1. Background
Melasma is a prevalent dermatologic ailment characterized by the development of brown or grayish - brown patches on the face, particularly on the cheeks, forehead, and upper lip. This disorder results from the synthesis of melanin, which is the pigment that gives color to the skin (1). In addition to genetic predisposition, other risk factors, such as sunlight exposure, pregnancy, hormonal disorders, sexual hormones, oral contraceptive pills, and some medications, can contribute to the development of melasma (2, 3).
The precise incidence of melasma has not been definitively identified; however, it has been reported to range from 8.8% to 40%, with a higher prevalence in individuals with darker skin tones, such as East Asians, Middle Easterners, and Mediterranean-Africans (4, 5). There is also a significant gender disparity, with women being more likely to develop melasma than men (6). Melasma can have a negative impact on patients’ self - esteem and quality of life, as it can affect their appearance (7, 8).
A wide range of treatments is available for the control of melasma, including topical medications, chemical peels, dermabrasion, and laser therapy (9, 10). However, treatments for melasma are challenging, and no single therapy exists to cure it. Conventional treatments might not achieve optimal results and sometimes have unwanted side effects (11). Therefore, numerous studies are being conducted to find alternative therapies (12). Due to their greater acceptance, fewer side effects, and lower costs, many individuals and even physicians have turned to natural therapies, such as herbal medicines (13, 14). As a modality of complementary and alternative medicine, herbal medicines have been traditionally used topically or orally to treat a variety of dermatological conditions (15, 16).
Herbal combinations have been used traditionally to improve melasma. One example is Chinese herbal medicine, a decoction of several medicinal plants, which was used in combination with laser therapy to achieve better effectiveness than laser therapy alone (17). Another example is an herbal combination in Traditional Persian Medicine (TPM) that includes lemon balm, fennel, and damask rose. This herbal syrup has been shown to have therapeutic effects on various skin problems, such as acne, psoriasis, and melasma. The efficacy of this polyherbal syrup has been demonstrated in a clinical trial for the treatment of psoriasis (18).
Lemon balm has antibacterial and anti-inflammatory effects due to its rosmarinic acid, flavonoids, phenolic, and terpenes compounds and, therefore, can be effective in skin rejuvenation (19). Damask rose has been shown to have beneficial effects on the skin, such as hydration, improvement of stretch marks and wrinkles, acne control, and pigmentation reduction (20). Furthermore, fennel has been used as a traditional medicine in different cultures for a variety of conditions, including digestive problems. Recent research has shown that fennel has anti - inflammatory and anti - melanogenesis effects, which might explain its traditional use in melasma (21, 22).
2. Objectives
No trial investigation has been conducted to elucidate the therapeutic effects of the aforementioned polyherbal syrup on melasma. Therefore, this randomized controlled clinical trial was planned to assess the efficacy and safety of this herbal syrup in patients with melasma.
3. Methods
3.1. Study Design
A controlled clinical study with a triple - blind randomization design was conducted at the Center for Research and Training in Skin Diseases and Leprosy, Tehran University of Medical Sciences, Tehran, Iran, from 2019 to 2021.
The study was conducted in accordance with the Declaration of Helsinki and its subsequent revisions. The research protocol was approved by the Ethics Committee of the Tehran University of Medical Sciences. The study was registered at the Iranian Registry of Clinical Trials (IRCT) with the registration number IRCT20180927041149N1. All participants provided written informed consent after being informed about the purpose, procedure, potential benefits, and risks of the study. Furthermore, the participants were aware of their withdrawal right at any stage of the study.
3.2. Study Subjects
Female participants who were aged 15 years or older and had melasma confirmed by a dermatologist were eligible to be included in the trial. However, the subjects were excluded if they had any of the features, including diabetes, pregnancy, lactation, hypermenorrhea, systematic or endocrine disorders, receiving any topical treatment from 2 weeks before the start of the study, and using contraceptive agents 1 month before entering the study.
3.3. Sample Size Estimation
Based on a previous study (23), assuming a mean difference of 1.77 on the melasma area and severity index (MASI) score with standard deviation (SD) values of 3.01 and 3.18, a power of 80%, and a type 1 error of 0.05, a total of 98 participants (49 per group) were required. To account for an estimated 10% attrition rate, a final sample size of 110 subjects was obtained.
3.4. Randomization and Intervention
The participants who met the inclusion criteria were randomly allocated to either the intervention or placebo group in a 1: 1 ratio using permuted block randomization with blocks of four. An allocation sequence was generated using a random number table. To ensure the integrity of the study, both the researchers and participants were blinded to the randomization schedules and the type of intervention.
During the 12-week period, the patients in the intervention group ingested 10 mL of polyherbal syrup three times a day, specifically before breakfast, lunch, and dinner. Conversely, patients in the placebo group consumed a placebo syrup in the same way.
The research team met with the participants every month. During these visits, the participants were provided with three bottles of syrup and asked to report any issues or concerns they might have had. To monitor adherence to the intervention, the participants were required to return the empty bottles at their next visit. Additionally, one of the researchers contacted the participants once a week to inquire about any potential side effects and remind them to take the syrup.
3.5. Syrup Preparation
The syrup was composed of three medicinal plants, including lemon balm leaves (Melissa officinalis L.), fennel seed (Foeniculum vulgare Mill.), and damask rose (Rosa damascena Mill). Herbs were purchased from a known market and were authenticated by a botanist, Melissa officinalis L. (voucher number: PMP-377), Foeniculum vulgare Mill. (Voucher number: PMP-1621), and Rosa damascene Mill (voucher number: PMP-531). The amount required for each ingredient to make syrup was 3, 8, and 1 g for Lemon balm, Damask rose, and Fennel seed, respectively.
To prepare the syrup, all the ingredients were mixed and boiled in 450 mL of water until the volume was reduced to 250 mL. During the boiling process, honey was added to enhance the sweetness. The placebo syrup was made using sugar, caramel coloring additive, and 1% rose water. The color, odor, viscosity, and bottle of the placebo syrup were indistinguishable from those of the original syrup.
3.6. Total Phenolic and Total Flavonoid Assay
The syrup’s overall phenolic content was assessed using the Folin - Ciocalteu assay and quantified in milligrams of gallic acid equivalents (GAE). Additionally, the total flavonoid content was calculated using the aluminum chloride colorimetric assay and expressed in milligrams of quercetin equivalents (QE) (13). Based on the analysis, each milliliter of syrup was observed to contain 185.22 ± 0.71 and 103.64 ± 1.32 mg of GAE and quercetin, respectively.
3.7. Outcome Assessment
Initially, all participants in both groups completed a sociodemographic questionnaire, which consisted of items on age, gender, employment, family history of melasma, length of disease, history of melasma, pregnancy, hormonal therapy, and sun exposure.
The melasma area and severity index were used as a primary outcome to measure the severity of melasma (14). This index assesses three factors of involvement area, intensity, and homogeneity of pigmentation on three areas of the face, including the forehead, chin, and cheek. The area of melasma involvement is graded from 0 to 6 (0: No involvement, 1: Less than 10% involvement, 2: 10 - 29% involvement, 3: 30 - 49% involvement, 4: 50 - 69% involvement, 5: 70 - 89% involvement). The darkness of pigmentation and homogeneity is graded from 0 to 4 (0: Absent, 1: Slight, 2: Mild, 3: Marked, 4: Maximum). The overall score of the MASI is calculated by the sum of pigmentation intensity and homogeneity scores multiplied by the area score and the multiplying factor for each region. The MASI score was measured by an independent dermatologist through visual inspection at the beginning and end of the study.
A Mexameter MX18 (Courage & Khazaka electronic GmbH, Cologne, Germany) was utilized to measure the melanin and erythema of participants at the baseline and the 12th week. Then, the values were transformed to mean melanin and erythema. In addition, to assess the lightness and pigmentation of lesions, photos of the face were taken by the VisioFace Quick system (Courage & Khazaka electronic GmbH, Cologne, Germany) and analyzed by the VisioFace CSI software before the intervention and 12 weeks after the intervention.
Furthermore, the Melasma Quality of Life (MELASQOL), which is a 10-item questionnaire designed specifically for melasma patients, was used to evaluate the participants’ quality of life. Each item takes a score from 1 (not bothered at all) to 7 (bothered all the time). A higher score on this questionnaire means a lower quality of life.
According to the Common Terminology Criteria for Adverse Effects (CTCAE version 4), the subjects were asked about systematic adverse effects.
3.8. Statistical Analysis
Version 20 of SPSS software for Windows was employed to analyze the study data. A statistical level below 5% was considered to be significant. Continuous values were reported as the mean ± SD or standard error (SE); however, discrete values were reported as the frequency and percentage. To compare the distribution of discrete values, the binomial test was implemented. To assess the normality of the data dispersion, the Kolmogorov-Smirnov test was applied. Intra - group comparisons were made using the paired t-test, and the Wilcoxon t-test was used for parametric and non-parametric variables. Inter-group analyses, on the other hand, involved using the independent sample t-test and the Mann - Whitney U test for parametric and non - parametric variables, respectively.
4. Results
4.1. General Characteristics
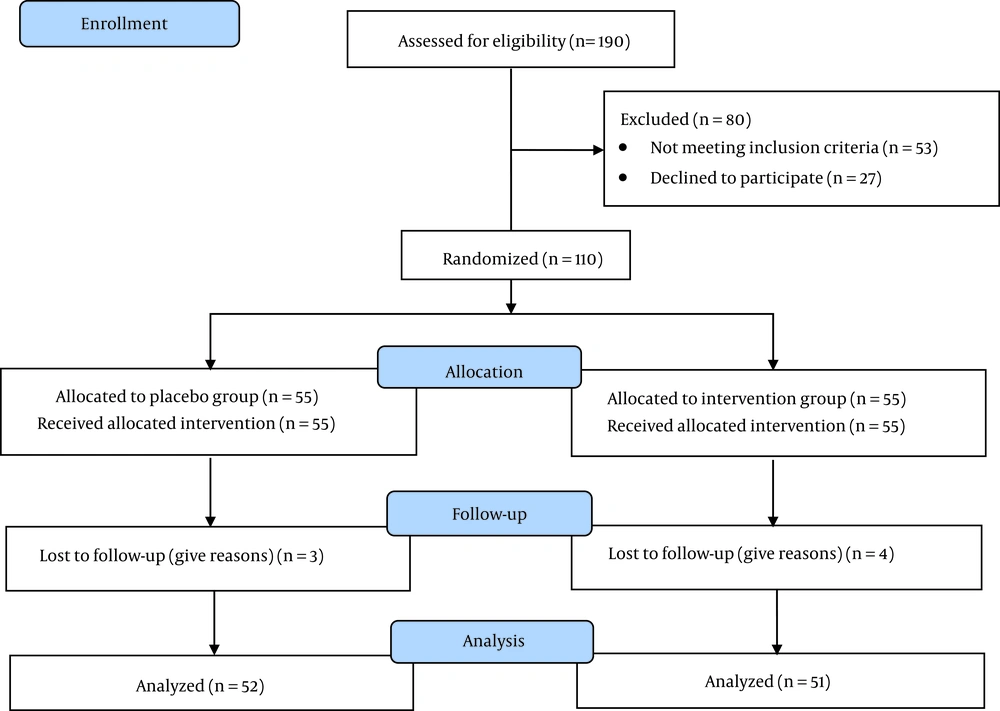
Out of the 190 individuals evaluated, a total of 110 patients met the study criteria and were randomly divided into either the intervention (n = 55) or placebo (n = 55) group. Upon the completion of the study, four individuals from the intervention group and three from the placebo group withdrew from the study. Figure 1 depicts additional details regarding participant enrollment, and Table 1 contains information about the general characteristics of the population. Overall, the distribution of participants between groups was statistically similar on most variables, except for a history of melasma in the malar area, duration of involvement in melasma of fewer than 5 years, and a family history of melasma.
| Variables | Treatment Group (N = 55) | Placebo Group (N = 55) | P – Value b |
|---|---|---|---|
| Age | |||
| < 20 | 4 (7.84) | 3 (5.76) | 0.776 |
| 20 - 39 | 18 (35.29) | 20 (38.46) | 0.650 |
| > 40 | 29 (56.86) | 29 (55.76) | 0.443 |
| Educational level | |||
| Illiterate | 5 (9.8) | 4 (7.6) | 0.506 |
| Under diploma or diploma | 7 (13.7) | 3 (5.7) | 0.868 |
| Academic | 39 (76.47) | 45 (86.53) | 0.213 |
| BMI (kg/m²) | |||
| 20 - 25 | 45 (88.23) | 48 (92.3) | 0.868 |
| ≥ 25 | 6 (11.7) | 4 (7.69) | 0.533 |
| Duration of melasma (y) | |||
| < 5 | 14 (27.45) | 17 (32.6) | 0.016 |
| > 5 | 37 (72.54) | 35 (67.30) | 0.484 |
| Distribution of melasma | |||
| Centrofacial | 9 (17.64) | 13 (25) | 0.312 |
| Mandibular | 16 (31.37) | 24 (46.15) | 0.999 |
| Malar | 26 (50.98) | 15 (28.84) | 0.037 |
| Baseline melasma score | |||
| Family history | 29 (56.8) | 28 (53.8) | 0.028 |
Abbreviations: SD, standard deviation; BMI, body mass index.
a Values are presented as No. (%) or mean ± SD.
b P-value is calculated by the binomial test.
4.2. Outcomes
The intra-group analysis showed a noticeable reduction in melanin and erythema values in the intervention group, from 254.07 ± 54.40 to 242.54 ± 51.05 (P = 0.013) and 398.20 ± 93.54 to 343.29 ± 71.55 (P < 0.001), respectively. The aforementioned scores did not change in the placebo group (P > 0.05). Additionally, the analysis of mean changes indicated a significant reduction in the melanin value (0.017). However, the reduction in erythema value was marginally insignificant (P = 0.060) (Table 2).
| Variables | Before | After | P - Value b | MD ± SE | P - Value c |
|---|---|---|---|---|---|
| Melanin | 0.017 | ||||
| Intervention | 254.07 ± 54.40 | 242.54 ± 51.05 | 0.013 | - 10.02 ± 5.88 | |
| Placebo | 245.26 ± 62.66 | 257.97 ± 70.26 | 0.487 | 11.31 ± 7.99 | |
| Erythema | 0.060 | ||||
| Intervention | 398.20 ± 93.54 | 343.29 ± 71.55 | < 0.001 | - 57.17 ± 9.57 | |
| Placebo | 378.77 ± 114.83 | 354.72 ± 86.37 | 0.223 | - 34.29 ± 14.12 | |
| Lightness | < 0.001 | ||||
| Intervention | - 8.31 ± 2.73 | - 6.27 ± 2.79 | < 0.001 | 2.04 ± 0.27 | |
| Placebo | - 5.17 ± 2.65 | - 6.92 ± 3.85 | < 0.001 | - 1.75 ± 0.32 | |
| Pigmentation | < 0.001 | ||||
| Intervention | 9.75 ± 3.00 | 7.44 ± 2.53 | < 0.001 | - 2.31 ± 0.28 | |
| Placebo | 7.25 ± 3.45 | 8.52 ± 3.92 | < 0.001 | 1.27 ± 0.31 |
Abbreviations: MD, mean difference; SD, standard deviation; SE, standard error.
a Values are presented as mean ± SD.
b P-value is calculated by the Wilcoxon signed-rank test.
c P-value is calculated by the Mann-Whitney U test.
Colorimetric analysis showed that the intervention group had a substantially higher lightness value (P < 0.001) and a significantly lower pigmentation value (P < 0.001) at the end of the study than their initial values. Nevertheless, the placebo group had significantly reduced lightness value and increased pigmentation value (P < 0.001). The comparison of mean changes demonstrated a significant difference between the intervention and placebo groups for both lightness and pigmentation variables (P < 0.001) (Table 2).
Within the intervention group, the MASI score decreased significantly from 4.34 ± 2.40 to 3.84 ± 2.02 (P = 0.001); however, the placebo group indicated a marginally insignificant increase from 3.63 ± 1.38 to 3.77 ± 1.65 (P = 0.051). Additionally, the intervention group showed a substantial reduction in MELASQOL score (P < 0.001); nonetheless, the placebo group demonstrated no significant change (P = 0.083). The comparison between the groups revealed a significant reduction in both MASI and MELASQOL scores (P < 0.05) (Table 3).
Abbreviations: MD, mean difference; SD, standard deviation; SE, standard error; MELASQOL, melasma quality of life; MASI, Melasma area and severity index.
a Values are presented as mean ± SD.
b P-value is calculated by the Wilcoxon signed - rank test.
c P-value is calculated by the Mann-Whitney U test.
No obvious side effects were reported over the treatment.
5. Discussion
In this study, a syrup containing Lemon balm, Damask rose, and Fennel that was based on TPM was utilized to treat melasma. After 12 weeks of consuming this polyherbal syrup, the analysis showed that individuals experienced a significant improvement in several biophysical and colorimetric parameters, such as melanin, erythema, lightness, pigmentation, and MASI score. As a result, their quality of life was enhanced, compared to before the study or the placebo group.
Melasma can be challenging to treat as the root cause remains unidentified. Traditional Persian Medicine literature suggests that skin weakness is the fundamental cause of various skin disorders, including melasma, eczema, and psoriasis. Traditional Persian Medicine is an ancient medical practice that is founded on the principles of temperament and humor. It maintains that the appearance of dark patches on the skin is an indication of an unfavorable temperament. It comprises four distinct approaches to treating health issues, including lifestyle changes, dietary interventions, herbal medicine, and physical therapy (24, 25). The present syrup contains lemon balm, fennel, and damask rose as its constituents, which have been recognized as potent herbs for managing certain skin conditions, such as melasma, in TPM manuscripts. Earlier studies have demonstrated the effectiveness of this syrup in ameliorating psoriasis (18).
Furthermore, the results of this clinical trial indicated that the polyherbal syrup based on TPM reduces certain parameters, such as melanin and pigmentation, and decreases the severity of melasma while improving skin lightness. Thornfeldt et al. also conducted a study that concluded that an herbal blend product was just as effective as conventional treatments, such as hydroquinone and tretinoin, in reducing hyperpigmentation (26). An experimental study evaluated the impact of lemon balm extract on melanogenesis in B16 - F1 murine melanocytes in vitro. The results indicated that the ethanolic extract of lemon balm could reduce the production of melanin in the cells by suppressing tyrosinase and its gene expression (27).
Tyrosinase and its related proteins are key enzymes involved in the pathways of melanogenesis (28). Some studies proposed that the most secure and efficient method of reducing hyperpigmentation is to hinder the function of the enzyme tyrosinase (29, 30). Furthermore, a study by Solimine et al. demonstrated that polyphenols present in roses, which is another component of the syrup, have tyrosinase inhibitory effects. Therefore, it could be used as a promising agent to alleviate disorders related to hyperpigmentation (31).
Moreover, scientific investigations have provided evidence of a significant correlation between inflammatory cytokines and skin pigmentation (32, 33). Studies have confirmed that specific inflammatory mediators, such as interleukin (IL)-1, IL-4, and IL-6, are capable of regulating the proliferation and differentiation of human epidermal melanocytes directly or indirectly. These mediators might also be involved in the regulation of melanogenesis in melanocytes (34, 35). According to the findings of a trial conducted by Zare Javid et al., the daily intake of 3 g of Melissa officinalis powder for a duration of 8 weeks led to a reduction in biomarkers of oxidative stress and inflammation in patients suffering from chronic stable angina (36). Furthermore, an animal study demonstrated that the simultaneous administration of lemon balm and dandelion resulted in a synergistic reduction in the messenger ribonucleic acid (mRNA) and protein levels of inflammatory cytokines, such as tumor necrosis factor - alpha, IL-1β, and IL-6 (37). Antioxidant and anti-inflammatory effects of damask rose and fennel have been reported in several studies (38, 39).
Recent discoveries have highlighted that an irregularity in the balance between reactive oxygen species and antioxidant defense mechanisms can be held responsible for the onset of melasma (40). The measurement of some stress oxidative enzymes revealed that the amount of these enzymes was markedly higher in melasma patients than in control patients (41, 42). Furthermore, skin aging and hyperpigmentation can also be attributed to the deterioration of dermal connective tissue caused by the expression of matrix metalloproteinase (43). Melissa officinalis L. comprises several bioactive compounds, primarily triterpenoids, phenolic acids, and flavonoids, and inhibits matrix metalloproteinase-2 (44).
To date, various plant extracts have been investigated as potential agents for skin lightness to improve skin pigmentation disorders (13, 45). In a particular study, a cosmetic formulation containing orchid plant extracts was observed to significantly enhance the size, brightness, color intensity, clarity, visibility, and overall appearance of pigmented spots (46). The aforementioned findings suggest that both the orchid and Melissa officinalis L. plant extracts are effective in the lightness of pigmented spots. Additionally, another study demonstrated that isoliquiritigenin extracted from licorice extract inhibited melanin biosynthesis, indicating that isoflavones and chalcones might be promising candidates for skin lightness (47).
This study has certain limitations, such as a short-term follow - up period, unavailability of spectroscopy to determine the extent of skin severity involvement, and a short interval between patient admissions, which caused some patients to discontinue the study. Additionally, further research is required to identify the active compound that is responsible for the therapeutic effects of the syrup, including the testing of various concentrations to determine the optimal effective dose.
5.1. Conclusions
To sum up, the results of this study suggest that a polyherbal syrup containing lemon balm, damask rose, and fennel at appropriate concentrations could be a viable complementary or alternative option for melasma treatment. However, further research is needed to explore the potential benefits of other medicinal plants and to identify the active components present in these plants.