1. Background
Nowadays, there is a great tendency to use natural products as medicinal plants instead of their chemical counterparts. Hypericum perforatum L., commonly known as St. John’s Wort, is a medicinal plant containing many active ingredients. It has antimicrobial, antiviral, anti- HIV, and anti-inflammatory activities and can treat various neurological disorders (1-3). Hypericin is a secondary metabolite isolated from Hypericum perforatum and belongs to napthodianthrones group. Hypericins and hyperforin were both considered to be responsible for the antidepressant effect of Hypericum perforatum (4). Also, hypericin has a protective effect on the plasmid DNA and E. coli against the reduction of SnCl2 (5).
Hypericin preparations were used as food supplement. However, the amount of Hypericin strongly depends on the source of plant material. Liu et al. found significant variations in the amounts of major components among 5 different brands of St. John’s Wort (6). Generally, most pharmaceutical manufacturers standardize the Hypericum perforatum products based on pseudohypericin and hypericin content (4). Hypericin is used as a standard to identify a genuine plant material, and thus is important from quality control point of view. The standardization of Hypericum perforatum is now based on both hypericin and hyperforin content. The herb must contain 0.3% hypericin.
Hypericin is soluble in ethanol, methanol, pyridine, acetone, ethyl acetate, butanone, and aqueous alkali solutions, but it is insoluble in water and methylene chloride (7). Different extraction methods such as maceration (4), solid phase extraction (8), and soxhelet extraction (7), using a wide variety of solvents, have been utilized to determine hypricin content of Hypericum perforatum; however, most of them are time consuming, use high volume of organic solvents, or need special equipment. Thus, the present study aimed at introducing a simple, rapid, and economic extraction and purification procedure for hypericin.
2. Methods
2.1. Reagents and Materials
All reagents were of analytical grade. Methanol, ethanol, diethyl ether, acetic acid, ammonia, acetonitrile, chloroform, and normal hexane were all provided from Merck (Darmstadt, Germany). Hypericin from Hypericum perforatum, 95% (HPLC grade), was a product of Fulka (Austria). TLC silica gel GF 254 aluminum sheet and silica gel with mesh size in the range of 35 to 70 were provided from Merck (Darmstadt, Germany). Nitrogen gas with 99.99% purity was used to evaporate the solvents. Hypericum triquetrifolium Turra leaves were collected from a wheat farm near Barangard village in Khuzestan province (Figure 1) by Khuzestan Agricultural Research Center and confirmed by botanical department of the center.
Hypericin standard solution: a 40 µg mL-1 hypericin solution was prepared by dissolving 1 mg pure hypericin (95%) in 25 mL HPLC mobile phase. Other dilution of hypericin was prepared from this stock solution.
2.2. Instruments
UV/Vis Spectra of the extract and standard hypericin were recorded on Shimadzu UV-PC (Japan). Metrohm 620 pH-meter (Swiss) was used for pH adjustments. Ultrasonic bath from L and R manufacturing company, T-14 (USA), and Kubota KN-70 centrifuge (Tokyo 113 Japan) were used whenever required.
2.3. Chromatographic System
HPLC measurements were performed on JASCO 880 PU HPLC system (Japan) equipped with 88-PU pump, 880-31 Vmixer, and 870-UV UV/Vis detector. Separation was performed on ODS C18 (250 × 4 mm, 5 µm) column. Mobile phase was composed of 5mM ammonium acetate (pH 5.4): acetonitrile: glacial acetic acid (25:75:0.1). Flow rate was 0.7 mL min-1. Hypericin calibration was linear in the range of 0.028 to 11.2 µg mL-1 at 590 nm.
2.4. Sample Preparation and Extraction
The leaves were dried at room temperature for about 14 days. Then, they were grinded and kept in a dark glass bottle before extraction.
Chlorophylls and some unwanted nonpolar components of the leaves were removed using dichloromethane as a solvent. To do so, about 1 g of leaf powder was poured into a dark glass, 50 mL of dichloromethane was added, the mixture was put in an ultrasonic bath for about 30 minutes, and was then centrifuged, and the supernatant, containing unwanted compounds, were discarded. Then, about 24 mL of MeOH: Aceton (2:1) was added to the plant residue, and sonicated for about 30 minutes. Finally, the red supernatant was separated and kept in a dark glass bottle for further purification. The extraction was repeated several times adding 24 mL portions of the above- mentioned solvent on the plant residue till colorless or pale purple supernatant was obtained. All portions were mixed and the solvents were evaporated to dryness using nitrogen gas. Then, dried residue was dissolved in 4 mL of HPLC mobile phase. The resulting solution was further purified by a procedure described below before injection to HPLC.
2.5. Hypericins Purification
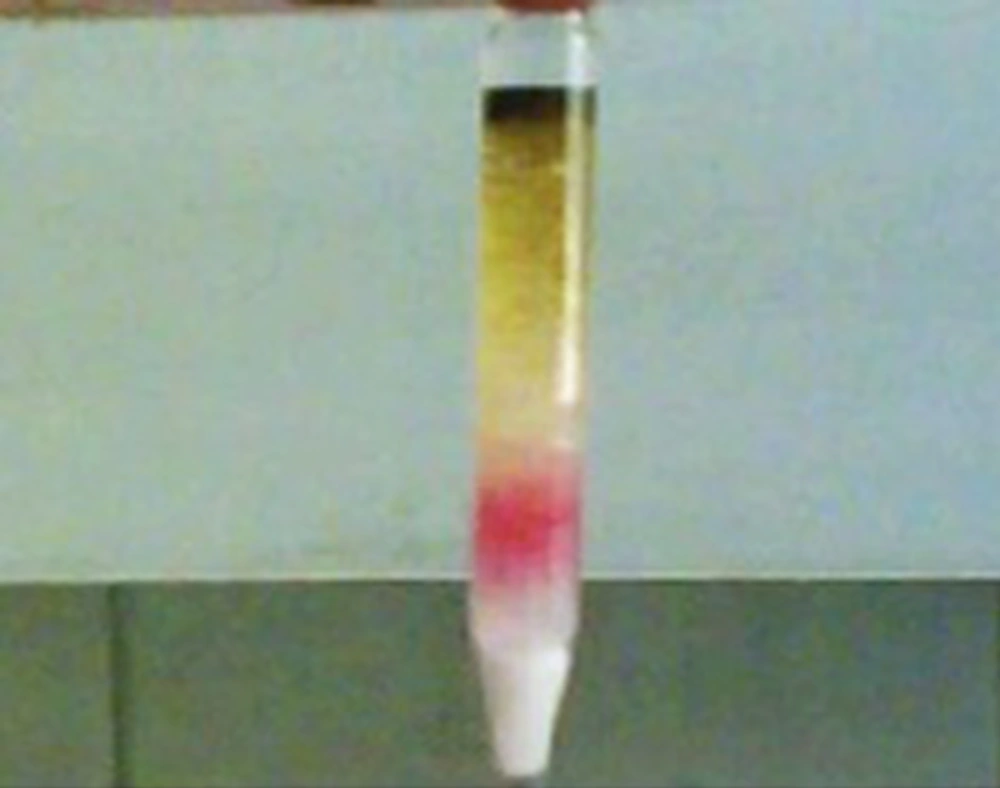
To obtain purified hypericins from the ultrasonic extract, 2 series of 70 × 5 mm glass columns, each filled with 800 mg silica gel, were used. About 10 µL of the extract or 100 µL of hyperan drop available in the Iranian pharmaceutical market was placed on top of the first column. The yellow colored compound was washed out on passing 4mL chloroform and 3 mL Chloroform: acetone (4:1) mixture through the columns. At that time, hypericins did not move down the first column. Finally, 3 mL MeOH: acetone: CH2Cl2 (75:10:15) was added to elute hypericins, while leaving another green and yellow colored compounds behind in the first column (Figure 2). The resulting red solution was collected and the solvent was evaporated to dryness using argon gas. Finally, 1 mL HPLC mobile phase was added to dry residue and 20 µL of the solution was injected to HPLC.
3. Results and Discussion
Hexane, dichloromethane, diethyl ether (9), as well as petroleum ether (10) were tested for maximum possible elimination of chlorophyll and nonpolar component including fatty acids. It was revealed that dichloromethane was the best solvent as it showed the least concomitant residue in the final crude extract.
Different SPE sorbents such as CG-400, CG-50, Amberlite XAD-7, activated carbon, and silica gel were tested for purification purposes. Among them, silica gel was shown the best band separation and purification ability. Thus, the effects of sorbent amount, column dimension, and elution solvent on purification of hypericin were optimized.
3.1. Elution Solvent
To find the best solvent for separation of hypericins from other components presented in the extract, using silica gel sorbent, we first examined the band separation ability of different solvents by TLC analysis of the extract (TLC aluminum sheets silica gel GF254). A brief explanation of the observations was as follows:
Chloroform as a developing solvent: All the sample components, except the yellow residue, which appeared in solvent front stay at the spotting line.
A mixture of Chloroform: MeOH (8:1) moved all the constituents, observing a broad continuous bond on the plate.
Acetonitril: A red spot (related to hypericins) was seen with no clear boarder line relative to other constituents, indicating poor separation.
EtOH: Acetonitril (1:1): showed the same results as acetonitrile alone.
CHCl3: Acetonitril (1:1): could not distinguish a border line between red and yellow spots on the TLC plate. They were mixed up.
EtOH: Eluting the entire constituent with the same rate, so all components were observed as a colored line in the solvent front.
CH2Cl2: Acetone: MeOH (15:10:75) showed the maximum migration differences of hypericins compared to the others.
From these observations, MeOH: acetone: CH2Cl2 (75:10:15) was selected as the best elution solvent to separate hypericins from other constituents in the purification step.
3.2. Column Specifications
Two glass columns with dimensions of 50 × 9 mm and 70 × 5 mm were checked for their resolution ability. High purification was obtained using 70 × 5 mm column. It was concluded that narrow bore and lengthy columns had better separation ability.
The purification extent was tested by a packing column with 0.8, 2, 3 g silica gel, and 2 × 0.8 gr (2 series of 800 mg silica columns). HPLC evaluation of the eluate indicated that 2 series of 800 mg silica column (70 × 5 mm) is more suitable for the hypericin purification.
3.3. Chromatographic Evaluations
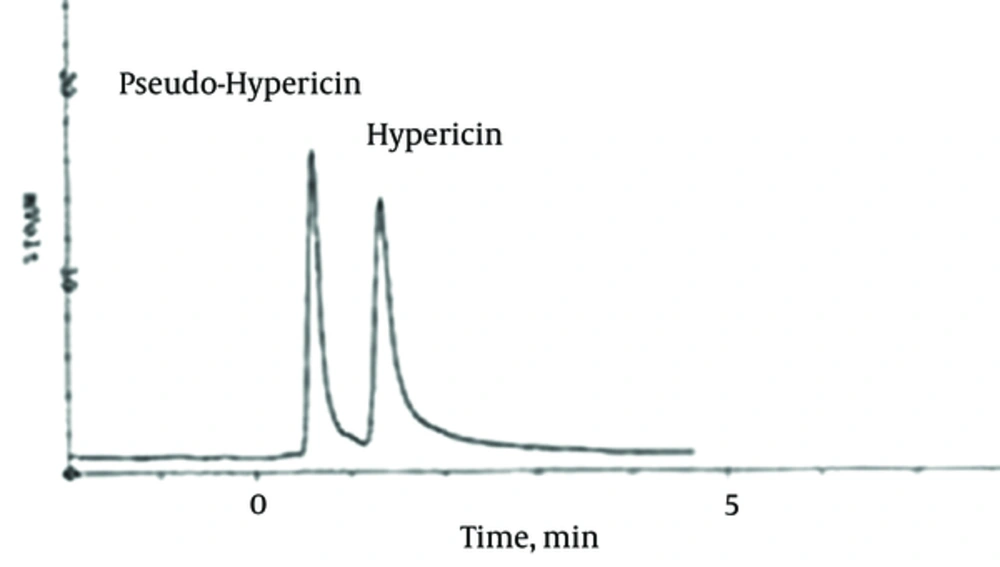
Each silica gel column eluate was injected into the HPLC instrument and the chromatograms at 254, 450, and 590 nm were recorded. As demonstrated in Figure 3, hypericin and pseudohypericin can obviously be seen at 590 nm, while no peaks at the other wavelengths were observed.
HPLC condition: ODS C18 (250 × 4 mm, 5 µm), Mobile phase: 5 mM amonium acetate: acetonitrile: glacial acetic acid (25:75:0.1) at pH 5.4. Flow rate: 0.7 mL min-1.
3.4. Method Reliability
Recovery of the present purification method was evaluated by adding precise amounts of hypericin standard to the ultrasonic extract before purification. Then, the amounts of standard recovered were measured; the results are reported as recovery in Table 1. As demonstrated by the the the present method has good purification ability.
| Sample | Hypericin Added, µg mL-1 | Determined, µg mL-1 | Recovery, % |
|---|---|---|---|
| Extract | - | 111.200 ± 0.025 | - |
| 0.112 | 111.314 ± 0.011 | 100.0 | |
| 5.600 | 120.064 ± 0.028 | 102.7 |
The hypericin contents of St Johns’ Wort leaf extract and hyperan drop, which was available in the Iranian pharmaceutical market, was determined as 5.104 ± 0.4 mg g-1 and 111.200 ± 0.2 µg mL-1, respectively. The sorbent capacity was 77.8 µg g-1.
3.5. Conclusion
A Simple, economic, and fast extraction and purification procedure was introduced for hypericin using readily available silica gel. The preliminary study revealed that scaling up is possible to have pure hypericins for use in the pharmaceutical preparations, food supplements, and other medical applications. Finally, this method can be considered as a rapid technique of hypericin extraction and purification suitable for teaching in laboratories and detecting the hypericins variations in different varieties and seasons from agricultural point of view.