1. Background
Depression is a prevalent psychiatric disorder with a global incidence of more than 20% (1). This disorder is associated with clinical symptoms, mainly decreased cognitive function and mood and social dysfunction (2). The pathophysiology of depression is still unknown; however, the monoaminergic system may have a pivotal role in its incidence (3). The most commonly prescribed antidepressants (such as fluoxetine [Flx] and imipramine [Imp]) decrease depression symptoms by enhancing one or more monoamine neurotransmitters (including serotonin, noradrenaline, and dopamine). On the other hand, antidepressants targeting monoamines directly affect the functional tone of the brain circuits, particularly in limbic and frontocortical areas that are associated with depression (4).
Despite this, studies have reported certain limitations that occur with the use of conventional antidepressants (e.g., drug reactions, sexual dysfunction, etc.) (5). Hence, it is preferable to use safer drugs instead of common antidepressants.
Medicinal plants and their derivatives are natural remedies for the treatment of psychological disorders such as depression (6). Animal research demonstrates the role of medicinal plants and their derivatives in treating depression through monoaminergic pathways (7, 8).
Rhus coriaria L. or sumac belongs to the family Anacardiaceae and is commonly used as a flavoring (9, 10). It has several pharmacological properties, such as anti-inflammatory and antioxidant effects (9, 11). Plants in the family Anacardiaceae are known to affect the psychological system (12). They are also known to have antioxidant properties that can help alleviate depression symptoms (13, 14). The phytochemical compounds in the extract are gallotannin derivatives and flavonoids (9, 15). Studies have reported the positive role of flavonoids and gallotannin as plant remedies for the treatment of depression (16-18).
Moreover, a study showed that R. coriaria essential oil led to an antidepressant effect in the forced swimming test in rats (19). However, the antidepressant potential of R. coriaria ethanolic extract and its mechanism of action remain unknown.
2. Objectives
The present study was designed to explore the antidepressant potential of the R. coriaria extract and the role of the monoaminergic system in its mechanism of action.
3. Methods
3.1. Animal
A total of 174 male NMRI mice (27 ± 3 g) were purchased from Urmia University (Urmia, Iran). Their feed was procured from Javaneh Khorasan Company (Mashhad, Iran). They had free access to food and water and were kept in a 12-hour light-dark cycle.
3.2. Chemicals and Drugs
Haloperidol (Hal), Imp, and Flx were purchased from Exir Company (Tehran, Iran), and SCH23390 (SCH), sulpiride (Sul), p-chlorophenylalanine (pCPA), WAY100635 (WAY), ritanserin (Rit), prazosin (Praz), yohimbine (Yoh), and reserpine (Res) were purchased from Sigma-Aldrich (St. Louis, MO, USA). All the chemicals were dissolved in 0.9% normal saline and administered intraperitoneally at a constant volume of 10 mL/kg. Saline was used as the vehicle (Veh).
3.3. Ethanolic Extract Preparation
Fresh sumac (R. coriaria) powder was procured from Diar Zagrous Company (Kermanhsh, Iran) and then added to ethanol (80%) and macerated. To mix the sample, the mixture was placed in a shaker for 48 hours. The sample was then cooled, filtered, and concentrated. The solvent was evaporated, removed, and kept in sterile glass containers at 2°C for further use (20). The percentage yield of the extract was 14.9% w/w dry matter.
3.4. Evaluation of the Antidepressant-Like Activity of the Rhus coriaria Ethanolic Extract
3.4.1. Tail Suspension Test
For the tail suspension test (TST), 42 mice were divided into 7 groups (n = 6 per group), and treatments were administered intraperitoneally as follows: group 1 received Veh (10 mL/kg), groups 2 - 5 received R. coriaria (25, 50, 100, and 200 mg/kg) (21), group 6 received Flx (20 mg/kg), and group 7 received Imp (30 mg/kg) based on previous studies (7, 8). Then, the animal was suspended 50 cm above the floor through adhesive tape positioned approximately 1 cm from the tip of the tail. After 2 minutes of acclimatization, the immobility time was recorded for 4 minutes (22). The desirable response observed at 100 mg/kg is recommended for future studies.
3.4.2. Open Field Test
To reduce the number of animals used, the open field test (OFT) was performed 5 minutes before the TST. In this regard, 4 groups of 6 mice received R. coriaria (25 - 200 mg/kg), and then the crossing and rearing numbers were recorded for 5 minutes (7).
3.5. Evaluation of the Possible Mechanism Involved in the Antidepressant-Like Activity of the Rhus coriaria Extract in Tail Suspension Test
3.5.1. Effect of Dopaminergic Antagonists
Thirty-six mice were divided into 6 groups (n = 6 per group) and received Hal as a non-selective dopamine receptor antagonist (0.2 mg/kg), SCH as a dopamine D1 receptor antagonist (0.05 mg/kg), and Sul as a dopamine D2 receptor antagonist (50 mg/kg) (7, 8). One hour after pretreating the mice with receptor antagonists, they received 100 mg/kg of R. coriaria and/or Veh, and TST was performed after 30 minutes.
3.5.2. Effect of Noradrenergic Antagonists
Twenty-four mice were divided into 4 groups (n = 6 per group) and received Praz (1 mg/kg) and Yoh (1 mg/kg) as α1- and α2-adrenoceptor antagonists, respectively (7, 8). One hour after pretreating the mice with receptor antagonists, they received 100 mg/kg of R. coriaria, and/or Veh and TST were performed after 30 minutes.
3.5.3. Effect of Serotonergic Antagonists
Thirty-six mice were divided into 6 groups (n = 6 per group) and received WAY (5-HT1A receptor antagonist, 10 mg/kg), Rit (5-HT2 receptor antagonist, 5 mg/kg), and pCPA (as a serotonin synthesis blocker, 150 mg/kg) (7, 8). Rhus coriaria (100 mg/kg) and Veh were also administered 1 hour after injecting Rit (5 mg/kg) and WAY (10 mg/kg); in addition, TST was performed after 30 minutes. Furthermore, the properties of pCPA were examined through its daily administration for 3 consecutive days. Twenty-three hours after the final administration, R. coriaria and Veh were administered, and the mice were subjected to the TST.
3.5.4. Effects of Res
Twelve mice were divided into 2 groups (n = 6 per group) and received Res (2 mg/kg) as a vesicular monoamine depleter 4 hours before treatment with 100 mg/kg of R. coriaria and/or Veh, and then the animals were subjected to the TST (7, 8).
3.6. Coadministration of Rhus coriaria and Subdoses of Common Antidepressants
Twenty-four mice were divided into 4 groups (n = 6 per group) and received R. coriaria (25 mg/kg) 15 minutes after administering 5 mg/kg of Flx and Imp as their sub-doses (7, 8). The mice were subjected to the TST 30 minutes after coadministration.
3.7. Data Analysis
To compare the groups, we used a 1 or 2-way analysis of variance (ANOVA), followed by a Tukey test. The collected data were expressed as mean ± SEM and analyzed using GraphPad Prism version 9.0. P values less than 0.05 were considered statistically significant.
4. Results
4.1. Tail Suspension Test Results
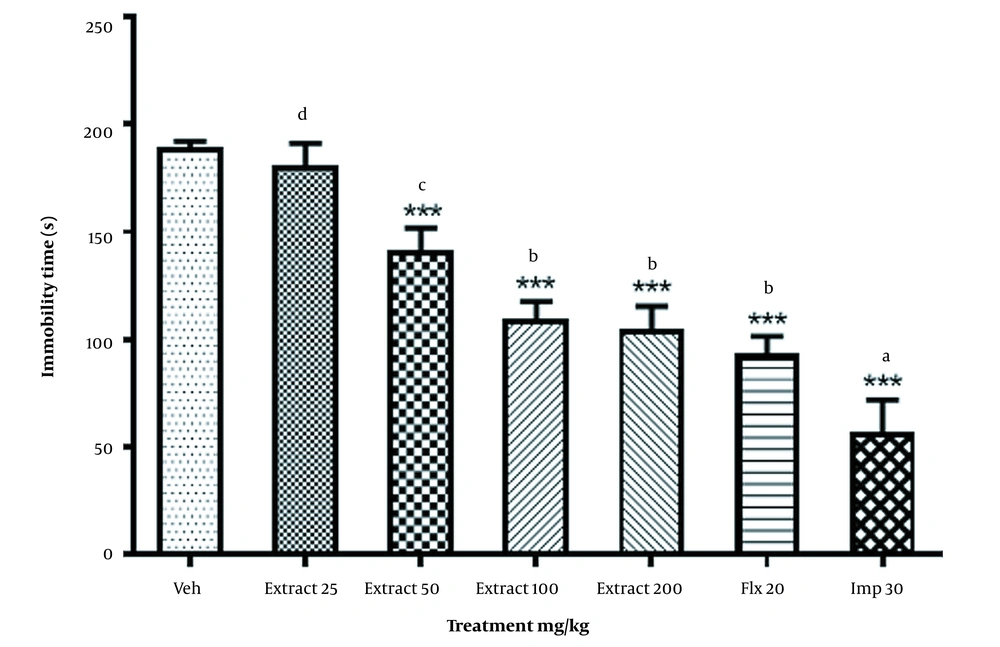
As shown in Figure 1 R. coriaria extract dose-dependently and significantly reduced immobility time at all doses tested, except for 25 mg/kg (F6,35 = 131; P < 0.001). Significant differences were observed between the mice receiving 50 - 200 mg/kg of the extract or Flx and those receiving 25 mg/kg of the extract (P < 0.001). Insignificant differences were found between 100 and 200 mg/kg of the extract (P = 0.984) or Flx (P = 0.125). Hence, a 100 mg/kg dose of R. coriaria should be used for future studies.
The effect of Rhus coriaria extract (25 - 200 mg/kg), imipramine (Imp; 30 mg/kg), and fluoxetine (Flx; 20 mg/kg) in the tail suspension test (TST). The data are expressed as mean ± SEM (n = 6) and were analyzed using a 1-way analysis of variance (ANOVA), followed by a Tukey post-hoc test. ***: Significant differences between the Veh group; a-d: significant vs extract (25 mg/kg; P < 0.05). Abbreviations: Veh, vehicle; Flx, fluoxetine; Imp, imipramine.
The mice that received Imp had the lowest immobility time compared to the other groups (P < 0.001).
4.2. Open Field Test Results
Table 1 shows that R. coriaria groups could not alter the numbers of crossings (P = 0.168) and rearing (P = 0.197) in OFT.
| Group | Dose | Number of Crossings | Number of Rearings |
|---|---|---|---|
| Vehicle | 10 (mL/kg) | 36.20 ± 10.10 | 12.30 ± 4.50 |
| R. coriaria | 25 (mg/kg) | 36.00 ± 15.10 | 14.00 ± 2.28 |
| R. coriaria | 50 (mg/kg) | 31.70 ± 9.05 | 13.70 ± 2.42 |
| R. coriaria | 100 (mg/kg) | 22.30 ± 13.7 | 13.00 ± 2.53 |
| R. coriaria | 200 (mg/kg) | 23.80 ± 8.50 | 11.7 ± 2.07 |
Results are expressed as mean ± SEM (n= 6) and were analyzed using a 1-way analysis of variance, followed by a Tukey post-hoc test.
4.3. Role of the Dopaminergic System
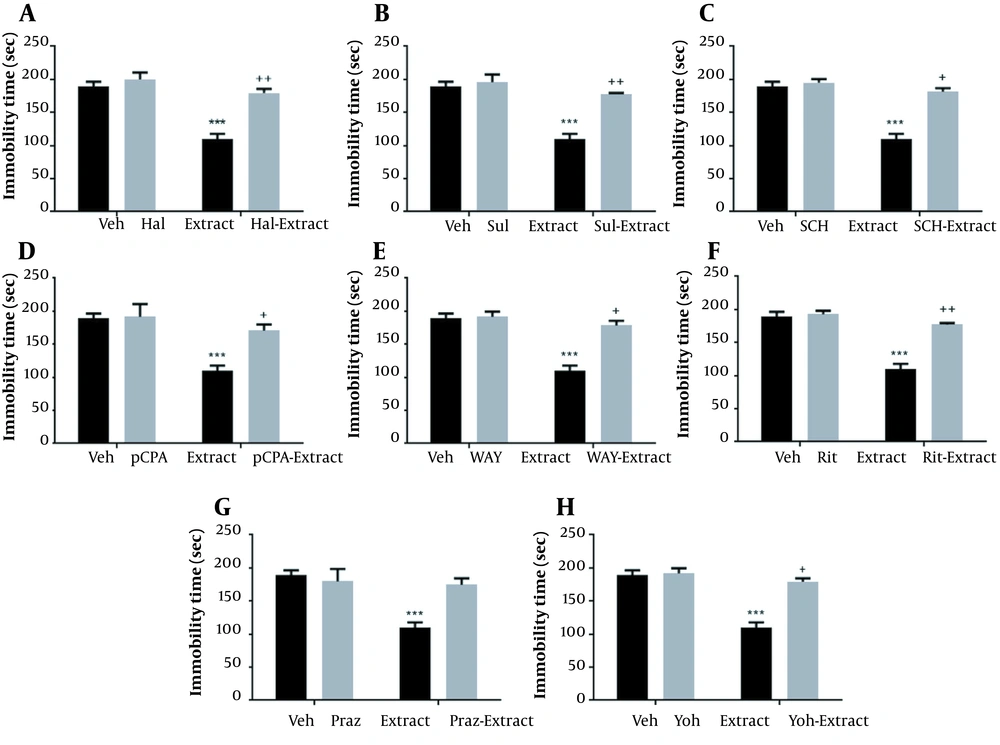
The results showed significant interactions between SCH 23390 pre-treatment (F1,20 = 145.10; P < 0.001) and extract treatment (F1, 20 = 205.60; P < 0.001) and SCH 23390 pre-treatment × extract interaction (F1,20 = 293.90; P < 0.001). In addition, significant effects were observed for Hal (P = 0.0001), Sul (P < 0.0001), extract (P < 0.001), and their interactions (P < 0.001; Figure 2A).
4.4. Role of the Serotonergic System
The results showed significant effects for extract (P = 0.0001), WAY (P < 0.001), Rit (P < 0.001), and pCPA (P < 0.001). Moreover, significant interactions observed included extract × WAY (P < 0.001), extract × pCPA (P < 0.001), and extract × Rit (P < 0.001; Figure 2B).
The effects of pretreatment with dopaminergic, serotonergic, and noradrenergic antagonists on the antidepressant impact of Rhus coriaria extract (100 mg/kg) in the tail suspension test (TST). The data are expressed as mean ± SEM (n = 6) and were analyzed using a 2-way analysis of variance (ANOVA), followed by a Tukey post-hoc test. *** P < 0.001 compared to Veh. + P < 0.05 and ++ P < 0.01 vs the extarct-treated group. (A) Haloperidol (Hal; 0.2 mg/kg), sulpiride (Sul; 50 mg/kg), and SCH23390 (SCH; 0.05 mg/kg). (B) P-chlorophenylalanine (pCPA; 150 mg/kg), WAY100635 (WAY; 10 mg/kg), and ritanserin (Rit; 5 mg/kg). (C) Prazosin (Praz; 1 mg/kg) and yohimbine (Yoh; 1 mg/kg). Abbreviations: Veh, vehicle; Hal, haloperidol; Sul, sulpiride; SCH, SCH23390; pCPA, p-chlorophenylalanine; WAY, WAY100635; Rit, ritanserin; Praz, prazosin; Yoh, yohimbine.
4.5. Role of the Noradrenergic System
The results showed significant effects for Yoh (F1,20 = 150.30; P < 0.001) and extract (F1,20 = 242.20; P < 0.001) and interactions between Yoh and extract (F1,20 = 128.60; P < 0.001). The results showed significant effects for Praz (F1,20 = 33.93; P < 0.001) and extract (F1,20 = 77.66; P < 0.001) and interactions between Praz and extract (F1,20 = 59.38; P < 0.001; Figure 2C).
4.6. The Role of Reserpine
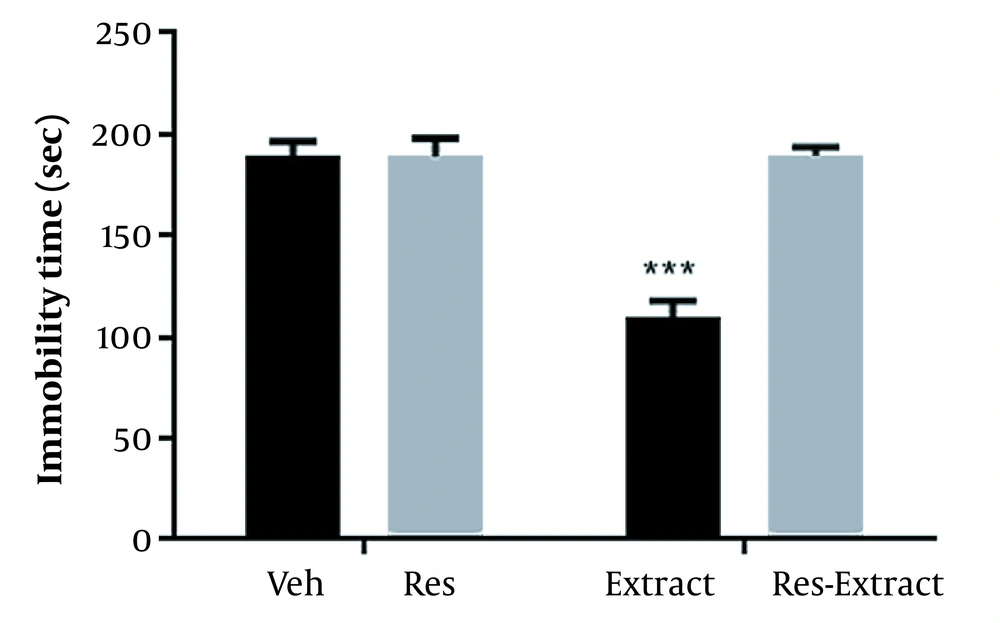
The results showed significant effects for Res (F1,20 = 176.80; P < 0.001) and extract (F1,20 = 173.70; P < 0.001) and interactions between Res and extract (F1,20 = 175.30; P < 0.001; Figure 3).
The effect of pretreatment with reserpine (Res; 2 mg/kg) on the antidepressant impact of Rhus coriaria extract (100 mg/kg) in the tail suspension test (TST). The data are expressed as mean ± SEM (n=6) and were analyzed using a 2-way analysis of variance (ANOVA), followed by a Tukey post-hoc test. *** P < 0.001 compared to Veh. Abbreviations: Veh, vehicle; Res, reserpine.
4.7. Coadministration of Rhus coriaria Extract and the Subdoses of Common Antidepressants
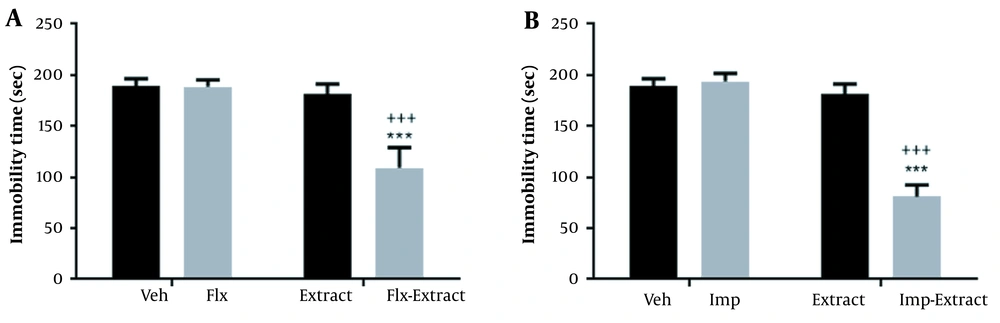
These findings showed the significant effects of the extract, Imp, and Flx (P < 0.001). The significant interactions observed included extract × Flx (F1,20 = 51.20; P < 0.001) and extract × Imp (F1,20 = 187.10; P < 0.001; Figure 4).
The interactions of the subdose of Rhus coriaria extract (25 mg/kg) and the subdoses of fluoxetine (Flx; 5 mg/kg) and imipramine (Imp; 5 mg/kg) in the tail suspension test (TST). The data are expressed as mean ± SEM (n = 6) and were analyzed using a 2-way analysis of variance (ANOVA), followed by a Tukey post-hoc test. ***, +++ P < 0.001 compared to Veh and extract-treated groups (RC), respectively.
5. Discussion
The findings showed that R. coriaria extract (50 - 200 mg/kg) induced antidepressant-like activity in TST without altering animal locomotion in OFT. Hence, the extract does not cause false positive results and is specific. Animal models (e.g., TST) have been commonly used to investigate the antidepressant impacts of different substances in the literature (23).
A study showed that faults in dopaminergic neurotransmitters were associated with major depression, suggesting the role of D1 and D2 receptors in the antidepressant impacts of substances (24). Our results indicated that the pretreatment of mice with SCH (dopamine D1 receptor antagonist), Sul (dopamine D2 receptor antagonist), and Hal (nonselective dopamine receptor antagonist) significantly blocked the antidepressant-like effect of the extract. In addition, the dopaminergic system appeared to be involved in the antidepressant-like effect of the extract. Flavonoids are a major component of sumac extract and can be involved in these responses. Consistent with our findings, a study showed that flavonoids exerted their antidepressant effects by interacting with D1 and D2 receptors (25).
Depression is also related to a hypofunction of the serotonergic system. On the other hand, different 5-HT receptors (e.g., 5-HT1A and 5-HT2) contribute to the mechanism of action of the antidepressants (26). Our findings also illustrated that the pretreatment of mice with WAY (5-HT1A receptor antagonist), pCPA (as a serotonin synthesis blocker), and Rit (5-HT2 receptor antagonist) reversed the antidepressant-like effect of the extract. In addition, the serotonergic system appeared to be involved in the antidepressant-like effect of R. coriaria extract. These findings can be associated with the flavonoid content of the extract. Consistent with the present research, a study found the role of flavonoids in the antidepressant-like effects of substances through 5-HT1A, 5-HT2A, and 5-HT3 receptors (27).
Apart from the dopaminergic and serotonergic systems, defects in the noradrenergic system are associated with depression (28). Animal studies have demonstrated that α1- and α2-adrenoceptors are involved in the antidepressant-like action of drugs or herbal remedies in animal models of depression (7, 8). According to our results, the pretreatment of mice with Praz (α1-adrenoceptor antagonist) and Yoh (α2-adrenoceptor antagonist) significantly blocked the antidepressant-like effect of the extract. In addition, the noradrenergic system appeared to be involved in the antidepressant-like effect of the extract. The antidepressant properties of R. coriaria can also be associated with its flavonoid content. In this regard, a study reported the involvement of flavonoids in α1-adrenergic receptors (29).
Furthermore, pretreatment of mice with Res (a vesicular monoamine depleter) blocked the antidepressant-like effect of the extract. Hence, it seems that R. coriaria may regulate the brain's monoamine neurotransmitters.
Apart from the role of several neurotransmitter systems, the antidepressant-like activity of the extract can be attributed to its antioxidant activity (10). Previous studies have confirmed the role of antioxidants as a positive factor in antidepressant-like activities (13, 14). In this regard, selective serotonin reuptake inhibitors (SSRIs, such as Flx) try to stabilize oxidative stress in a valance state (30). Moreover, a study showed that reactive oxygen species (ROS) modulate the activity of brain monoamines (e.g., noradrenaline, serotonin, and dopamine) (31).
Furthermore, our results showed a synergistic interaction between common antidepressants and the extract. Common antidepressants exert their effects via the monoaminergic system, and R. coriaria seems to exert its effects through the same pathway. Previous studies have reported a positive relationship between common antidepressants and plant derivatives (7, 8). The extract and antidepressant agents can thus have a synergistic interaction and can both be used in the formulation of drugs.
5.1. Conclusions
Rhus coriaria (sumac) ethanolic extract exerts its antidepressant-like effects via the monoaminergic system and can have synergistic effects with common antidepressants. This study was conducted on mice, and the results cannot be generalized to humans. We recommend clinical studies on this subject and the application of the extract in the preparation of drugs after the clinical studies yield positive findings.