
The GC-MS analysis of the whole plant extract (ethanolic) was conducted at the Sophisticated Analytical Instrument Facility (SAIF) laboratories. The temperature was initially set at 40°C and increased at 4.3°C per minute until it reached 250°C. The linear velocity and the input pressure were 48.1 m/s and 100.0 kPa, respectively. The linear velocity and input pressure were maintained at 48.1 m/s and 100.0 kPa, respectively. The interface temperature was set at 250°C, the detector gain at 0.70 kV + 0.10 kV, and the A-scan speed at 1666, which commenced at 50 m/z and concluded at 800 m/z (12).
3.2. In-vitro Antimicrobial Activity of the Formulation
Staphylococcus aureus (ATCC 6538, a non-enterotoxin strain) was procured from the Microbiology Department of the Pasteur Institute of Iran. The bacteria were cultured and incubated on Müller-Hinton agar for 24 hours at 37°C. The McFarland standard, as outlined in reference (13), served as a benchmark. To create this standard, 0.05 mL of dehydrated barium chloride was mixed with 9.95 mL of 1% sulfuric acid, yielding a turbid solution due to the precipitation of Barium Chloride.
3.2.1. Determination of Growth Inhibition Zones using the Kirby-Bauer Test
In this study, 40 µL of each plant extract and the formulated substance were dispensed onto sterile 6 mm discs coated with Müller-Hinton agar. These discs were subsequently allowed to air dry for 30 minutes at a temperature of 30°C. Following this, an incubation period of 24 hours at 37°C was employed.
3.2.2. Minimal Bactericidal Concentration (MBC) and Minimal Inhibitory Concentration (MIC)
The formulated extracts' concentrations for MICs ranged from 60, 30, 15, 7.5, 3.75, 1.87, 0.93, 0.46, 0.23, to 0.112 mg/mL. These concentrations were incorporated into sterile Müller-Hinton agar contained within test tubes. In this experimental setup, the positive control group comprised media and bacteria, while the negative control group comprised media with antibacterial agents.
Each test tube was filled with 9 mL of Müller-Hinton agar medium, 0.5 mL of the various formulation concentrations, and 0.5 mL of bacterial dilution equivalent to 0.5 mL of McFarland, after which they were incubated at 37°C for 24 hours. Subsequently, an ELISA reader assessed all the tubes at a wavelength of 630 nm.
To ascertain the MBC, 100 μL of the media culture derived from each well of the micro-broth assay was subcultured onto Müller-Hinton agar tubes after 24 hours of incubation. The Müller-Hinton tubes were then subjected to an additional 24 hours of incubation, with the lowest concentration of extracts that displayed no bacterial growth being designated as the MBC.
3.3. Preparation of PVA and Herbal Formulation
Polyvinyl Alcohol (PVA) with CAS No: 9002-89-5 was procured from Sigma-Aldrich.
A 10% (w/v) PVA powder was slowly introduced into distilled water and stirred at 450 rpm. Subsequently, it was maintained at 60°C for approximately 2 hours until achieving a transparent solution, which was then filtered using filter paper. Based on the MIC (1.87 mg/mL), the herbal formulation was incorporated into the solution and stirred for approximately 90 minutes at 500 rpm. The formulation/PVA, with a specific viscosity, was loaded into a syringe. The nanofiber membrane was electrospun onto aluminum foil at 20 KV voltage, maintaining a 19 cm distance between the needle and the collector.
3.4. Animal Study Design
Twelve male Wistar rats weighing 200 and 250 grams were procured from the Animal Husbandry Department at the Pasteur Institute in Iran. They were divided into four groups:
G1: Negative control group (healthy mice without infections and wounds).
G2: Positive control group (wound infection model with S. aureus without treatment).
G3: Treatment group 1 (wound infection model with S. aureus received Povidone-iodine) G4: Treatment group 2 (wound infection model with S. aureus received herbal formulation on PVA microfibers).
The formulation with PVA was applied to adhesive wound dressings and used for daily treatment 24 hours after bacterial infection. Wound contraction was measured on critical days (0, 7, and 14) to estimate the healing rate.
The animals were housed in temperature-controlled rooms at 21°C with a 12: 12 light/dark cycle. The rats were provided with balanced commercial pellets and ozonated drinking water. Iran's National Research Ethics Committee (IRPII.REC.1395.108) approved all procedures and experimental protocols.
For the model groups, the rats were anesthetized with 10% Ketamine (100 mg/kg) (Manufacturer: Kepro B.V., Netherlands) and 2% Xylazine (18 mg/kg) (Manufacturer: Alfasan) administered intraperitoneally. To induce wounds, the dorsal skin was shaved, followed by anesthesia, and a full-thickness wound with a diameter of 10 mm was created on the back using a biopsy punch. Subsequently, the wound was inoculated with S. aureus (1/5 × 108 μL) (14).
3.5. Pathological Assessment of Wound Healing
Standard protocols were used to perform the histopathological examination (15). The Histological sections were acquired from the paraffin-embedded surgical specimen and stained in hematoxylin-eosin (a thickness of 4 - 6 μ) (code No. ab245880).
3.6. Immunohistochemistry (IHC)
Silane-coated slides were initially rinsed with 10% goat serum in phosphate buffer saline (PBS) to block nonspecific protein binding. Tissues were incubated overnight at four degrees Celsius with the following primary antibodies targeting CD3 (Supplier: Biorbyt, Catalog No: orb228923, UK). At a 1: 100 dilution, the primary antibodies were combined with PBS and refrigerated overnight. Subsequently, secondary antibodies (Supplier: Biorbyt, Catalog No: orb688925, UK) were applied at a 1: 150 dilution and incubated for 1 hour and 30 minutes. After a dark-field washing step, DAPI was introduced, and slides were observed under a fluorescence microscope.
3.7. Statistical Analysis
GraphPad Prism 5.04 software was utilized for one-way ANOVA and multiple Tukey comparison test post hoc analyses. Data were presented as the mean ± SE and compared to the control group. A P-value of less than 0.05 indicated a significant difference in all studies.
4. Results
4.1. GC-MS Analysis
The GC-Mass data for all plant specimens are presented in Table 1. In terms of their relative abundance, the primary constituents identified in the ethanolic extract of E. purpurea were Melezitose (24.95%), Palmitic acid (14.44%), and Stigmasterol (22,23-dihydro-12.65%).
In the case of the ethanolic extract derived from A. sativum, the predominant compounds detected were Isoamyl formate (40.78%), followed by (23S)-ethylcholest-5-en-3 beta-ol (31.27%), and n-Hexadecenoic acid (6.89%).
| No. | Echinacea purpurea | Allium sativum | Arnebia euchroma | |||
|---|---|---|---|---|---|---|
| Percentage | Component | Percentage | Component | Percentage | Component | |
| 1 | 4.01 | Di-2-propenyl disulfide | 40.78 | Isoamyl formate | 2.36 | 2-Heptanone, 3-hydroxy-3-methyl- |
| 2 | 1.84 | Cyclopropanecarboxylic acid, dodecyl ester | 5.73 | 3-Allyl-6-methoxyphenol | 2.01 | 4-Vinyl guaiacol [4VG] |
| 3 | 1.99 | Trisulfide, di-2-propenyl | 4.20 | D-mannose | 2.98 | 1,2,5-Trimethoxypentane |
| 4 | 24.95 | Melezitose | 4.29 | Neophytadiene | 3.42 | 2-Methyl-2-butenolide |
| 5 | 4.14 | Octyl phenyl ether | 6.89 | n-Hexadecenoic acid | 12.61 | Benzyl benzoate |
| 6 | 14.44 | Palmitic acid | 3.45 | 3-Nitrophthalic acid | 41.47 | Mannitol |
| 7 | 2.79 | Bis[2-ethylhexyl] phthalate | 3.40 | Gibberellin A3 | 3.74 | Palmitic acid |
| 8 | 6.50 | 3,6,9,12-Tetraoxo tetra decan -1-ol, 14-[nonylphenoxy]- | 31.27 | [23S]-Ethylcholest-5-en-3.beta.-ol | 1.80 | Methyl 9,12,15-octadecatrienoate |
| 9 | 2.76 | 8-Methoxy-2,4-diphenylquinoline | _ | _ | 2.67 | Phytol |
| 10 | 6.50 | 1,1,3,3,5,5,7,7,9,9,11,11,13,13-Tetradecamethylheptasiloxane | _ | _ | 4.63 | Bicyclo[4.3.1]dec-1[9]-ene |
| 11 | 3.85 | 1,1,3,3,5,5,7,7,9,9,11,11,13,13,15,15-Hexadecamethyloctasiloxane | _ | _ | 2.80 | Palmitic acid β-monoglyceride |
| 12 | 2.69 | 2',4'-Dimethyloxanilic acid N'-veratrylidenehydrazide | _ | _ | 5.79 | Bis[2-ethylhexyl] phthalate |
| 13 | 12.65 | Stigmasterol, 22,23-dihydro- | _ | _ | 6.18 | Vitamin E acetate |
| 14 | 5.82 | Gibberellin A3 | _ | _ | 7.56 | γ-Sitosterol |
| 15 | 3.80 | 1,3-Dimethyl-4-phenanthrene | _ | _ | _ | _ |
Meanwhile, the ethanolic extract of A. euchroma featured mannitol (41.47%) as the most abundant compound, along with Benzyl benzoate (12.61%) and γ-Sitosterol (7.56%) as the second and third principal constituents, respectively.
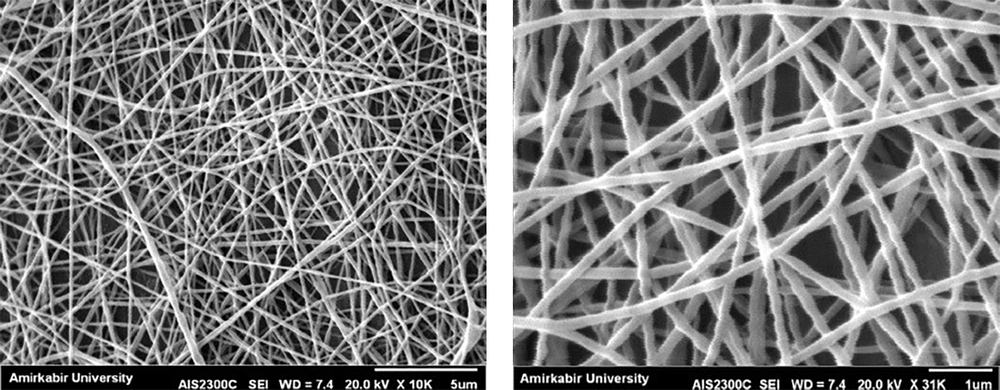
4.2. The Scanning Electron Microscope
The scanning electron microscope (SEM) was operated at 20 kV× 10K (5 μm and 1 µm magnification) under high vacuum conditions to analyze the morphological characteristics of the electrospun PVA nanofiber formulation (Figure 1).
4.3. Immunohistochemistry
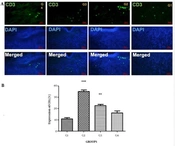
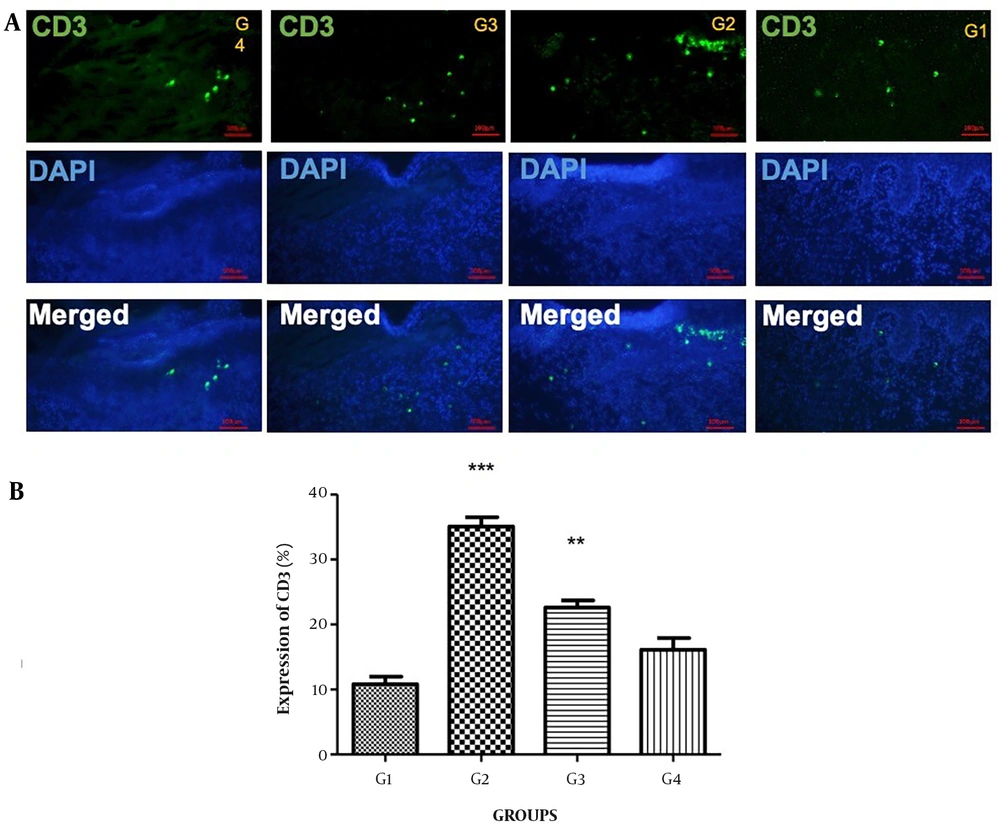
CD3, as seen in Figures 2A and B, served as the positive marker for validating the skin wound within a 1×1 cm2 area. A small amount of CD3 was reported in the skin of groups G1 and G4. The expression of CD3 exhibited a significant increase in G2 (P < 0.001) and G3 (P < 0.01) as compared to G1 and G4 (Figure 2).
(A) Immunohistochemically expression of CD3 was observed throughout the wound repair process up to day 14 post-wounding. On day 14, Unwounded skin (G1), wound + Staphylococcus aureus (G2), wound + S. aureuss + Povidone-iodine (G3), and wound + S. aureus + formulation of herbals on PVA microfibers (G4). The upper panels illustrate CD3 (green), the central panels represent DAPI (blue), and the lower panels show merged images (400×); (B) Bar graph representation of the CD3 scores in all three groups, ***P < 0.001 and **P < 0.01in G2, G3, and G4 compare to G1
4.4. Histological and Morphometrically Study
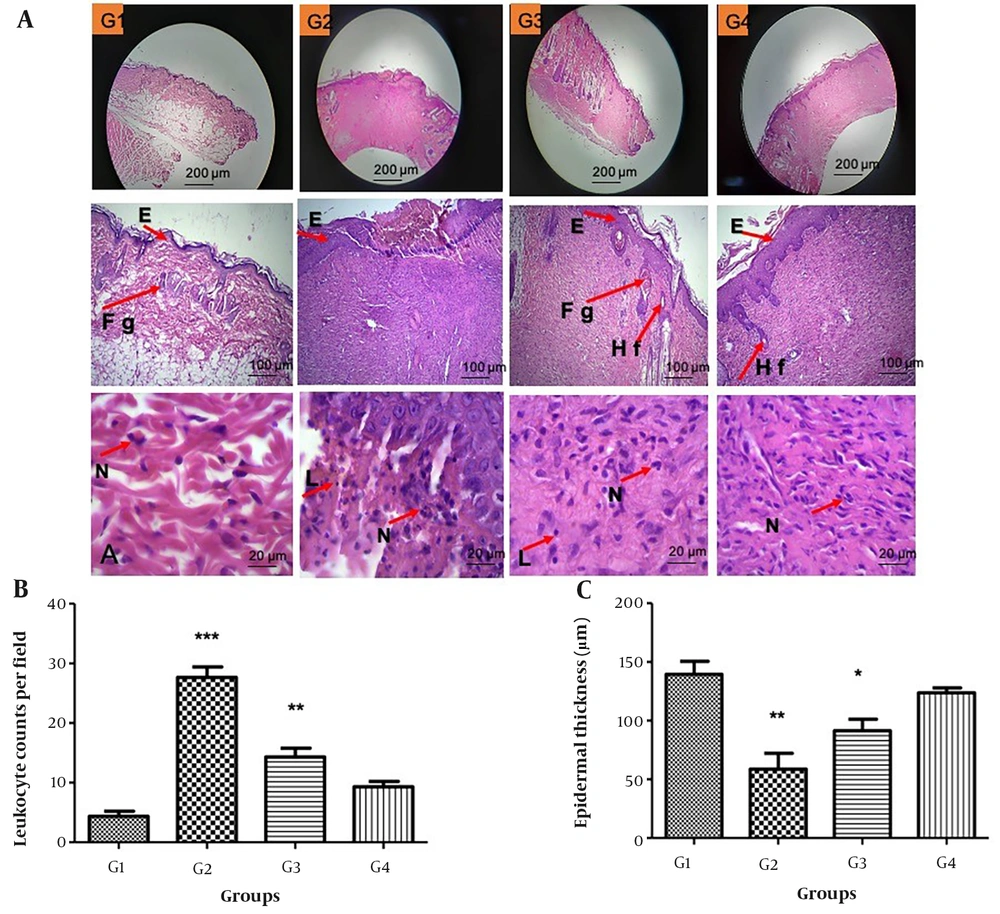
On day 14, inflammatory cells and granulation tissue in the PVA/formulation nanofiber group disappeared, and its follicles began growing new hair (Figure 3). The epidermal thickness in the G2 and G3 compared with G1 decreased respectively by P < 0.01 and P < 0.05.
(A) Skin samples from rats stained with H&E in four groups on day 14 post-wounding (100X, 400X). Nine sections/skin/rats were examined for each group (n = 3). (E = Epithelium, FG = Fatty gland, Hf = Hair follicle, N = Neutrophil, L = lymphocyte); (B) Significant increase in leukocyte numbers were observed in G2 and G3 groups compared to G1 (***P < 0.001 and **P < 0.01), respectively. There are not any differences between G1 and G4. (C) The thickness of the epidermal decreased in the G2 and G3 compared with G1 (**P < 0.01 and *P < 0.05), respectively. There are not any differences between G1 and G4.
Moreover, the G2 (P < 0.001) and G3 (P < 0.01) groups displayed a notable increase in leukocyte numbers compared to G1. No significant distinctions were observed between G1 and G4.
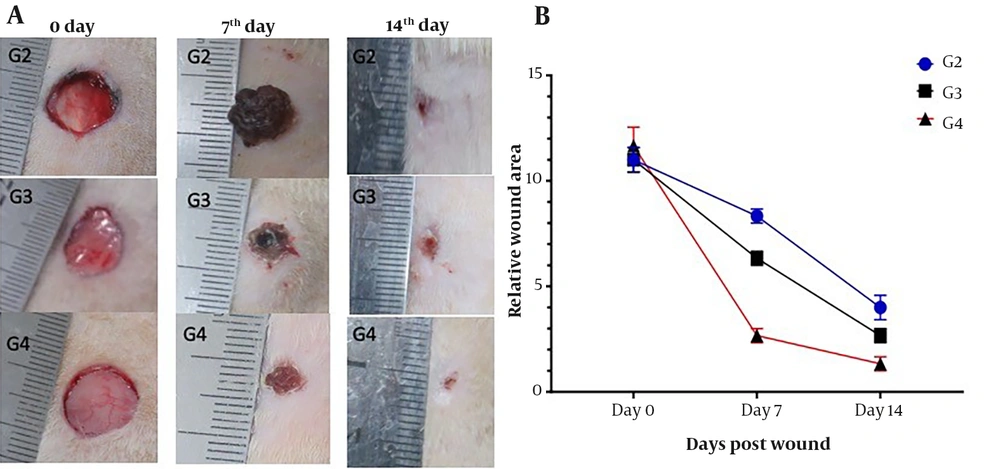
On day zero, 10 mm of macroscopic morphology of the dorsal wound was seen in all three groups. The average ulcer area on the seventh day was 8, 6, and 3 mm in G2, G3, and G4, respectively. On day 14, the wound size had notably decreased, and the healing rate was more rapid in G4 compared to the other groups. The wound area in G4 was significantly smaller than the one in G2 and G3(P < 0.01) (Figure 4).
The wound healing progression in all G2, G3, and G4 groups in rats after 0, 7, and 14 days. Herbal formulation/ PVA accelerated wound healing in the G4 group compared to G3 with Povidone-iodine and G2 without any treatment. B) Each rat's wound area was calculated in millimeters (n = 3). mean ± SD **P < 0.01 in all groups
4.5. Antibacterial Activity of Formulated Plant Extracts
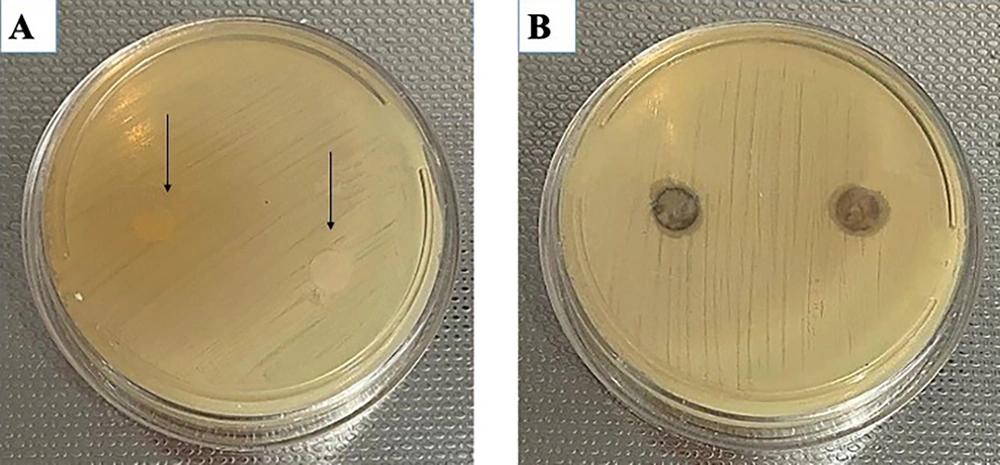
The diameter of the inhibition zone, measured in millimeters (mm), was employed to quantify inhibitory activity. The diameter of a droplet containing the S. aureus strain lacking antibacterial properties served as the control. Notably, the formulated extract exhibited a significantly greater inhibitory zone against S. aureus, with a measurement of 7 mm, in contrast to the negative control group devoid of the formulated material (Figure 5).
Antimicrobial effects of the herbal formulation on Staphylococcus aureus (ATCC: 6538) by inhibition zones test. The average diameter (mm) of the inhibition zones in the negative control group with S. aureus = 0 mm is indicated by arrows (A), while both formulated extracts exhibit an inhibition zone of 7 mm (B).
4.6. Minimal Inhibitory Concentration and Minimal Bactericidal Concentration of Formulated Plant Extracts
The results of MIC and MBC values indicated that the alcoholic extracts of the formulated substance are the most potent in suppressing the growth of S. aureus. The tested solutions exhibited identical MIC and MBC values against S. aureus at 1.87 mg/mL concentrations.
5. Discussion
In light of the economic infeasibility of industrial dressings, this study aimed to develop a suitable herbal formulation, combined with electrospinning PVA, to cover rat skin-induced wounds. On day 14, while all ulcers exhibited macroscopic repair, microscopic investigation revealed a significant increase in leukocyte numbers in wounds infected with S. aureus in the povidone-iodine group.
After 14 days, leukocyte numbers did not significantly differ between the control group and the infected wounds treated with formulated PVA nanofibers.
The histopathological analysis of the formulation-treated group also demonstrated wound healing attributed to increased epidermal thickness. The results of this study revealed that the attenuated effects of formulated PVA microfibers on rat-infected wounds could be achieved by day 14 of treatment, compared to routine wound healing procedures.
Based on prior assessments, active plant extract compounds with appropriate antibacterial activity can be transformed into possible prescriptions for wound healing.
Antimicrobial agents in wound dressing can play an essential role in controlling the proliferation and colonization of microorganisms (16). The A. euchroma, A. sativum, and E. purpurea extracts possess antibacterial, anti-inflammatory, and wound-healing properties. However, their beneficial effects occurred after 14 days in mice and rat models (6, 17).
We decided to use the potency of all three herbs through this nano-coating for treatment. This method proved highly effective in reducing the treatment duration compared to reported cases and displayed favorable healing symptoms.
According to previous research, designing and developing electrospun nanofibers has no antibacterial effect, and it is very suitable for wound dressing with natural antimicrobial agents that help prevent wound infection. The plant extracts combined with nanofiber membranes that can maintain moisture at the desired level will help skin wounds heal better (18, 19). In the present experimental study, herbal wound dressing reduced CD3+ activity at the wound site during epidermal healing in rats. Our findings align with a 2021 study on the LiangXue JieDu (LXD) formula, which demonstrated reduced CD3+ levels during wound healing (20).
Neophytadiene and 3-nitrophthalic acid revealed antibacterial properties against S. aureus, as evidenced by the inhibition zone diameter evaluation (21-23). In the present study, GC-MS analysis detected 3-nitrophthalic acid and Neophytadiene in A. sativum, suggesting these components may bolster the antibacterial effects of the formulation. On the other hand, A. euchroma demonstrated beneficial wound-healing properties, including antibacterial characteristics, fibroblast proliferation, and vascularization in the wound (24, 25). Yan et al. tested three new compounds derived from the Arbania plant, revealing their cytotoxic capabilities (26, 27).
Numerous in vivo studies on the immunomodulatory and anti-inflammatory properties of E. purpurea suggest that it enhances innate immunity and strengthens the immune system against pathogenic infections through the activation of neutrophils, macrophages, polymorphonuclear leukocytes, and natural killer cells (28, 29).
Arnebia euchroma extract stimulates collagen synthesis and fibroblast proliferation in wound healing (30). Allium sativum promotes granular tissue formation while reducing leukocyte penetration into the repaired tissue during wound healing. Also, according to the therapeutic approach of A. sativum, a noticeable amount of collagen production is predictable, especially in the deeper layers of the dermis (31). Echinacea purpurea was previously employed as an herbal remedy for skin wounds (32).
However, in this study, the combination of A. sativum, E. purpurea, and A. euchroma increased efficiency and accelerated the remodeling stage of the skin. In all groups, assessment of wound recovery on days 7 and 14 showed that wound mats with nanofibers and herbal extraction exhibited superior macroscopic healing ability. The wound measurement showed that G4 has a significant statistical difference compared to G2 and G3 (P < 0.01). In the last phase of wound healing, leukocyte counts decrease, collagen production reaches equilibrium, and epithelialization through collagen absorbance is enhanced, resulting in scar tissue healing facilitated by herbal wound dressing. Microscopic evaluations of histopathology revealed that the epidermal thickness in G4 did not have any significant differences with G1, which showed perfect healing with a high rate of epithelialization on day 14.
5.1. Conclusions
The electrospun medicated nanofibers with alcoholic extraction of A. euchromaA. sativum, and E. purpurea were utilized in animal models for infectious wound healing.
Histopathological and immunohistochemical studies revealed that formula/PVA wound dressing might reduce inflammation and accelerate the entrance of the proliferation phase, regenerative response, tissue granulation, and wound contraction. The advantages of this new membrane include its simplicity, versatility in various sizes and shapes, full foldability, and relatively low cost.
Therefore, the co-supplementation of PVA and plant extracts can effectively regulate abnormal protein expression and correct clinical symptoms, suggesting that this co-supplementation wound dressing could be a potential nutraceutical in overcoming the harmful effects of bacterial infectious beyond pharmacological interventions for ameliorating CD3 and improving tissue histopathology evaluations.
Consequently, it is advisable to conduct further human studies to recommend using adjunctive therapeutic agents with their corresponding protective impact, devoid of side effects, and at a reasonable price for treating infectious wounds.