1. Background
The complex structure of human eye in terms of anatomy, physiology, and biochemistry makes it almost immune to foreign agents, including drugs. Due to different removal mechanisms of precorneal area, ophthalmic drug delivery is complicated and usually leads to decrease in drug efficacy. In spite of many scientific efforts, effective ocular drug delivery is still a challenging task for pharmaceutical scientists. Most ocular diseases are cured by topical drug implementation in the shape of ointment, solutions, and suspensions. Poor bioavailability of drugs due to anatomical and physiological barriers, including nasolacrimal drainage, short presence time in precorneal area, and corneal low permeability are the main difficulties of conventional drug delivery to eye (1). The cornea is a significant mechanical and chemical obstacle and its main task is the safeguard of the intraocular tissues of the eye. The cornea is depicted by lipophilic and hydrophilic structures and reflects an effectual obstacle to the absorption of both hydrophilic and lipophilic molecules (2, 3). Because of the tightness of the corneal barrier and quick loss of the instilled drug solution from the precorneal area, the bioavailability lessens. The poor bioavailability of drugs from ocular dosage forms is primarily because of the tear production, nonproductive absorption, transient residence time, and impermeability of corneal epithelium. Corneal bioavailability is estimated to vary between 1% and 5% for lipophilic molecules and to be less than 0.5% for hydrophilic molecules (4). Ophthalmic drug delivery might benefit from the qualities of nanotechnology-based drug delivery systems. The nano-size indicates a state of matter depicted by higher amounts of solubility, surface area, dissolution, and corneal penetration. Nanocarrier systems can be categorized as nanosuspensions, nanoparticles, liposomes, niosomes, dendrimers, micelles, microemulsions, and solid lipid nanoparticle (5-7). The demanding objective targeted at dealing with these problems is to develop topical drug delivery systems with improved ocular retention, increased corneal drug absorption, and reduced systemic side effects (8, 9).
Azithromycin is a widely-used semi-synthetic macrolide antibiotic employed in the treatment of infections caused by both Gram-positive and Gram-negative organisms (10). Azithromycin attaches to 50S subunit of the 70S bacterial ribosome and impedes the synthesis of RNA-dependent protein of micro-organisms (11). It has been shown that Azithromycin has bactericidal effects on Staphylococcus aurous, Pseudomonas aeruginosa, and respiratory pathogens (12, 13). In ophthalmology, oral administration of Azithromycin has been shown effective for the treatment of trachoma. The treatment of ocular surface infections with topical Azithromycin appealing in medicine is restricted because of systemic exposure to the drug (14). Major topical formulation problems originate from the fact that Azithromycin is hydrophobic and rarely soluble in water at neutral pH (15). Thus, it is necessary to formulate it as a new drug delivery system, which could deliver the drug topically. Despite the large usage, azithromycin has certain physicochemical qualities to be modified due to its poor water solubility and comparatively low oral bioavailability of about 37% after application (16). Therefore, we tried to design a suitable drug delivery system to improve its solubilization with enhanced stability and thereby, the efficiency of the drug could be ameliorated. In spite of the antimicrobial and anti-inflammatory effects displayed in the systemic preparation of azithromycin, many ophthalmic clinical situations would make the topical application of azithromycin to shrink potential systemic side effects and to direct azithromycin to the site of ophthalmic infection. Commercially available azithromycin 1.0% ophthalmic solution (AzaSite®) is available for the treatment of bacterial conjunctivitis originated from susceptible isolates (17).
Microemulsion, first described by Hoar and Schulman, is a colloidal dispersion composed of the oil phase and aqueous phase that need surfactant and co-surfactant agents in order to stabilize the interfacial area (18). Microemulsions (MEs) have a single optically isotropic, thermodynamically stable composites with a droplet diameter size usually within the range of 10 - 100 nm (19). Microemulsions have very low surface tension and small droplet size, which lead to high absorption and permeation.
Due to unique structure and properties of MEs, they may be considered as proper formulations for ocular delivery of many drugs. MEs are easily prepared and sterilized, relatively stable, and capable of embracing both hydrophilic and lipophilic molecules (20). The presence of surfactant and co-surfactant in O/W MEs enhances drug permeation and uptake through biomembrane (21). Hence, they may be considered as ophthalmic absorption enhancers. In addition, due to their lowered surface tension, MEs are readily spread on the surface of cornea, and mixed with precorneal fluid, increasing the contact area between the drug and the corneal epithelial surface (21). Furthermore, microemulsions result in the prolonged release of drugs administered to the cornea and higher penetration into the deeper layers of the ocular structure and the aqueous humor compared to the native drug. The aim of the present study was to design a novel ME for ocular delivery of Azithromycin and evaluate its physicochemical characteristics and rabbit corneal permeability in order to enhance the penetration of the drug.
2. Objectives
The aim of the current study was to design and evaluate physicochemical characteristics and rabbit corneal permeability of a novel ME formulation for ocular delivery of Azithromycin.
3. Methods
3.1. Materials
Azithromycin powder was bought from Exir Company (IR Iran). Tween 80, span 20, and oleic acid were purchased from Merck (Germany). Diethylene glycol monoethyl ether (Transcutol P) was gifted from GATTEFOSSE Company (France). Fresh double distilled water was utilized in the experiments. Dialysis bag was purchased from Tuba Azma Co. (Tehran, Iran). Minitab 17 software was employed for experimental design and the evaluation of the effect of variables on responses. Sigma plot 12 software was used for providing pseudo-ternary phase diagrams.
3.2. Animals
Male New Zealand white rabbits weighing 2.5 - 3.0 kg were utilized in the current study which was conducted with the approval of the animal ethical committee, Ahvaz Jundishapur University of Medical Sciences (permit no. 826).
3.3. Azithromycin Assay
The amount of Azithromycin was measured by UV spectrophotometer apparatus at 204 nm.
3.4. Azithromycin Solubility
Solubility of Azithromycin was measured in different oils (Transcutol-P, Oleic acid), surfactants (Span 20, Tween 80), and a co-surfactant (Propylene glycol) by dissolving an excess amount of Azithromycin in 5 mL of oil, surfactant, and co-surfactant using a stirrer at 37°C ± 0.5 for 72 hours (6). The equilibrated samples were then centrifuged at 10,000 rpm for 30 minutes to remove undissolved drug. In the next step, the clear supernatants were filtered through a polytetrafluoroethylene membrane filter (φ = 0.45 µm) and the filtrates were analyzed using UV spectrophotometry (22).
3.5. Pseudo Ternary Phase Diagram Construction
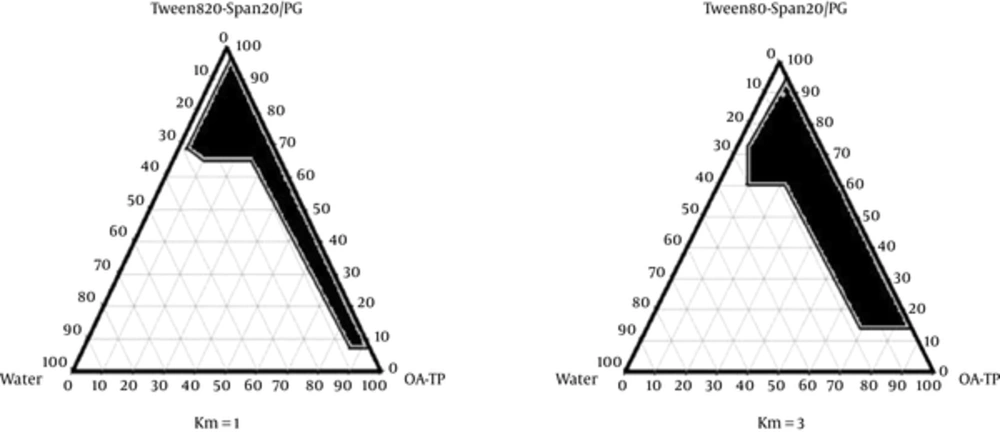
Different pseudo-ternary phase diagrams of unloaded microemulsions were prepared to investigate the concentration range of the components for the existing boundary of microemulsions. Therefore, two phase diagrams were prepared with 1:1 and 3:1 weight ratios of Tween80/Span20 to Propylene glycol. For each phase diagram, the surfactant mixture was added to the oil blended (Oleic acid-Transcutol-P) (10:1) and the surfactant/co-surfactant mixtures were then prepared at the weight ratios of 1:9, 2:8, 3:7, 4:6, 5:5, 6:4, 7:3, 8:2, and 9:1. These mixtures were vigorously mixed using a magnetic stirrer and diluted dropwise with double distilled water at 25 ± 1°C. The samples were classified as microemulsions when they appeared as clear liquids (23). The microemulsion region of the system was constructed on a triangular graph using SigmaPlot®12.0 software.
3.6. Preparation of Azithromycin-Loaded Microemulsions
Several parameters can affect the final quality of microemulsions. After obtaining the microemulsion region in the phase diagram, a full factorial design was employed using 3 variables at 2 levels for preparing 8 formulations. The main variables taking part in the determination of microemulsion’s qualities included surfactant/co-surfactant ratio (S/C), percentage of oil (%Oil), and water percentage (%W). The 8 formulations at low and high levels of oil (5% and 50%), water (3%, 7%), and S/Co mixing ratio (1:1, 3:1) were selected for preparing microemulsion formulations. Various MEs were selected from the pseudo-ternary phase diagram with 1:1 and 3:1 weight ratio of Tween 80/Span20 to propylene glycol (Table 1). Azithromycin (1%) was added to the oil phase and then, S/Co mixture as well as a suitable amount of double distilled water was added to the mixture dropwise. Stirring the mixtures continued at ambient temperature until a uniform mixture was obtained (24).
3.7. Particle Size Determination
The average droplet size of MEs was determined at room temperature by SCATTER SCOPE 1 QUIDIX apparatus. The measurements were triplicated for checking reproducibility.
3.8. Viscosity Measurements
The viscosity of MEs was determined at 25 ± 1°C with a Brookfield viscometer apparatus (DV-II + Pro Brookfield, USA) using spindle no. 34 at shear rate of 50 rpm (25).
3.9. Surface Tension Determination
The surface tension of MEs was determined at 30 ± 1°C using a Torsion balance (WHITE ELEC Model NO. 83944E). A 5 mL volume sample was used for surface tension measurements.
3.10. Physical Stability Study
The physical stability of each ME formulation was evaluated by centrifuge stress test and temperature stability test. Microemulsions were kept in various temperature conditions (4°C, 25°C , 37°C and 75% ± 5% RH) as per the ICH guidelines for six months and then evaluated by monitoring time- and temperature-dependent changes of the physicochemical properties, such as phase separation, flocculation, precipitation, and particle size. Additionally, ME samples were centrifuged by high speed brushless centrifuge (MPV-350R, POLAND) at 15,000 rpm for 30 minutes at 25 ± 1°C. Following the centrifugation, the physical instability of the MEs was visually assessed by the degree of phase separation (26).
3.11. In vitro Release Experiments
Fabricated Franz diffusion cells (contact area 3.46 cm2) with a cellulose membrane were utilized to determine the drug release rate of Azithromycin from different MEs. Prior to each experiment, the cellulose membrane was hydrated in double distilled water at 25°C for 24 hours. Then, it was mounted between donor and receptor compartments. Azithromycin samples (5 g microemulsion) were accurately weighed and placed on the membrane. Each diffusion cell was filled with 25 mL of buffer phosphate solution (pH = 7.4). The receptor fluid was continuously stirred by externally driven magnetic bars at 200 rpm throughout the experiment. At definite time intervals ( 0.5, 1, 2, 3, 4, 5, 6, 7, 8, and 24 hours), 2 mL sample was withdrawn from the receptor compartments, analyzed spectrophotometrically and then, replaced immediately with an equal volume of fresh receptor medium to maintain sink conditions. The samples were studied by UV spectrophotometer at 204 nm. The obtained results were plotted as cumulative percentage of released drug versus time. The cumulative percentage of released drug was plotted versus time and their behavior was described by fitting to different kinetic models. The maximum r2 was considered as the most probable release mechanism (27).
3.12. Differential Scanning Calorimetry (DSC)
Differential scanning calorimetry determinations were administered by means of a Metller Toldo DSC apparatus equipped with refrigerated cooling system (until -45°C). Approximately 5 - 10 mg of each ME sample was weighted into aluminum pans and quickly sealed to prevent water evaporation from ME samples. Simultaneously an empty hermetically sealed pan was used as a reference. ME samples were exposed at a temperature ranging from +30°C to -60°C (scan rate: 5°C/min). Changes of enthalpy amounts (∆H) were obtained from endothermic and exothermic peaks of DSC thermograms (27).
3.13. The Ex Vivo Cornea Permeation Studies
The eye cornea with sclera ring was separated from a newly sacrificed Male New Zealand Albino rabbit. The subcutaneous tissue was completely removed without causing any injury using the scissors or a scalpel. The rabbit corneas were kept in a DexSol solution (chondroitin-sulphate-based, commercial storage media for preservation of corneal epithelium, Chiron Ophthalmic, Irvine, California) (28, 29). The ex vivo cornea permeation study was performed using modified Franz diffusion cells fabricated in house with an effective diffusion area of roughly 0.348 cm2. The excised rabbit corneas were mounted between the donor and receptor compartments of the cell in a manner that sclera ring clamped between two chambers and cornea facing the receptor without any damage due to diffusion cell apparatus. The receptor chamber was filled with 7 mL of buffer phosphate solution (pH = 7.4) (PBS), and its temperature was maintained at 37 ± 0.5°C. Azithromycin ME samples (0.5 g containing 1% AZ)) were accurately weighed and transferred onto the corneas. The receptor medium was constantly stirred using the externally driven magnetic beads at 200 rpm throughout the experiment. The experiments were performed under non-occlusive condition to allow air permeation to corneal tissues. At each interval time (0.5, 1, 2, 3, 4, and 5 hours), a 0.5 mL sample was withdrawn from the receptor medium for spectrophotometric determination, and immediately replaced with an equivalent volume of fresh PBS. Sink conditions were maintained in the receptor compartment during ex vivo permeation studies. The samples were analyzed by UV visible spectrophotometer at 204 nm. The free drug microemulsion was used as blank. The same test was performed for the suspension of Azithromycin (1%) and thus the amounts of permeated drug between ME and suspension were compared. The obtained results were outlined as cumulative percentage of drug permeated versus time (30, 31).
3.14. Ex Vivo Data Analysis
The cumulative amount of Azithromycin permeated per unit cornea area was measured and plotted against time. Different corneal permeability parameters were measured through corneal permeation studies that include flux (Jss), permeability coefficient (P), lag time (Tlag), and diffusivity coefficient (D). The cornea permeation rate at steady state (Jss, mg/cm2h) was determined from the linear portion of the slope of the permeation curve. Since the cornea thickness (h) does not show the actual pathway for drug permeation, diffusivity coefficient is defined as appearance D (Dapp). Apparent permeability coefficient (Papp, cm/s) and apparent diffusivity coefficient (Dapp cm2/h,) parameters were calculated from the equations of Papp= Jss/C0 and Dapp = h2/6 Tlag, respectively. The lag time (Tlag, h) was determined by extrapolating the steady-state line to the time axis.
3.15. Statistics
All the experiments were repeated three times and data were expressed as the mean value ± SD. One-way analysis of variance (ANOVA) was applied to identify any significant difference and P < 0.05 was the level of significance with 95% confidence intervals.
4. Results
4.1. Solubility of Azithromycin
The solubility of Azithromycin is shown in Table 1.
| Phase Type | Excipient | Solubility, mg/mL |
|---|---|---|
| Oil | Transcutol p | 3.034 ± 0.01 |
| Oleic Acid | 1.05± 0.001 | |
| Oleic Acid + Transcutol p (10:1) | 3.9 ± 0.1 | |
| Surfactants | Tween 80 | 0.087 ± 0.002 |
| Span 20 | 0.01 ± 0.001 | |
| Co-surfactant | Propylene Glycol | 5.02 ± 0.1 |
4.2. Phase Studies
The pseudo ternary phase diagrams of Oleic acid-Transcutol P (10:1)/Tween 80 Span 20/Propylene glycol/Water are presented in Figure 1.
4.3. Characterization of the Azithromycin-Loaded Microemulsion Formulations
8 different MEs were selected from the pseudo-ternary phase diagrams with 1:1 and 3:1 weight ratios of Tween 80-Span 20/PG. The composition of selected MEs is shown in Table 2. The viscosity, mean particle size, polydispersity index (PI), pH, and surface tension of Azithromycin MEs are presented in Table 3. The ME samples in this study showed the average viscosity range of 115 - 361 cps, pH range of 5.1 - 5.7, and particle size range of 6.78 - 26.65 nm. Analysis of variance showed that the correlation between the mean particle size and the independent variable (S/C ratio) was significant (P < 0.05). It means that the mean particle size increases with less S/C ratio in ME formulation. Also, analysis of variance showed that the correlation between pH and the independent variable (%Water) was significant (P < 0.05). It indicates that pH increases with less percentage of water phase in some MEs.
| Formulation | Factorial Design | (S:C) | % Oil | %S+C | %Water |
|---|---|---|---|---|---|
| MEA-1 | +++ | 3:1 | 50 | 43 | 7 |
| MEA-2 | ++- | 3:1 | 50 | 47 | 3 |
| MEA-3 | +-+ | 3:1 | 5 | 88 | 7 |
| MEA-4 | +-- | 3:1 | 5 | 92 | 3 |
| MEA-5 | --+ | 1:1 | 5 | 88 | 7 |
| MEA-6 | --- | 1:1 | 5 | 92 | 3 |
| MEA-7 | -+- | 1:1 | 50 | 47 | 3 |
| MEA-8 | -++ | 1:1 | 50 | 43 | 7 |
| Formulation | pH | Viscosity, cps | Mean Particle Size, nm | Surface Tension, dyne/cm | Polydispersity Index |
|---|---|---|---|---|---|
| MEA-1 | 5.1 ± 0.1 | 142 ± 1.1 | 11.3 ± 0.7 | 33 ± 0.3 | 0.353 ± 0.001 |
| MEA-2 | 5.1 ± 0.2 | 135 ± 1.3 | 17.45 ± 0.77 | 31 ± 0.1 | 0.347 ± 0.002 |
| MEA-3 | 5.5 ± 0.1 | 325 ± 1.5 | 4.95 ± 1.92 | 34 ± 0.4 | 0.363 ± 0.001 |
| MEA-4 | 5.7 ± 0.3 | 361 ± 1.4 | 26.65 ± 16.75 | 36 ± 0.5 | 0.383 ± 0.003 |
| MEA-5 | 5.6 ± 0.2 | 210 ± 1.3 | 7.095 ± 1.47 | 33 ± 0.3 | 0.359 ± 0.003 |
| MEA-6 | 5.4 ± 0.1 | 225 ± 1.5 | 17.35 ± 8.98 | 34 ± 0.3 | 0.361 ± 0.004 |
| MEA-7 | 5.3 ± 0.1 | 120 ± 0.98 | 8.57 ± 2.58 | 37 ± 0.4 | 0.345 ± 0.002 |
| MEA-8 | 5.2 ± 0.2 | 115 ± 0.78 | 6.78 ± 2.89 | 39 ± 0.5 | 0.365 ± 0.005 |
Analysis of variance also represented that the correlation between viscosity and the independent variables (%Water, %Oil) was significant (P < 0.05). It means that viscosity increases with less percentage of water phase and more percentage of oil phase in Azithromycin MEs. The correlation between surface tension and the independent variables was not significant (P > 0.05).
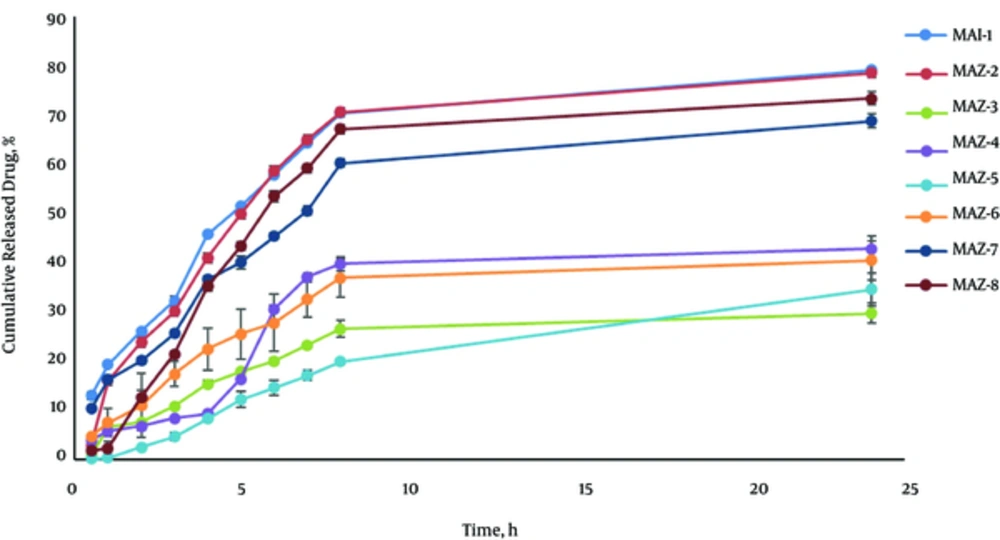
Figure 2 shows the release profile of Azithromycin MEs. Drug release profile revealed that 79.066% of the drug were released in 24 hours of the experiment for MEA-1. MEA-1 obeyed the Weibull kinetic. The percentage of the drug released and kinetics of release in the selected ME formulation are summarized in Table 4.
| Formulation | % Release, 24 h | Kinetics of Release | R2 | %Release, 2 h |
|---|---|---|---|---|
| MEA-1 | 79.066 ± 0.53 | Weibull | 0.9549 | 26.13 ± 0.29 |
| MEA-2 | 78.523 ± 1.72 | Log Wagner | 0.9510 | 23.86 ± 1.09 |
| MEA-3 | 29.646 ± 2.03 | Log Wagner | 0.8894 | 7.63 ± 0.12 |
| MEA-4 | 42.775 ± 2.62 | Peppas | 0.8362 | 6.78 ± 0.94 |
| MEA-5 | 34.525 ± 3.37 | Log Wagner | 0.9759 | 2.55 ± 0.03 |
| MEA-6 | 40.460 ± 3.92 | Log Wagner | 0.9478 | 10.92 ± 1.49 |
| MEA-7 | 68.760 ± 1.49 | Weibul | 0.9567 | 20.09 ± 0.66 |
| MEA-8 | 73.307 ± 1.33 | Log Wagner | 0.9338 | 12.67 ± 1.39 |
Analysis of variance represented that the correlation between drug released in 2 hours (R2h) and the independent variables (S/C, %Oil) was significant (P < 0.05). It means that R2h increases with less S/C ratio and more percentage of oil phase in Azithromycin formulations. Also, the correlation between drug released in 24 hours (R24h) and the independent variable (%Water) was significant (P < 0.05) so that R24h increases with more percentage of water phase.
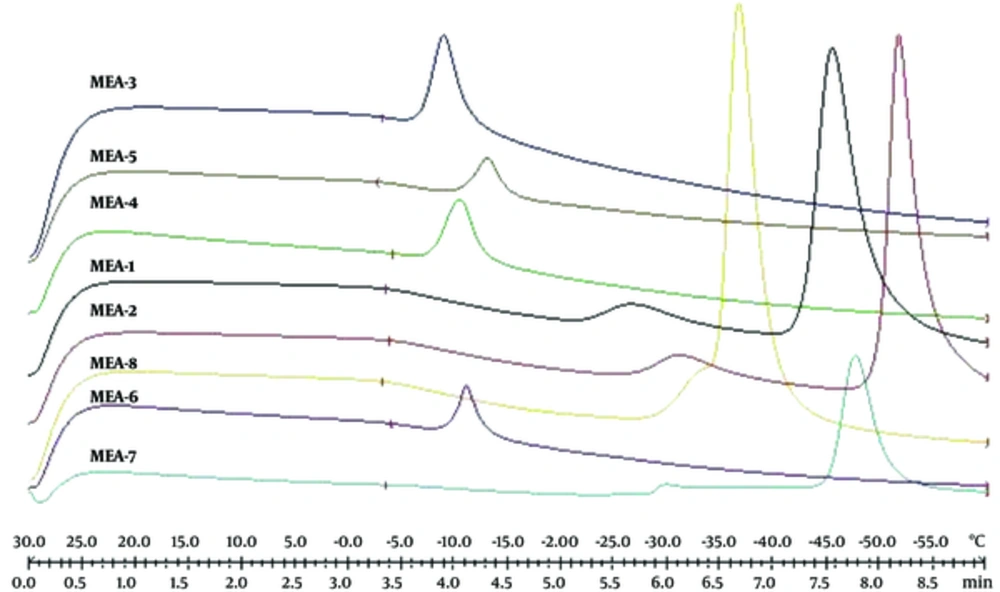
Figure 3 shows DSC cooling thermograms of Azithromycin ME formulations. Cooling microemulsion’s transition temperature and enthalpy are provided in Table 5. In cooling curves of the ME samples, bulk water (free water) and bound water are obtained at -1 to -6°C and -9 to -33°C, respectively. According to ANOVA results, a significant correlation (P < 0.05) was found between bulk (free) and bound melting transition temperature (Tm1 and Tm2) and independent variables, so that any increase in oil amount and any decrease in water phase significantly increased temperature. Also, the effect of independent variables on affected enthalpy of exothermic peak of free water showed that the enthalpy increased significantly (P < 0.05) due to the increase of oil, water phase percentage, and S/C ratio.
| Formulation | TM1, °C | ∆H1, mJ/mg | TM2, °C | ∆H2, mJ/mg | TM3, °C | ∆H3, mJ/mg |
|---|---|---|---|---|---|---|
| MEA-1 | -4 ± 0.05 | 1.03 ± 0.15 | -27 ± 0.1 | 2.71 ± 0.5 | -46 ± 1.1 | 33.15 ± 1 |
| MEA-2 | -4 ± 0.06 | 0.61 ± 0.04 | -31 ± 0.12 | 2.47 ± 0.4 | -52 ± 1.04 | 23.24 ± 0.9 |
| MEA-3 | -1 ± 0.01 | 0.16 ± 0.02 | -9 ± 0.1 | 4.73 ± 0.7 | - | - |
| MEA-4 | -3 ± 0.02 | 0.04 ± 0.01 | -11 ± 0.5 | 3.22 ± 0.4 | - | - |
| MEA-5 | -5 ± 0.01 | 0.05 ± 0.01 | -11 ± 0.4 | 2.13 ± 0.1 | - | - |
| MEA-6 | -4 ± 0.03 | 0.01 ± 0.005 | -13 ± 0.6 | 2.51 ± 0.12 | - | - |
| MEA-7 | -6 ± 0.04 | 0.09 ± 0.01 | -30 ± 08 | 0.27 ± 0.2 | -48 ± 1.1 | 9.44 ± 0.4 |
| MEA-8 | -4 ± 0.03 | 0.77 ± 0.14 | -33 ± 0.9 | 0.007 ± 0.001 | -37 ± 0.9 | 18.4 ± 0.5 |
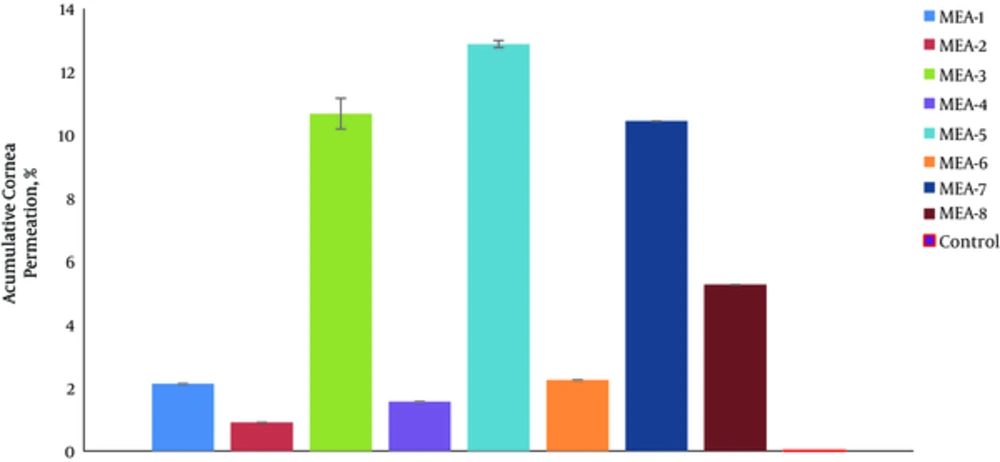
Figure 4 shows the percentage of drug permeated in 5 hours (%P5h) through rabbit cornea for various MEs. The permeability parameters of various ME formulations are represented in Table 6. The amount of AZ permeated through the rabbit cornea membrane per the area of ocular cells (mg/cm2) was plotted as a function of time (h).
| Formulation No. | Jss, mg/cm2 h | TLag, h | Dapp, cm2/h | Papp, cm/h | ERflux | ERD | ERP |
|---|---|---|---|---|---|---|---|
| Control | 0.3 ± 0.001 | 3.4 ± 0.1 | 0.0005 ± 0.0001 | 0.068 ± 0.001 | - | - | - |
| MEA-1 | 2.186 ± 0.574 | 0.367 ± 0.352 | 0.00882 ± 0.0077 | 0.437 ± 0.114 | 7.287 ± 1.91 | 17.65 ± 15.53 | 6.43 ± 1.69 |
| MEA-2 | 1.822 ± 0.752 | 0.956 ±0.081 | 0.00175 ± 0.0001 | 0.364 ± 0.150 | 6.07 ± 2.5 | 3.5 ± 0.3 | 5.36 ± 2.21 |
| MEA-3 | 7.823 ± 0.638 | 0.65 ± 0.122 | 0.0026 ± 0.0005 | 1.564 ± 0.127 | 26.08 ± 2.12 | 5.27 ± 1.1 | 23.01 ± 1.87 |
| MEA-4 | 2.517 ± 0.947 | 1.903 ± 0.051 | 0.0008 ± 0.00002 | 0.503 ± 0.189 | 8.39 ± 3.15 | 1.75 ± 0.04 | 7.4 ± 2.78 |
| MEA-5 | 9.929 ± 1.272 | 0.68 ± 0.346 | 0.00315 ± 0.002 | 1.985 ± 0.254 | 33.1 ± 4.24 | 6.3 ± 4.1 | 29.2 ± 3.74 |
| MEA-6 | 4.177 ± 0.242 | 2.096 ± 0.083 | 0.00079 ± 0.00003 | 0.903 ± 0.113 | 13.92 ± 0.8 | 1.59 ± 0.06 | 13.29 ± 1.67 |
| MEA-7 | 11.958 ± 0.486 | 2.116 ± 0.116 | 0.00079 ± 0.00004 | 2.391 ± 0.097 | 39.86 ± 1.62 | 1.57 ± 0.08 | 35.17 ± 1.43 |
| MEA-8 | 3.296 ± 0.093 | 0.973 ± 0.020 | 0.00171 ± 0.00003 | 0.659 ± 0.018 | 10.99 ± 0.31 | 3.42 ± 0.07 | 9.69 ± 0.27 |
In permeability studies, the correlation between Papp and the independent variable (%Oil) was significant (P < 0.05). Hence, Jss of Azithromycin for MEA-7 was 11.958 ± 0.486 mg cm-2h-1, which was 39.86 times higher than that of control (AZ suspension, 1%). The correlation between Jss and the independent variables was not significant (P > 0.05).
The correlation between Tlag and the independent variables (%Oil and S/C ratio) was significant so that, any decrease in oil phase percentage and S/C ratio significantly increased the Tlag parameter. The correlation between apparent diffusivity coefficients (Dapp) and the independent variable (S/C ratio) was significant (P < 0.05). Hence, any decrease in oil phase percentage (%Oil) significantly increased the Tlag parameter.
Dapp and Papp parameters in MEA-1 and MEA-7 formulations were 0.00882 cm2h-1 and 2.391 cmh-1, which were 17.65 and 35.17 times higher than those of control (AZ suspension, 1%), respectively.
The correlation between drug permeated percentage in 2 hours (%P2h) and the independent variables (%Water, S/C ratio) was significant (P < 0.05); therefore, %P2h increased with any decrease in water percentage phase and more S/C ratio. Also, the correlation between drug permeated percentage in 5 hours (%P5h) and the independent variable (S/C ratio) was significant (P < 0.05); therefore, %P5h increased with more S/C ratio.
5. Discussion
To develop and design ME formulations, the suitable oil was selected by determining the concentration of Azithromycin that would be dissolved. Based on the solubility experiments of Azithromycin in oil, surfactant, and co-surfactant, we found that oleic acid-Transcutol P (10:1), Tween 80, Span 20, and PG could be the most appropriate combinations for preparation of Azithromycin ME. It seems that the phase behavior depends on surfactant to co-surfactant weight ratios. The weight ratio of surfactant/co-surfactant is an important and critical parameter affecting the phase behaviors of ME. The extent of ME boundary increasing with the increase of relative amount of surfactant was also reported in previous research (21, 32). The phase diagrams revealed that ME region extended with higher weight ratios of surfactant/co-surfactant (km = 1 - 3). The phase diagrams showed more width of ME region with a rise in S/C ratio.
The results of ME formulations indicated the average viscosity range of 115 - 361cps, pH values of 5.1 to 5.7, and particle size of 6.78 - 26.65 nm. Analysis of variance showed that the correlation between the mean particle size and the independent variable (S/C ratio) was significant (P < 0.05). It means that the mean particle size increases with decreasing S/C ratio in ME formulations. Particle size is one of the most important properties in nano-sized drug delivery systems. The decrease in particle size is related to a great increase in surface area that would lead to improved bioavailability (33). In the current study, the droplet size of all ME formulations was below 30 nm. The droplet sizes of the MEs prepared were far below the particle size of 10 µm that could cause irritation (33). The polydispersity value described the uniformity of the droplet size. All polydispersity values were smaller than 0.5. Therefore, the obtained results show the narrow distribution of droplet size in ME formulations. Also, multivariate regression displayed a significant correlation between pH and the independent variable (%Water) (P < 0.05), so that any decrease in water phase percentage of MEs significantly increased the pH parameter. The finding is consistent with the previous reports (27). The pH values of all ME formulations were around 5.1. In the present study, viscosity in ME samples increased significantly with less percentage of water phase and more percentage of oil phase. The findings are in agreement with the previous reports by Dong et al. (34). Increased viscosity might help improve the preocular retention time and thus, increase the amount of the drug permeated through corneal. All of the ME systems prepared in our study were more viscose in comparison with aqueous suspension. The correlation between surface tension and the independent variables was not significant (P > 0.05). Surface tension is an important property. In our study, surface tension of ME formulations was in the range of 31 - 39 dyne/cm that was lower than that of azithromycin suspension (52 dyne/cm). The lower surface tension caused the better spreading of the product on the cornea and the more contact between them.
Analysis of variance represented that the correlation between drugs released in 2 hours (R2h) and the independent variables (S/C, %Oil) was significant (P < 0.05). It means that R2h increased with less S/C ratio and more percentage of oil phase in Azithromycin formulations. Also, the correlation between drug released in 24 hours (R24h) and the independent variable (%Water) was significant (P < 0.05), so that R24h increased with more percentage of water phase. In the current study, very small Azithromycin MEs droplet size was obtained. It is known that small particle size contributes to the fast release. Additionally, it can be stated that the improved viscosity of MEs lowered the diffusion of the drug through the formulation and slowed the release from the membrane. This phenomenon was observed in No.3, 4, 5, and 6 formulations.
In cooling curves of the ME samples, bulk water (free water) and bound water were obtained at -1 to -6°C and -9 to -33°C, respectively. According to ANOVA results, a significant correlation (P < 0.05) was found between the bulk (free) and bound melting transition temperature (Tm1 and Tm2) and independent variables, so that any increase in oil amount and any decrease in water phase significantly increased temperature. Also, the independent variables affected enthalpy of exothermic peak of free water (P < 0.05); i.e., the enthalpy increased due to the increase of oil, water phase percentage, and S/C ratio.
It was shown that all of the MEs have proper characteristics regarding their homogeneity and six-month duration stability. There was no significant difference between mean droplet sizes at the beginning and after six months storage of the MEs (P > 0.05). Visual inspection during the storage showed no change in clarity, precipitation, phase separation, and flocculation. Centrifugation of the samples at 15,000 rpm for 30 minutes caused no phase separation and the MEs remained homogenous during and after the examination. In the stability studies, narrow polydispersity index values were observed for MEs. This parameter could be utilized as an indication of stability of ME systems. The ME systems were isotropic, transparent dispersions, and after centrifugation, no phase separation was observed. In the previous studies regarding the stability of MEs, it was demonstrated that there is a complex relationship between zero interfacial tension and thermodynamic stability (35).
In permeability studies, the correlation between apparent corneal permeability coefficients (Papp) and the independent variable (%Oil) was significant (P < 0.05). Hence, Jss of Azithromycin for MEA-7 was 11.958 mg cm-2h-1, which was 39.86 times higher than that of control (AZ suspension, 1%). The correlation between Jss and the independent variables was not significant (P > 0.05).
The correlation between Tlag and the independent variables (%Oil and S/C ratio) was significant so that any decrease in oil phase percentage and S/C ratio significantly increased the Tlag parameter. The correlation between apparent diffusivity coefficient (Dapp) and the independent variable (S/C ratio) was significant (P < 0.05). Hence, any decrease in oil phase percentage (%Oil) significantly increased the Tlag parameter.
Dapp and Papp parameters in MEA-1 and MEA-7 formulations were 0.00882 cm2h-1 and 2.391 cmh-1, which were 17.65 and 35.17 times higher than those of control (AZ suspension, 1%), respectively. The correlation of ERp and ERflux with the independent variables was not significant. The relationship between ERD and the independent variable (S/C ratio) was significant (P < 0.05). Therefore, any increase in S/C ratio significantly increased the ERD parameter. The results showed that drug flux of all MEs through skin increased more than diffusion. The results showed that drug flux of all ME formulations through rabbit cornea increased more than diffusion. All ME formulations with different compositions and properties significantly increased partitioning, flux, and permeability coefficient from rabbit cornea. The correlation of drug permeated percentage in 2 hours (%P2h) with the independent variables (%Water, S/C ratio) was significant (P < 0.05); therefore, %P2h increased with any decrease in water phase percentage and any increase in S/C ratio. Also, the correlation of drug permeated percentage in 5 hours (%P5h) with independent variable (S/C ratio) was significant (P < 0.05); therefore, %P5h increased with higher S/C ratio. Also, multivariate regression showed the significant correlation between drug permeated percentage in 5 hours (%P5h) and droplet size of MEs, so that a decrease in droplet size significantly increased the total percentage of drug permeated in 5 hours (%P5h). The staining test of Azithromycin MEs established oil-in-water ME structures. In previous studies, it was demonstrated that the O/W MEs may be beneficial because the presence of surfactant and co-surfactant compositions increases barrier permeability (21). In this research, Azithromycin ME formulations could act as permeation enhancers to improve corneal drug delivery. Our findings are in agreement with those of previous reports by Naveh et al. (35). They showed the increase of the corneal absorption of pilocarpine by an especial oil-in-water ME system. The rabbit cornea model was employed for drug delivery studies because of its similarity to human corneas (36).
5.1. Conclusions
This study established that the amount of components of S + C, oil, and water in ME formulation plays a vital role in the physicochemical properties, in vitro release, and drug permeability through rabbit cornea. Also, this study showed that microemulsions could be alternatively used as ocular drug carriers for AZ.