1. Background
Since many years ago, people have been found herbal products to be promising agents for treatment of disease. Many natural medicines have low adverse effects, price and good efficacy in different human diseases (1). Anti-aging features of some herbs which are used traditionally have been mentioned in new experimental clinical studies (2).
Nigella sativa L. which is also known as black seed belongs to the Ranunculaceae family. Remarkable biological properties of N. sativa seeds is attributed to thymoquinone (TQ). Up to now, it was applied for various diseases and conditions such as gastrointestinal problems, asthma, high blood pressure, diabetes, inflammatory conditions, fever, infection (3-5) and convulsion (6).
From the pharmacological point of view, TQ is a quinone showing anti-inflammatory and analgesic effects (7), protective effects against chemical induced carcinogenesis (8), and also is a powerful inhibitor of eicosanoid production, especially thromboxane B2 and leukotriene B4 (9). TQ has more inhibitory effect on PGE2 production than indomethacin (10). Because of antioxidant effect, TQ is able to protect hepatocyte membranes from peroxidation (11, 12).
In a model of hippocampus injury following transient forebrain ischemia in rats, TQ showed protective effects. It was concluded that TQ elevated GSH content, SOD and catalase activity (13). TQ markedly inhibited amyloid beta peptide (Aβ) 1-40 induced apoptosis in cerebellar granule neurons mainly via intrinsic/extrinsic caspase cascades (14).
Aging is a complex biological event which demonstrated by induction of harmful changes during the time. Oxidative stress theory of aging and free radical generation have been mentioned as the most important interpretation for the process of aging (15). Deregulations of immune system followed by elevation of proinflammatory cytokines, reduction of sex hormone levels and induction of lipid peroxidation are different factors which can be changed during this process (16). A typical model of aging using D-galactose was selected in the present study.
D-galactose as a reducing sugar can be metabolized at normal concentrations. Aldose and hydroperoxidase are by-products of D-galactose, which induce the production of superoxide anion and oxygen-originated free radicals (17).
2. Objectives
In regard to potent antioxidant properties of TQ, this study was conducted to examine the anti-aging potential effect of TQ on D-galactose induced aging model in mice using GSH and MDA concentration, sex hormone and inflammatory indices determination.
3. Methods
3.1. Chemicals
D-galactose, TQ, malondialdehyde tetrabutylammonium and DTNB (5, 5’-dithiobis- (2-nitrobenzoic acid)) were obtained from Sigma. TNF-α ELISA kit and IL-6 ELISA kit were obtained from eBioscience.
3.2. Animal
Male mice, 25 - 30 g were housed in colony rooms with (21 ± 2°C) under a 12/12 h light/dark cycle with free access to food and water. All experiments were performed according to Mashhad University of Medical Sciences, Ethical Committee Acts (920979).
All animals for this study were provided from Faculty of Pharmacy, Mashhad University of Medical Sciences.
3.3. Experimental Design
Induction of aging by D-galactose has been considered as a valuable model of aging in different studies (2, 18, 19). Therefore in the current experiment, aging was induced following administration of D-galactose (500 mg/kg, SC, daily) for 42 days to mice (2).
For our study, animals were divided into 6 groups (n = 10) randomly and treatment was done as described below:
1- Control, normal saline, SC.
2- D-galactose (500 mg/kg) SC for 42 days.
3- D-galactose (500 mg/kg) SC for 42 days + TQ (2.5 mg/kg) intraperitoneally (IP) (11).
4- D-galactose (500 mg/kg) SC for 42 days + TQ (5 mg/kg) IP (11).
5- D-galactose (500 mg/kg) SC for 42 days + TQ (10 mg/kg) IP (11).
6- TQ (10 mg/kg) IP.
TQ was administrated daily to animals intraperitoneally. After the treatment period, cardiac blood samples were collected under anesthesia. Serum samples were taken by centrifuging blood at 4°C for 10 minutes and were transfered to microtubes for separated biochemical assay. By means of liquid nitrogen, separated liver and brain tissues were snap-frozen and kept in -80°C freezers until analyzing day.
3.4. Lipid Peroxidation Assay
Using malondialdehyde (MDA) content determination, the level of lipid peroxidation was assessed. The reaction of MDA with thiobarbituric acid (TBA) results in a complex with pink color which can be detected in λmax of 532 nm.
At the first step, brain or liver tissue homogenate 10% in KCl was prepared. Then 3 mL phosphoric acid (1%), 1 mL TBA (0.6%) and 0.5 mL of tissue homogenate were mixed. After that, the mixtures were heated in a boiling water bath for 45 minutes. N-butanol was added to the mixtures and vortex-mixed for 1 minute. The mixtures were centrifuged at 3000 g for 10 minutes. Finally, the absorbance of organic layers were measured at 532 nm (20). Malondialdehyde tetrabutyl ammonium was used to plot calibration curve. MDA quantity was reported as nmol/g tissue.
3.5. Reduced Glutathione (GSH) Assay
With some modification, samples were analyzed for determination of brain and liver GSH content by use of previously published method (Moron et al.). For this method, homogenized samples were mixed with 10% tricolor acetic acid (TCA) [600 µL, 1:1 (v:v)] and vortexed. The supernatants of these mixtures were obtained by centrifuge at 2500 g for 10 minutes. Then the supernatants were separated and mixed with reaction mixtures including 2 mL phosphate buffer (pH: 8) and 500 µL DTNB indicator [5,5 ′ di thiobis- (2-nitrobenzoic acid)]. The absorbance of final complex was recorded at 412 nm (21) using a spectrophotometer (Jenway 6105 uv/vis, UK). Commercially available GSH was used to plot calibration curve. GSH Levels were reported as nmol/g tissue.
3.6. Measurement of TNF-α and IL-6
Determination of TNF-α and IL-6 concentrations in serum conducted by TNF-α ELISA kit and IL-6 ELISA kit (eBioscience). The absorbance of the final colored complex was calculated in 450 nm as the primary wave length and 650 nm as the reference wavelength. TNF-α and IL-6 levels were expressed as pg/mL.
3.7. Measurement of Testosterone and Dehydroepiandrosterone - Sulfate (DHEA-S)
Levels of these sex hormones were determined in accordance with kit protocols. Data were expressed as ng/mL (testosterone) and ng/dL (DHEA-S).
3.8. Measurement of ALT and AST (IU/L)
These markers were measured by Mindary Autos Analyzer (BS 800) according to kit protocols. Data were expressed as IU/L.
3.9. Statistical Analysis
Statistical analysis was performed using one-way ANOVA followed by Tukey post-hoc test for multiple comparisons to evaluate the discrepancy of the data. P-value less than 0.05 were considered statistically significant.
4. Results
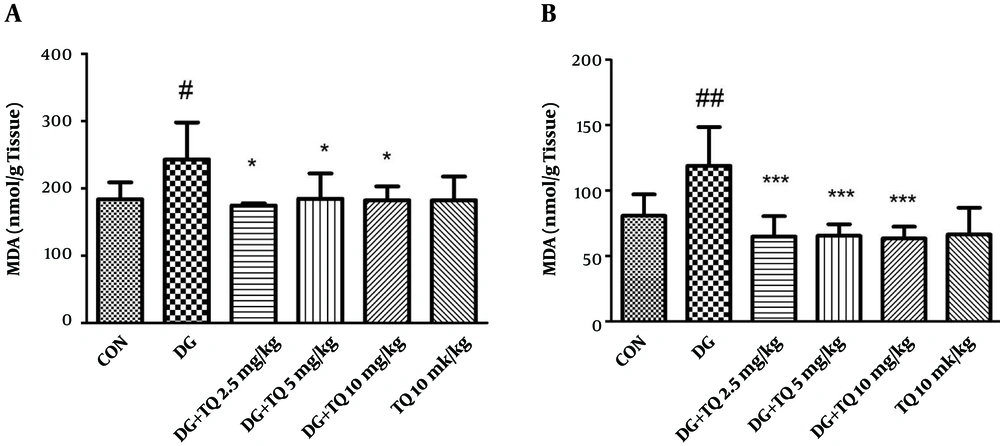
4.1. Effect of TQ on Lipid Peroxidation Induced by D-Galactose in Mice
As shown in Figure 1A, MDA level in D-galactose treated group was significantly higher than control one, (P < 0.05). Treatment with 2.5, 5 and 10 mg/kg of TQ reduced lipid peroxidation in brain tissue, when compared to aged mice (P < 0.05).
In addition, exposure to D-galactose for 42 days significantly increased lipid peroxidation in liver tissue in comparison to control group (P < 0.01). Interestingly, TQ (2.5, 5 and 10 mg/kg) markedly inhibited lipid peroxidation when compared to D-galactose treated animals (P < 0.001). Results have been shown in Figure 1B.
TQ (10 mg/kg) alone did not induce lipid peroxidation in both brain and liver tissues in comparison with control group.
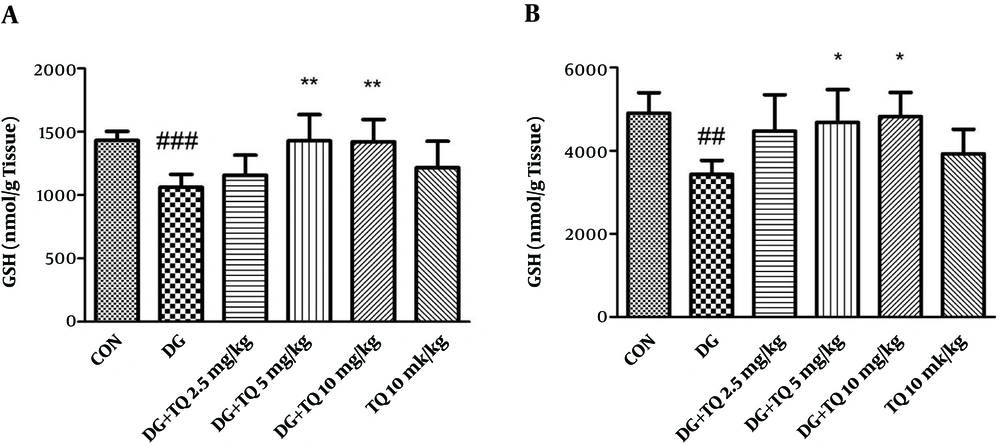
4.2. Effect of TQ on Tissue GSH Content in Aged Mice
As presented in Figure 2A and B, TQ administration (5 and 10 mg/kg) significantly increased the reduced content of GSH in brain and liver of aged animals, (P < 0.05).
Effect of TQ on brain (A) and liver (B) GSH content following treatment of animals with D-galactose. Data are expressed as mean ± SD (n = 7). ## P < 0.01 and ### P < 0.001 vs. control, * P < 0.05 and ** P < 0.01 vs. D-galactose treated animals. DG (D-galactose), TQ (thymoquinone), CON (control).
Administration of TQ (10 mg/kg) alone did not change GSH content when compared to control group.
4.3. Effect of TQ on ALT and AST Levels in Serum of Aged Mice
AST and ALT levels markedly increased in D-galactose- received group. In comparison with D-galactose aged mice, treatment with TQ (5 mg/kg) diminished level of AST and ALT (P < 0.05 for AST and P < 0.01 for ALT). As depicted in table 1, no remarkable change was detected in level of serum ALT and AST following treatment with TQ (10 mg/kg) alone when compared to control animals.
| Test | AST (IU/L) | ALT (IU/L) | Testosterone (ng/dL) | DHEA-S (ng/mL) |
|---|---|---|---|---|
| Control | 246.4 ± 7.57 | 120.3 ± 7.09 | 15 ± 2.68 | 40 ± 1.63 |
| D-galactose (DG) | 335 ± 32.66b | 176.37 ± 14.59b | 6 ± 3.22b | 26 ± 9.29c |
| DG+ TQ 2.5 mg/kg | 256.6 ± 57.12d | 134.5 ± 30.28 | 13.1 ± 2.5d | 36.85 ± 4.74d |
| DG+ TQ 5 mg/kg | 259.42 ± 31.96d | 109.75 ± 37.76e | 13.66 ± 3.7d | 36.28 ± 5.4d |
| DG+ TQ 10 mg/kg | 289.57 ± 48.8 | 175.57 ± 57.34 | 6.4 ± 4.24 | 26.4 ± 2.45 |
| TQ 10 mg/kg | 249 ± 29.48 | 110.3 ± 17.53 | 17.8 ± .6.16 | 35.71 ± 8.44 |
Abbreviations: DG, D-galactose; TQ, thymoquinone; CON, control; AST, aspartate aminotransferase; ALT, alanine aminotransferase; DHEA-S, dehydroepiandrosterone- sulfate.
a Values are expressed as mean ± SD (n = 7).
b P < 0.05 vs. control.
c P < 0.01 vs. control.
d P < 0.05 vs. D-galactose treated animals.
e P < 0.01 vs. D-galactose treated animals.
4.4. Effect of TQ on Sex Hormone Levels of Aged Mice
Sex hormones as another marker that can be changed following aging were determined using Elisa kit. Treatment of animals with D-galactose reduced level of testosterone from 15 ± 2.68 ng/dL in control animals to 6 ± 3.22 ng/mL in aged ones. Also, the level of DHEA-S was 40 ± 1.63 ng/mL in control group that reduced to 26 ± 9.29 ng/mL in aged animals. TQ (2.5 and 5 mg/kg) significantly recovered both levels of testosterone and DHEA-S in aged animals (Table 1).
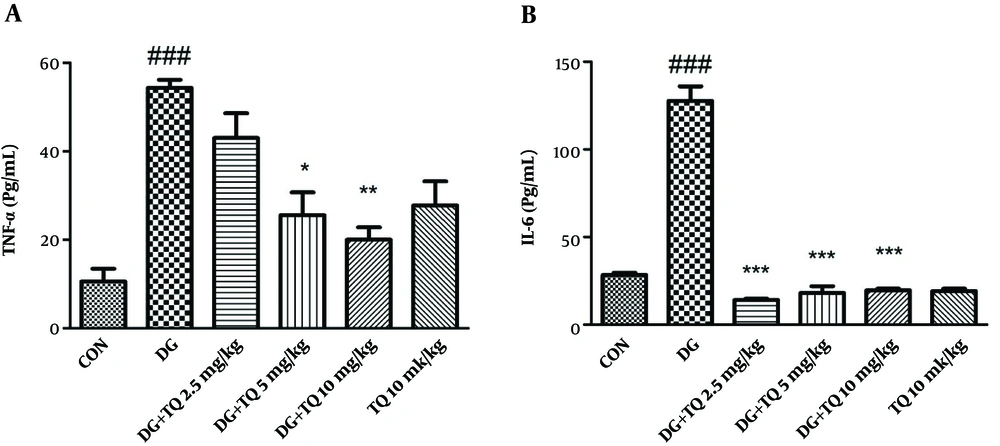
4.5. Effect of TQ on Level of TNF-α and IL-6 in D-Galactose-Received Mice
As presented in Figure 3A and B, TNF-α and IL-6 levels in aged mice were higher than control group.
As shown in Figure 3A and B, the level of TNF-α and IL-6 were considerably higher in D-galactose-received mice in comparison to control group (P < 0.001), while administration of TQ (5 and 10 mg/kg) significantly reversed the mentioned inflammatory markers. No significant change was observed in the level of IL-6 and TNF-α following exposure to TQ (10 mg/kg) alone.
5. Discussion
The anti-aging effect of TQ in model of aging induced with D-galactose (SC, for 42 days) in mice was illustrated in this study. Aging process is known by significant oxidative stress that is observed with reduction of GSH content in brain and liver tissues and consequently elevation of MDA level (marker of lipid peroxidation). Biochemical factors including ALT, AST, IL-6 and TNF-α increased following the aging process. TQ with doses of 5 and 10 mg/kg significantly inhibited brain and liver injury, reduced inflammation and increased level of sex hormones.
Importance of oxidative stress in aging pathogenesis is undeniable. It has been exhibited that aging is associated with a remarkable reduction in mitochondrial function, decline of GSH, enhancement of free radical production and progression of lipid peroxidation (2).
It should be mentioned that liver injury can be observed following aging (22). Change in MDA and GSH levels in aged animals of our study was in accordance with other works. Also, ALT and AST concentrations were remarkably elevated in aged animals in comparison with control group. Interestingly, treatment of animals with TQ (5 and 10 mg/kg) could reduce liver injury which was determined with reduction of serum enzymes, elevation of GSH content and finally diminishing lipid peroxidation.
Hepatoprotective effects of TQ have been evaluated in different studies. For instance, administration of TQ to Sprague-Dawley rats reduced MDA content in liver, which means inhibition of lipid peroxide generation (23). TQ reduced the level of serum enzymes, increased the hepatic GSH content and consequently recovered hepatotoxicity of carbon tetrachloride (24).
There is a strong association between oxidative stress and neurodegenerative diseases (25).
Nowadays, induction of aging with D-galactose is well accepted to provide a model of neurotoxicity, memory dysfunction, and oxidative damage (26). We observed that D-galactose administration to mice could induce brain damage, while TQ significantly inhibited lipid peroxidation in aged animals.
TQ exhibited neuroprotective effects in a model of hippocampus injury induced by transient forebrain ischemia. Enhancement of GSH level and CAT and SOD activity have been revealed in TQ-neuroprotective mechanisms. TQ markedly reduced Aβ 1-40 induced apoptosis in cerebellar granule neurons (14). In cerebral cortex of Wistar rats, TQ decreased peroxidation of lipid induced with acrylamide (27).
Aging process is related with a disturbance in immune system function, induction of inflammation and elevation of proinflammatory cytokines (e.g., IL-6, TNF-α). While the data are not uniform, IL-6 and TNF-α are thought to be involved in morbidity and mortality in the elderly, and IL-6 has been regarded as the strongest risk marker in healthy elderly people (28). Studies indicated that, in healthy old populations, elevation in circulating IL-6 represents a systemic response to local pro-inflammatory action. Long term elevated TNF-α and IL-6 have several biological activities that induce age-associated pathology and mortality (28).
In the current study level of these important cytokines elevated in aged animals. Interestingly, TQ (2.5, 5 and 10 mg/kg) could reduce the level of increased cytokines.
In the inflammation cascade, TQ plays a protective role with inhibition of production of leukotriene B4 and thromboxane B2 in eicosanoid biosynthesis pathway (9).
Changes of the level of sex hormones have been considered as an important marker during aging (29). Also, there is a correlation between inflammatory markers and testosterone concentration (30). In regard to these facts, we decided to evaluate serum testosterone and DHEA-S in aged mice. In agreement to recent reports, the level of these factors markedly diminished in aged animals in comparison to control, but treatment with TQ (5 and 10 mg/kg) significantly increased sex hormone levels. A significant elevation in testosterone concentration in rats was observed following oral administration of water extract of N. sativa (31).
5.1. Conclusions
The present study clearly indicated that TQ possess significant anti-aging effects in the model of aging induced by D-galactose especially through enhancement of GSH content, decline of lipid peroxidation and inhibition of inflammation.