1. Background
Epilepsy is one of the most common neurological disorders. Also, seizure patients are at high risk of cognitive impairment, depression, anxiety, and sudden death. Despite various antiepileptic therapies, there are still severe barriers to the successful treatment of epilepsy, like side effects and recurrence of seizures. Also, 30 to 40% of epileptic patients exhibit drug resistance (1). Therefore, new treatments with minimal toxicity are necessary for people with epilepsy. Polyunsaturated fatty acids (PUFAs) have been suggested as dietary supplements without side effects for epilepsy treatment (2). Polyunsaturated fatty acids are essential nutrients with critical functional and structural roles in the body (3). There are three classes of PUFAs; omega-3, -6, and -9. Because the human and mammalian bodies do not have an efficient enzymatic system for the biosynthesis of omega-3 and omega-6, (3) it is essential to have sufficient and balanced amounts of PUFAs in the diet. Fish oil and seed oil are the dietary supplement of omega-3 and omega-6, respectively. Also, human and animal studies have indicated the anticonvulsant effects of the acute and chronic administration of oils containing omega-3 and their dietary supplements, such as fish oil (4-9). Although fish oil leads to more health improvement than seed oils, there are limitations in consuming marine resources, such as overfishing, marine environmental pollution, inadequate access, and high oxidation rates (9). Also, the dietary intake of fish oil lowers the omega-6 levels in the body. Notably, the balanced omega-3/omega-6 ratio is critical for physiological function in the body, and its imbalance affects the pathophysiology of some disorders (3). Therefore, there is a need to find alternative sources that contain the essential omega-3 and omega-6 PUFAs. Vegetable seeds could be considered as the replacement with the balanced ratio of omega-3/omega-6. Although several studies have reported the anticonvulsant effects of PUFAs, little is known about the effect of seed oils as PUFA sources against seizure. Recently, seed oil from Echium species (Boraginaceae) has been considered a source of considerable omega-3 and omega-6. Echium amoenum Fisch & C. A. Mey, a member of the Boraginaceae family, is a well-known Iranian traditional herbal medicine. The Iranian Echium amoenum's seed oil has considerable PUFAs (10). Stearidonic acid (SDA) and alpha-linolenic acids (ALA) constitute a significant portion of omega-3 and γ-linolenic acid (GLA), and linoleic acid (LA) makes up most of the omega-6 content in Echium seed oil (EO) (11). Unlike many seed oils, EO has a high level of SDA and ALA, which are the precursors of the main essential omega-3 in the body, like docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA). Polyunsaturated fatty acids can easily pass through the brain-blood barrier and be incorporated into the neuronal membrane. Then, PUFAs modulate the voltage-gated ion channels and attenuate the excitability in the neurons (12, 13).
Furthermore, oxidative stress is one of the causes of the pathophysiology of epilepsy (14). Polyunsaturated fatty acid administration inhibited the oxidative stress induced by pentylenetetrazole (PTZ) (6). Recently, PUFAs, due to their antioxidant effects, have been considered to prevent and treat seizures. Echium oil has a significant amount and variety of PUFAs. Thus, it is expected that the antioxidant properties of Echium oil could be effective against seizures.
2. Objectives
Based on the mentioned background, this study evaluated the possible anticonvulsant property of EO pretreatment and its probable regulatory action on the anti-oxidative defense system. Therefore, we investigated the potential effects of oral consumption of Iranian EO on the threshold of seizures induced by intravenous injection of PTZ and the activity of superoxide dismutase (SOD) as an antioxidant system enzyme in mice. The seizure threshold is related to the balance of inhibitory and excitatory neuronal stimuli and is a clinical parameter to evaluate seizure susceptibility. The seizure threshold can be measured based on the amount of convulsive substance needed to cause a seizure. Pentylenetetrazole, a GABAA receptor antagonist, induces the absence of seizures and generalized epilepsy in mice and is used to determine the seizure threshold (15). Also, the long-term consumption of fatty acids and the omega-3/omega-6 imbalance may lead to dysfunction in some of the body organs, such as the liver and kidney (16). Thus, the measurement of liver and kidney parameters is necessary following the sub-chronic intake of EO. Subsequently, we evaluated the serum levels of liver enzymes, creatinine, SOD, and lipid concentrations.
3. Methods
The Echium amoenum seeds were obtained from Pakanbazr Company, Isfahan, Iran (herbarium code: 2hc.1400.her). The oil was extracted using the cold press method at a temperature below 40 - 45°C and under low pressure. Finally, the extracted EO was filtrated and then stored at 4°C. For more solubility, EO was mixed with sesame oil as a vehicle. Sesame oil was obtained from the local oil refining market. Pentylenetetrazole was purchased from Sigma-Aldrich. Chemical constituent analysis of the oil samples was done by gas chromatography (GC) using an Agilent 7890A gas chromatograph equipped with a DB-WAX fused polyethylene glycol column (60 m × 0.25 mm i.d., film thickness: 0.25 μm). The temperature of the oven was programmed as follows: The initial temperature was held at 170°C for 5 min, then increased to 220°C at a rate of 1°C/min. Detector and injector temperatures were both 220°C; Nitrogen was used as carrier gas with a linear velocity of 1 mL/min. Quantification data were obtained from GC-FID area percentage without correction factors.
3.1. Experimental Design
3.1.1. Animals and Treatment
Adult male NMRI mice (20 - 25 g) were purchased from the Pasteur Institute, Amol, Iran. Mice were kept in the polypropylene cages under controlled temperature (23 ± 2.0°C) and humidity and a 12-h light/dark cycle (6:00 - 18:00), with free access to a standard pellet diet and water. All experiments involving animals were approved by the Institutional Animals Ethics Committee of Amol University of Special Modern Technologies (ethics code: IR.ASMT.REC.1401.002) to minimize the number of animals and their suffering. Animals were randomly chosen and allocated to five groups (n = 10 per group): Control (no treatment), vehicle (sesame oil), and EO (1, 3, 5 g/kg). Administration of the vehicle and EO was performed by oral gavage (at a volume of 10 mL/kg) once daily for four consecutive weeks. We selected these doses using previous human studies (17, 18) and the dose conversion formula (19).
The threshold of PTZ-induced-colonic seizures was determined. We first inserted the 30 gauge needle connected to the insulin syringe via a polyethylene tube into the tail vein of the restrained mice based on the established method (20). After fixing the needle, we placed the animal in a chamber and injected PTZ (10 mg/kg) at a rate of 100 μL/min until the onset of general clonic seizures. Finally, we recorded the volume of PTZ solution required to induce general clonus. The amount of PTZ (mg) per mouse body weight (kg) was considered the seizure threshold. The performance of animals in all experimental groups was closely monitored during the EO administration in terms of abnormal behaviors, such as aggression, sensitivity to external stimuli, such as sound and light, cleaning body hair, muscle tremors, hyperactivity, lack of exploratory activity, and isolation.
3.2. Biochemical Analysis
At the end of the treatment, the animals were sacrificed. Then, the blood samples were taken from cardiac puncture and centrifuged at 5000 rpm for 15 min to obtain serum, and then biochemical parameters, such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatases (ALP), creatinine, triglycerides, total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), and very low-density lipoprotein (VLDL) were determined using an automated biochemical analyzer (HITACHI 7180, Japan) with Pars Azmoon kits (Pars Azmoon Inc., Tehran, Iran). The serum SOD activity was assessed using commercially available kits (ZellBio GmbH, Germany) based on the spectrophotometry method.
3.3. Statistical Analysis
The data obtained were analyzed using SPSS version 22.0 and expressed as the mean ± SEM. Statistical analysis was done using a one-way analysis of variance (ANOVA) followed by Tukey's test. In all experiments, a P < 0.05 was considered statistically significant. All graphs were drawn using Graph Pad Prism version 8 for Windows.
4. Results
4.1. Chromatographic Analysis
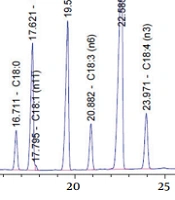
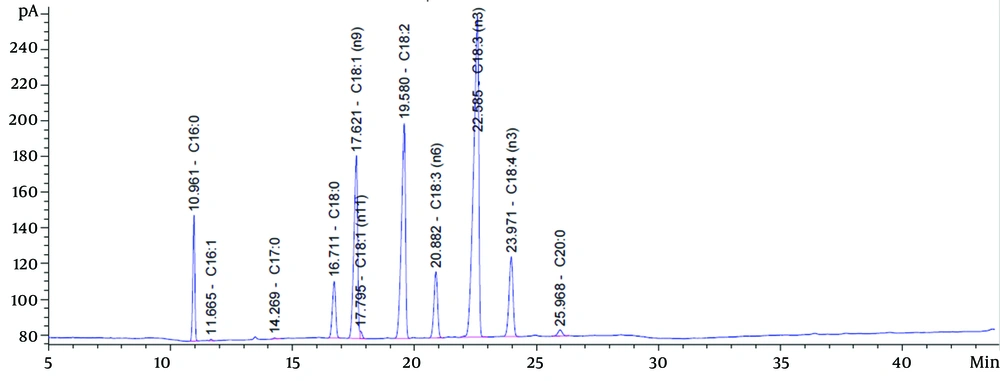
The fatty acid composition of EO is shown in Table 1. The highest constituents of PUFAs in EO were as follows: ALA > LA > OA > SDA > GLA, respectively (Figure 1).
| S. No. | Fatty Acid | Formula | Percentage |
|---|---|---|---|
| 1 | Palmitic acid | C16:0 | 5.86410 |
| 2 | Palmitoleic acid | C16:1(Δ9) | 0.05956 |
| 3 | Margaric acid | C17:0 | 0.06274 |
| 4 | Stearic acid C | 18:0 | 4.38450 |
| 5 | cis-Vaccenic acid | C18:1 | 0.35577 |
| 6 | Oleic acid | C18:1(Δ9) | 15.90194 |
| 7 | Linoleic acid | C18:2(Δ6) | 20.46961 |
| 8 | γ-Linolenic acid | C18:3(Δ6) | 5.65756 |
| 9 | α-Linolenic acid | C18:3(Δ3) | 39.19477 |
| 10 | Stearidonic acid | C18:4(Δ3) | 7.42315 |
| 11 | Arachidic acid | C20:0 | 0.62630 |
The Fatty Acid Composition of Echium Oil
The effect of EO on the threshold of seizure induced by PTZ:
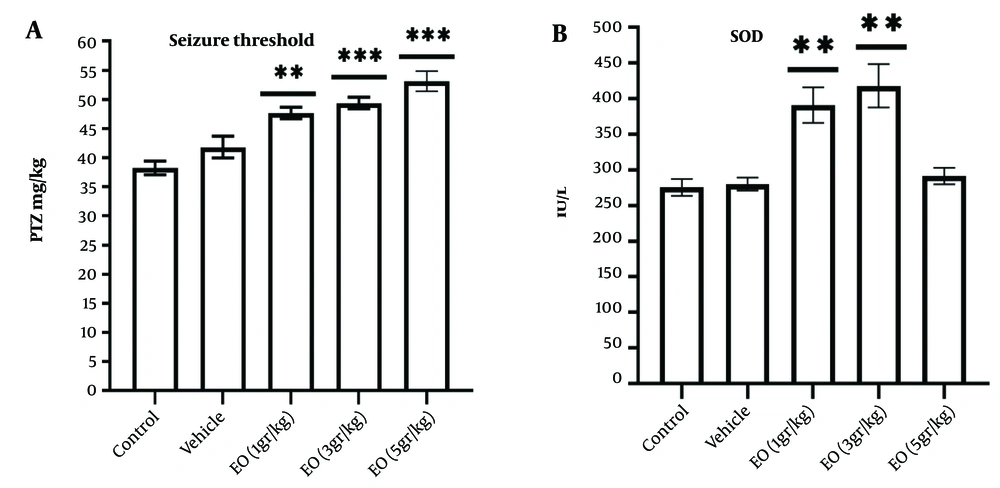
Compared with the control and vehicle groups, the sub-chronic oral pretreatment with EO raised the threshold of PTZ-induced general clonus dose-dependently. However, this elevation was statistically significant compared to the vehicle group only at 3 and 5 g/kg (P < 0.01 and P < 0.001, respectively) (Figure 2A).
Effects of Echium oil (EO) on the clonic seizure threshold (A); and serum superoxide dismutase activity (B). The sub-chronic oral administration of EO increased A, the seizure threshold dose-dependently; and B, the superoxide dismutase (SOD) activity at the doses of 1 and 3 g/kg. Data are represented as mean ± SEM. * P-value < 0.05, ** P-value < 0.01, and *** P-value < 0.001 vs. the vehicle group.
No abnormal behaviors were observed in the animals during the treatment.
4.2. Biochemical Analysis
4.2.1. The Serum SOD Activity
Pretreatment with EO at 1 and 3 g/kg dose-dependently increased the SOD activity (P < 0.01). However, it did not alter SOD activity at 5 g/kg (Figure 2B).
4.2.2. The Liver and Kidney Biochemical Markers
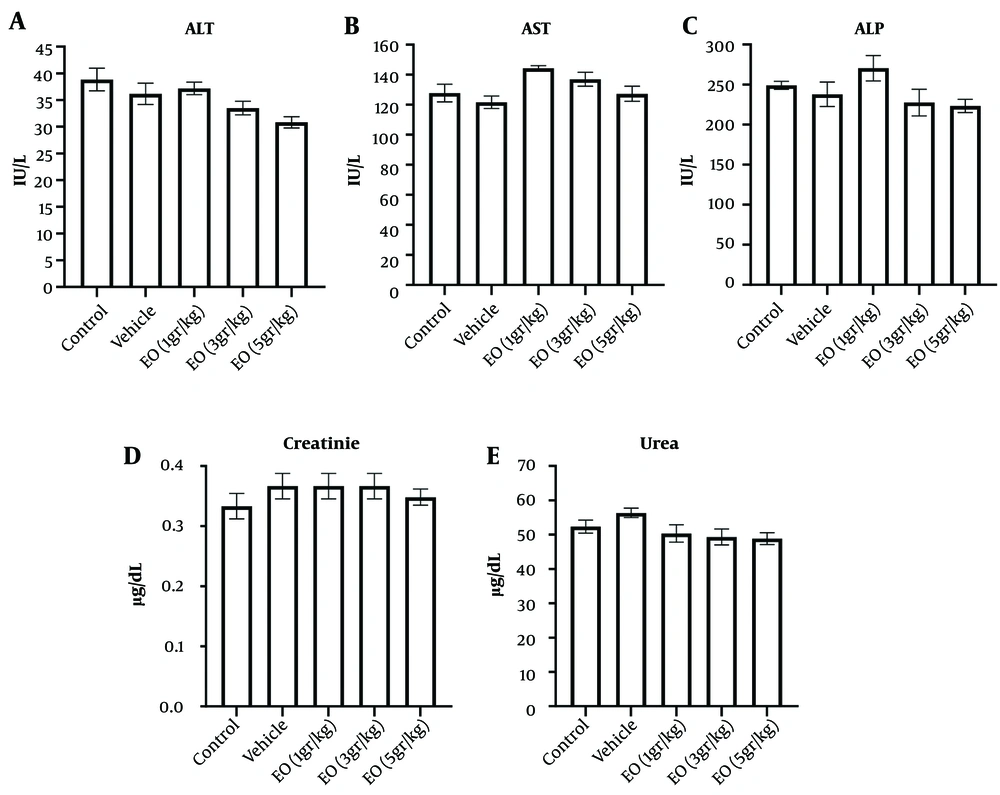
Compared to the vehicle group, EO did not significantly alter the serum's ALT, ALP, and creatinine levels (Figure 3).
Effect of Echium oil (EO) on the serum levels of the liver (A-C); and kidney parameters (D and E). The sub-chronic oral administration of EO did not change the A, alanine aminotransferase (ALT); B, aspartate transaminase (AST); C, alkaline phosphatase (ALP); D, creatinine; and E, urea levels. Data are represented as mean ± SEM.
4.2.3. Serum Cholesterol and TG Concentrations
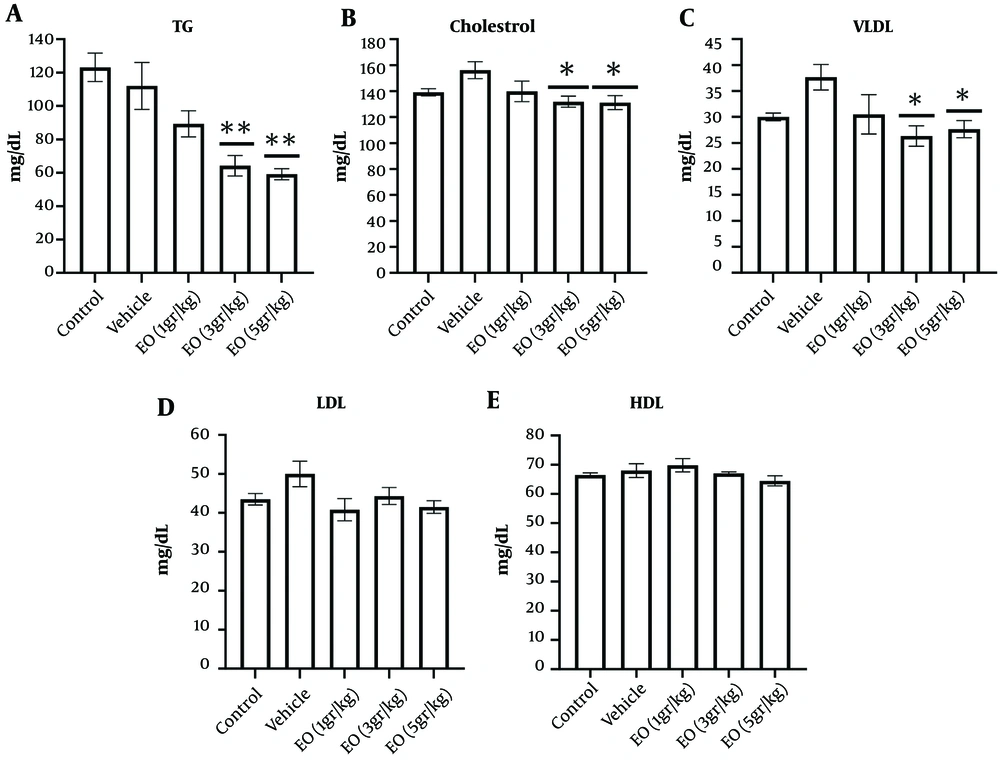
Compared to the vehicle group, the serum levels of cholesterol, TG, VLDL, and LDL decreased dose-dependently by EO consumption. Echium oil decreased the serum concentrations of cholesterol (P < 0.05), TG (P < 0.01), and VLDL (P < 0.05) at 3 and 5 g/kg. However, EO consumption did not significantly change HDL and LDL (Figure 4).
Effect of Echium oil (EO) on the serum levels of lipids (A-E). The sub-chronic oral administration of EO at the doses 3 and 5 g/kg decreased A, triglyceride (TG); B, cholesterol; and C, very low-density lipoprotein (VLDL) compared to the vehicle group. Echium oil did not change D, low-density lipoprotein (LDL); and E, high-density lipoprotein (HDL) levels. Data are represented as mean ± SEM. * P-value < 0.05 and ** P-value < 0.01 vs. the vehicle group.
5. Discussion
For the first time, we indicated that the sub-chronic administration of EO exerts the anti-seizure effect against PTZ with no negative impact on the liver and renal function. Furthermore, high doses of EO could lower serum cholesterol, TG, and VLDL levels. The serum SOD activity as the antioxidant enzyme increased at 1 and 3 g/kg of EO doses and showed no change at the highest dose of 5 g/kg. Because EO contains different types of omega-3 and omega-6 fatty acids, these results agree with the previous studies indicating the anticonvulsant effects of PUFAs in human and animal models (2). The acute and chronic administration of PUFAs inhibited the seizures induced by PTZ (4, 5, 7, 20, 21). Pentylenetetrazole induces seizures by inhibiting the gabaergic system (15) and modulating the metabotropic receptor glutamate (22). Gabaergic drugs and T-type calcium channel blockers inhibit PTZ-induced seizures (15). Polyunsaturated fatty acids could block the ion channels, such as T-type calcium channels, and modulate the function of the neurotransmitter system in the brain (12, 13). The chromatographic analysis showed that the EO had significant amounts of ALA, SDA, GLA, LA, and OA, which account for its anti-seizure effect reported in some studies. The incubation of zebrafish larvae at 10 and 20 µM concentrations of ALA inhibited the PTZ-induced seizure dose-dependently (5). Daily administration of the formula with a 4/1 ratio of LA to ALA decreased the latency of PTZ-induced seizure in rats (7). The chronic treatment of fish oil and oleic acid for 75 days in rats decreased the mean amplitude of ictal EEG recordings (23). In this regard, besides fish oil, the plant sources of PUFAs have been found with anticonvulsant effects. Pretreatment with argan oil with a high amount of LA and OA inhibited the seizures and the mortality rate after status epilepticus induced by pilocarpine (24).
In addition, adopting a diet enriched with herbal oils, such as rapeseed oil as the n-3 PUFA source and sun oil/ corn oil as the n-6 PUFA source for one month significantly improved the anticonvulsant effects of carbamazepine in magnesium-deficient mice (25). Notably, ALA and SDA in EO could endogenously convert to DHA and EPA. DHA is the main form of PUFAs in the cell membrane of the brain, and both DHA and EPAs have been commonly used as supplement for seizure prevention (26). Therefore, the formulation of PUFAs in EO as a sub-chronic supplement can suppress the seizure induced by PTZ. A few studies have investigated the supplementary EO effects on oxidative stress status. Intake of EO for four months in LDL-KO mice fed with a high-fat diet significantly increased the catalase (CAT) levels and decreased malondialdehyde (MDA) levels in the liver. However, SOD activity remained unchanged (27). Catalase and MDA are the anti-oxidative enzymes and the oxidative factor, respectively. The gestation and lactation diet enriched with 1% of EO did not significantly change the PUFA profile and oxidative status in sows' maternal and piglet plasma (28). Contrarily, our results indicated that a four-week intake of EO at 1 and 3 g/kg increased the SOD activity, a key anti-oxidative enzyme, in mice serum. The administration route and the amount of oil are the main difference between previous studies and the present work. Further, the antioxidant effect of EO confirms the previous reports, indicating the antioxidant activity of some herbal oils with a considerable amount of PUFAs. The diet enriched with rapeseed oil containing ALA or fish oil containing DHA + EPA increased the SOD activity in the liver of pups born to dams fed a diet low in omega-3 (29).
Additionally, the dietary consumption of flaxseed oil, which contains a high amount of ALA, for ten days significantly improved the oxidative stress induced by ethanol in the liver of alcoholic mice. It also decreased thiobarbituric acid-reactive substances (TBARS) as the oxidative stress indicator and increased SOD activity and glutathione (GSH) levels (30). Also, argon oil with a significant amount of LA and OA attenuated the oxidative stress by decreasing the nitrite level and lipid peroxidation and increasing the CAT concentration in the rat hippocampus (24). Pretreatment with SDA could increase the EPA levels in amyloid-β (A-β)-induced neurotoxicity in rat hippocampal cells. Further, Aβ-induced oxidative stress was inhibited by SDA through the significant increase in the SOD1 mRNA expression and CAT activity in rat hippocampal cells (31).
Interestingly, the antioxidant effect of EO could be correlated with its anticonvulsant effect. Seizure increases the formation of reactive oxygen species (ROS) and inhibits the antioxidant defense system in the brain; both consequently lead to oxidative stress. Oxidative stress results in damage to the neuronal cell membrane and hyper-excitability. On the one hand, n-3 PUFA was able to restore the oxidative stress induced by PTZ by increasing the amount and activity of antioxidant enzymes, like SOD, and decreasing ROS production. Also, the anticonvulsant effects of n-3 PUFAs in the PTZ kindling model were attributed to their antioxidant activity (6, 32). Similarly, the single i.p. administration of omega-3 significantly ameliorated the oxidative stress induced by lipopolysaccharide (LPS) via moderating the SOD activity and GSH and MDA levels in the neonatal rat hippocampal tissue (31). Thus, the PUFAs in EO are probably responsible for elevating the SOD activity. Consequently, the increased level of SOD activity could be considered one of the factors involved in the anticonvulsant effect of EO. On the contrary, EO at 5 g/kg could not elevate the SOD activity. This may be due to the inhibitory effects of PUFAs on the activity and production of oxidative stress parameters. Thus, the intake of the highest dose of EO may lead to a lower need for antioxidant production.
Despite the positive effects of PUFAs on health, the chronic consumption of high amounts of PUFAs may cause side effects, such as higher lipid metabolism. The liver has a critical role in fatty acid metabolism, like oxidation. The high intake of PUFAs causes hyper-activation and more synthesis of liver enzymes, inducing oxidative stress and negatively changing the plasma lipid profile (16). Therefore, safety assessments should be carried out about the effect of chronic consumption of EO on organs, such as the liver and kidney. The present result showed that daily oral administration of EO for four weeks does not change the levels of liver and kidney parameters in the serum, including ALT, ALP, and creatinine. These results demonstrate the safety of EO.
Furthermore, EO reduced the levels of lipid parameters in mice serum except for HDL and LDL. EO at 3 and 5 g/kg significantly lowered TG, cholesterol, and VLDL levels. The present results, similar to the previous studies, indicated that the supplementation with PUFAs positively affects the serum lipid profile (33). Administration of EO for four weeks to mice fed with the high-fat diet lowered the serum levels of cholesterol, TG, and VLDL (34). Also, a four-month supplementation with EO in LDLR-knockout mice significantly decreased TG, VLDL, and omega-6/omega-3 levels (27). Supplementation knockout mice with mild hypertriglyceridemia with EO for eight weeks could reduce TG, VLDL, cholesterol, and phospholipids levels in the plasma (35).
Unlike the previous works, the present study investigated the impact of EO on healthy animals with a normal diet. Therefore, it is concluded that pretreatment with EO is associated with an improvement in the lipid profile and prevention of related diseases in healthy animals and humans. Some mechanisms have been suggested for the beneficial effects of PUFAs on the lipid profile. Omega-3 inhibited the hepatic enzymes related to TG synthesis and degraded apolipoprotein B. Thus, the hepatic secretion of VLDL and TG decreased following omega-3 administration (36). Also, a diet with high fish content reduced the concentration of apoB-100 and apoB-48 in TG-rich lipoprotein and VLDL (35). Accordingly, PUFAs are the endogenous ligands of the peroxisome proliferator-activated receptors (PPARs). Peroxisome proliferator-activated receptors are the transcription factors involved in lipid metabolism, such as fatty acid β-oxidation, lipoprotein metabolism, and TG breakdown (37). The administration of PUFAs causes the activation of PPARs and increases the expression of PPARs (38). Thus, the positive effect of the sub-chronic administration of EO on serum lipids could be explained by the activation of PPARs by the PUFAs. Furthermore, we did not observe any abnormal behaviors in the animals of all experimental groups during the study. Therefore, it could be proposed that the sub-chronic administration of EO is well tolerated.
5.1. Conclusions
In conclusion, the sub-chronic pretreatment of EO inhibits the threshold of PTZ-induced seizures in a dose-dependent manner. This protective effect might be associated with enhancing SOD activity as an antioxidant enzyme. Also, it has no adverse effect on liver and kidney function and lowers TG, cholesterol, and VLDL levels. Thus, EO is a safe alternative source of PUFAs against seizures with a beneficial impact on the antioxidative system and lipid profile.