1. Background
Ulcerative colitis (UC) is a type of inflammatory bowel disease (IBD) that results in inflammation and ulceration of the intestinal mucosa. It is chronic, recurrent, and progressive, and its main clinical hallmarks include mucous diarrhea with blood, weight loss, abdominal pain, anemia, and fatigue (1-3). Based on a recent systematic review by Ng et al., the highest prevalence rates of UC were in North America (0.29%) and Europe (0.51%) (4). In Iran, the incidence of UC has increased over the past two decades (5, 6). The etiology of the disease is complex and unclear. However, epidemiological studies found that environmental and genetic predisposition plays a key role in inflammation-related diseases. The pathological features include immunologic abnormalities (7), abnormal microflora of the GI (8), oxidant/antioxidant imbalance (9), elevated pro-inflammatory cytokines, and defects in mucosal integrity (10, 11).
Emerging evidence indicates that oxidative stress plays a crucial role in the development of intestinal inflammation via underlying mechanisms, including overproduction of reactive oxygen species (ROS), infiltration of immune cells, and upregulation of inflammatory cytokines (12, 13). Furthermore, many studies have shown a positive correlation between high concentrations of nitric oxide (NO) in the serum and intestinal mucosa and increased levels of pro-inflammatory cytokines in the induction of chronic UC (14, 15). Currently, immunosuppressive agents, anti-inflammatory drugs, corticosteroids, aminosalicylates, and probiotics are commonly used to control inflammation and alleviate the risk of recurrence. However, the response rate to available drugs is poor, and there are various side effects. Therefore, it is significant to search for potential therapeutic strategies for UC. Medicinal plants are worthy and effective in treating diseases and are the source of most newly discovered drugs (16). The use of natural products to treat various gastrointestinal diseases offers an alternative therapy.
Artemisia vulgaris (known as mugwort) is a well-known genus of the Asteraceae family, with many species found in Iran, most of which are aromatic. This genus is native to the temperate regions of Europe, Asia, North Africa, and Africa and contains saponins, alkaloids, essential oils, phenolic acids, coumarins, and flavonoids (17, 18). Further, A. vulgaris called the "mother of herbs," is used as an anti-inflammatory, antioxidant, and immunomodulatory agent (19). It has also been used as an analgesic and anti-ulcerogenic (20, 21). Researchers have confirmed that A. vulgaris exhibits antibacterial, antiseptic, anticancer, and hepatoprotective properties, as well as therapeutic effects in metabolic disorders such as diabetes (18, 22). In this regard, recent studies have demonstrated that A. vulgaris can attenuate inflammatory mediators, such as cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) (23, 24), increase antioxidant activity by upregulating nuclear factor erythroid 2-related factor 2 (Nrf2) (25), and improve damage levels in an experimental model of colitis.
2. Objectives
Considering the medicinally useful properties of the plant and due to the limited number of studies on its anti-ulceration effects in rats, this study investigated the efficacy of A. vulgaris hydroalcoholic extract on oxidative stress and inflammatory damage in an experimental model of acetic acid-induced colitis.
3. Methods
3.1. Animals
Male Sprague-Dawley rats (8 - 10 weeks old; 180 - 200 g) were purchased from the animal laboratory of the Faculty of Pharmacy, Kermanshah University of Medical Sciences (Iran). The rats were housed in a temperature-controlled room (22 ± 2°C) with a 12-h light/dark cycle (lights on at 7:00 AM) and food and tap water ad libitum throughout the experiments. All the procedures were conducted in compliance with the National Institutes of Health guidelines for the care and use of laboratory animals (NIH; publication No. 82-23, revised 1985 and again implemented in 1996) and approved by the Ethics Committee of Ardabil University of Medical Sciences (IR.ARUMS.REC.1400.202).
3.2. Preparation of A. vulgaris Extract
A. vulgaris was collected in May 2021 from Kermanshah province in western Iran and identified by the herbarium of the Department of Pharmacology, Kermanshah University of Medical Sciences, Kermanshah (voucher No. 075-014-034). Briefly, the aerial parts of the plant were powdered, extracted with 70% ethanol using the maceration technique, and filtered. The resulting dried extract was using a centrifugal evaporator (Heidolph-Laborota 4001, Germany) and stored at 4°C for further experiments (26).
3.3. Induction of Ulcerative Colitis
After 24 h of fasting, the animals were anesthetized with ketamine/xylazine (40/10 mg/kg, IM). Then, an acute UC model was induced by intrarectal administration of acetic acid (AA, 2 mL, 3% v/v, via the medical-grade polyurethane catheter, Sigma-Aldrich). After the AA injection, the rats were physically monitored to check for weight loss, diarrhea, and anorectal bleeding, which indicated the induction of colitis (27, 28).
3.4. Experimental Groups
The rats were randomly assigned to 6 groups (n = 5 rats/group): (1) control group: Healthy rats that received 1 mL of normal saline orally (gavage); (2) colitis group: Colitis was induced with acetic acid intrarectally (2 mL of 3% in 0.9% NaCl) and the rats received 1 mL of normal saline orally; (3) sulfasalazine group: Ulcerative colitis-induced rats that received sulfasalazine as a reference drug [1 mg/kg/day; intraperitoneally (i.p.)]; (4) AV50 group: Ulcerative colitis-induced rats that received 50 mg/kg of A. vulgaris orally; (5) AV100 group: Ulcerative colitis-induced rats that received 100 mg/kg of A. vulgaris orally; (6) AV200 group: Ulcerative colitis-induced rats that received 200 mg/kg of A. vulgaris orally. The treatment period was 7 days before induction and 3 days after. The doses of A. vulgaris extracts and the duration of treatment were selected according to previous studies (29-31). The body weight of the rats was measured at the beginning and end of the interventions using an FEW (Japan) scale with a sensitivity of 1 g.
3.5. Collection of Samples
On the last day of the experiment, the rats were sacrificed under deep anesthesia with ketamine and xylazine (100 and 10 mg/kg i.p., respectively). Then, trunk blood was collected into a microtube, centrifuged at 3000 rpm for 10 min, and kept at -80°C for evaluation of the pro-inflammatory mediator and oxidative stress markers. In addition, the colon tissue was immediately removed and fixed in a formalin solution for macroscopic and histopathological studies.
3.6. Measurement of Serum NO and Tumor Necrosis Factor-α Levels
Serum NO and tumor necrosis factor-α (TNF-α) levels were measured using a rat ELISA kit (ZellBio GmbH, Germany) according to the manufacturer's protocols and expressed as µM and Pg/mL, respectively.
3.7. Measurement of Serum Oxidative Stress Markers
Levels of serum malondialdehyde (MDA), glutathione (GSH), and superoxide dismutase (SOD) activity were determined using a colorimetric kit (ZellBio GmbH, Germany) as per the manufacturer's instructions and expressed as nmol/mL and U/mL, respectively.
3.8. Macroscopic Assessment of Colonic Damage
The macroscopically visible damage of the colon was examined under a stereomicroscope and scored as described by Farzaei et al. (28). Mucosal edema, thickening, hemorrhage, hyperemia, erosions, shortening, and necrosis were assessed based on the Gerald scoring system (32).
3.9. Histopathological Examination of Colonic Damage
Colonic tissue samples were collected for microscopic (histopathological) evaluation and fixed in 10% buffered neutral formalin. The segments were then processed, embedded in paraffin blocks, sectioned (5 - 7 µm) with a rotary microtome, and counterstained with hematoxylin-eosin (H&E) for analysis under a light microscope. The description and scoring of lesions were conducted based on El-Akabawy and El-Sherif (33) as follows: Morpho-architectural distortion of crypts, submucosal edema, ulceration, cryptitis, and glandular atrophy: Absent (score 0), mild (score 1, ≤ 10%), moderate (score 2, 10 - 15%), and intense (score 3, > 50%). The final scores were calculated by summing the scores for each sample. The degree of inflammatory cell infiltration was also scored according to the following scale: 0, normal; 1, presence of inflammatory cells confined to the mucosa; 2, present in both mucosa and submucosa; and 3, infiltrate extended into the traversal of the entire length of the colonic wall.
3.10. Statistical Analysis
The data were analyzed using GraphPad Prism v. 8. One-way analysis of variance (ANOVA; considering the drug as the independent factor) and two-factor mixed-model ANOVA (drug as the independent factor and time as the repeated factor), followed by Tukey's post-hoc test for multiple comparisons, were used. The results are expressed as mean + standard error of the mean (SEM), and P-values below 0.05 were considered significant.
4. Results
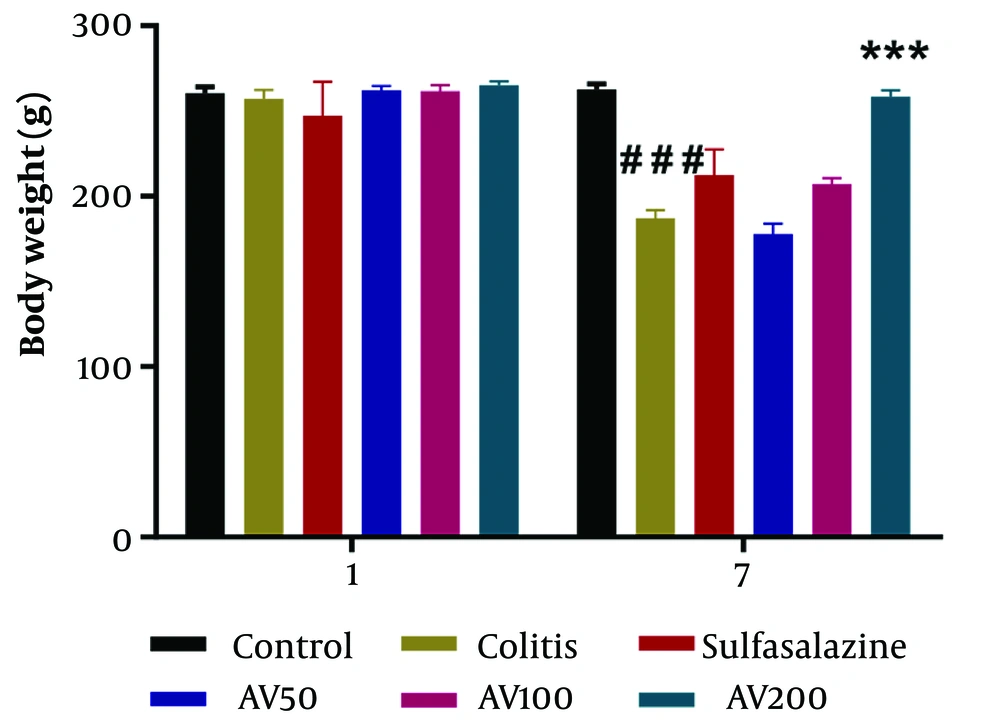
4.1. A. vulgaris Increased Body Weight in the Rat Model of Colitis
Two-way repeated-measures ANOVA showed the significant effect of time [F (1, 48) = 78.77, P < 0.0001] and treatment [F (5, 48) = 10.89, P < 0.0001] and the significant effect of time × treatment interaction [F (5, 48) = 9.202, P < 0.0001] on the body weight of the experimental groups. Based on Figure 1, the body weights of the animals were not significant in any assigned groups on day 1 of the experiment. On the other hand, colitis rats showed significantly decreased body weights compared to control groups on day 7 of the study (P < 0.001). However, A. vulgaris treatment for 3 days at a 200 mg/kg concentration caused a significant increase (P < 0.001) in the body weight of the animals compared to the rat model of colitis at the end of the study (P < 0.001) (Figure 1).
Effect of A. vulgaris extract treatment on body weight in acetic acid (AA)-induced colitis rats. Data are represented as mean ± standard error of the mean (n = 5 each); data were analyzed using two-way repeated measures analysis of variance, followed by Tukey's post-hoc test. *** P < 0.001 vs. colitis group; ### P < 0.001 vs. control group. AV, A. vulgaris.
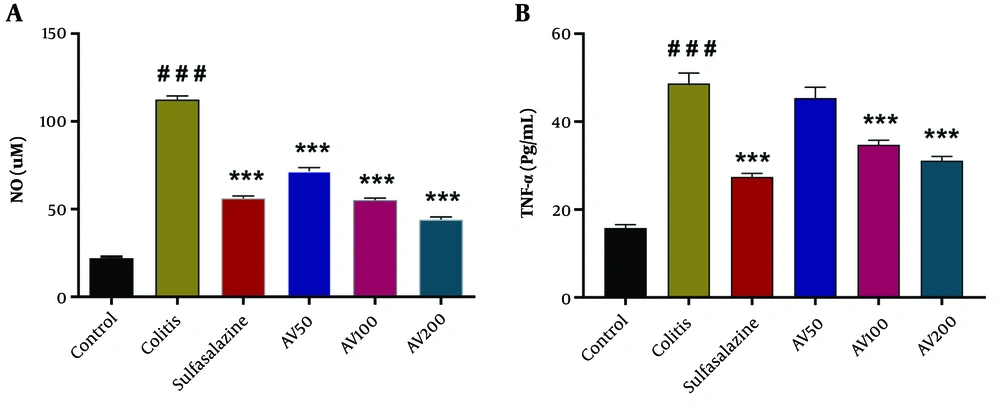
4.2. A. vulgaris Diminished Serum NO and TNF-α Levels in the Rat Model of Colitis
As shown in Figure 2A, B, a significant difference was shown by one-way ANOVA in serum NO [F (5, 24) = 352.9, P < 0.0001] and TNF-α levels [F (5, 24) = 63.58, P < 0.0001] between the study groups. The post-hoc analysis indicated that serum levels of NO and TNF-α significantly increased in the colitis group when compared with the control group (P < 0.001). At all doses tested, treatment with A. vulgaris resulted in a significant decrease in serum NO and TNF-α levels (except in the AV50 group) in colitis rats compared to untreated colitis rats (P < 0.001) (Figure 2A, B). In addition, there was a significant difference between the sulfasalazine group and the colitis group (P < 0.001) (Figure 2A, B).
Effect of A. vulgaris extract treatment on serum levels of nitric oxide (NO) (A); and tumor necrosis factor-α (TNF-α) (B) in the acetic acid (AA)-induced colitis rats. Data are presented as mean ± standard error of the mean (n = 5 each); data were analyzed using one-way analysis of variance, followed by Tukey's post-hoc test. *** P < 0.001 vs. colitis group; ### P < 0.001 vs. control group. AV, A. vulgaris.
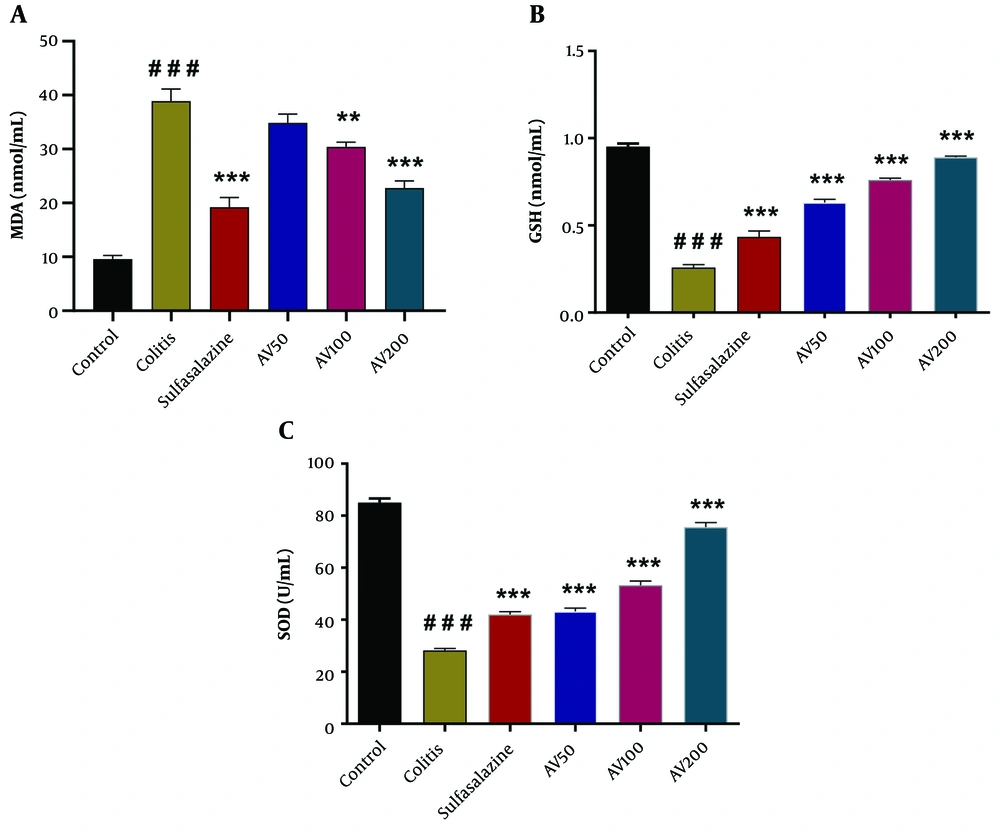
4.3. A. vulgaris Amended Serum Levels of MDA, GSH, and SOD Activity in the Rat Model of Colitis
One-way ANOVA revealed a significant difference in serum levels of MDA [F (5, 24) = 51.49, P < 0.0001], GSH [F (5, 24) = 191.2, P < 0.0001], and SOD activity [F (5, 24) = 229.9, P < 0.0001] among the groups. The post-hoc analysis revealed that the colitis rats had significantly increased serum levels of MDA (P < 0.001) compared to the control group. However, A. vulgaris treatment at 100 and 200 mg/kg significantly decreased MDA levels compared to the colitis group (P < 0.001 to P < 0.01) (Figure 3A).
Effect of A. vulgaris extract treatment on serum levels of malondialdehyde (MDA) (A); glutathione (GSH) (B); and superoxide dismutase (SOD) (C) activity in the acetic acid (AA)-induced colitis rats. Data are expressed as mean ± standard error of the mean (n = 5 each); data were analyzed using one-way analysis of variance, followed by Tukey's post-hoc test. ** P < 0.01, *** P < 0.001 vs. colitis group; ### P < 0.001 vs. control group. AV, A. vulgaris.
According to Figure 3B, C, a significant reduction in the serum GSH levels and SOD activity was observed in the colitis group compared to the control (P < 0.001) (Figure 3B, C). Nevertheless, the administration of AV50, AV100, and AV200 resulted in a significant increase in serum GSH levels and SOD activity in colitis rats compared to untreated colitis rats (P < 0.001) (Figure 3B, C). Furthermore, these parameters were significantly higher in the sulfasalazine-treated group compared to the colitis group (P < 0.001) (Figure 3B, C).
4.4. A. vulgaris Improved Macroscopic Alterations in the Rat Model of Colitis
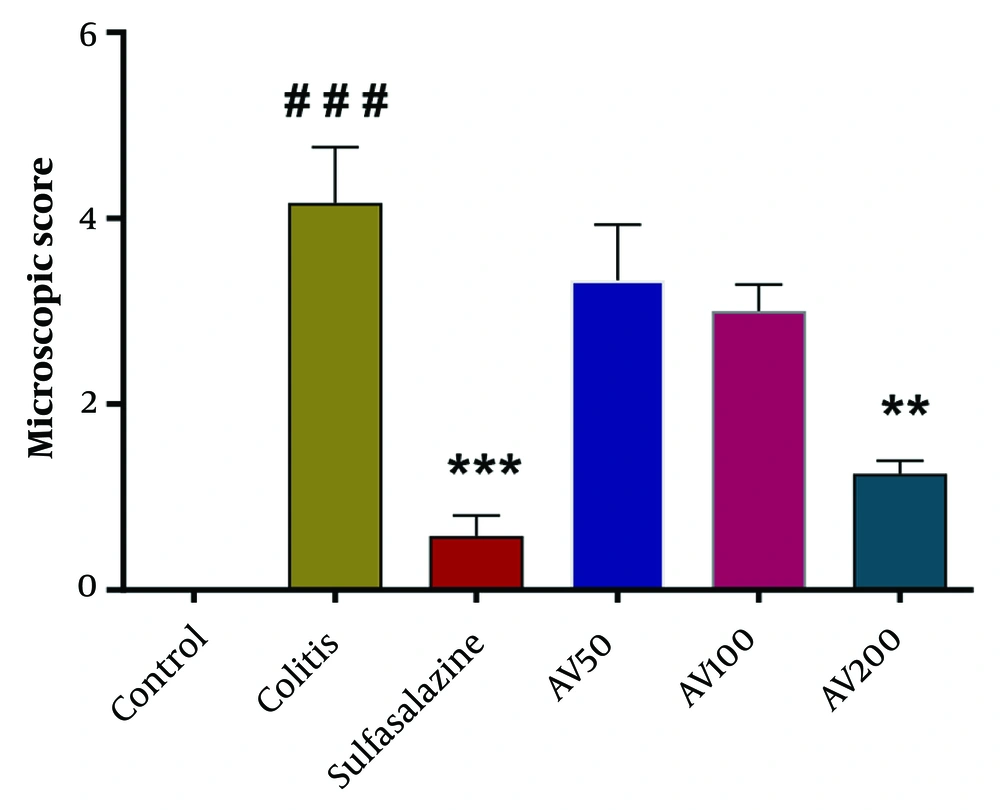
The results of one-way ANOVA of the macroscopic score indicated a significant difference between the groups [F (5, 12) = 19.23, P < 0.0001]. The post-hoc analysis revealed that compared to controls, acetic acid-induced colitis rats displayed severe ulceration and inflammation in the colonic damage (P < 0.001) (Figure 4). Meanwhile, A. vulgaris-treated groups showed an amended severity of lesion score compared to the colitis group, with the highest healing effect seen in the groups treated with 200 mg/kg A. vulgaris (P < 0.01) (Figure 4). Additionally, the rats treated with sulfasalazine had significantly lower macroscopic scores as compared to the colitis group (P < 0.001) (Figure 4).
Effect of A. vulgaris extract treatment on the microscopic score of colonic injury in the acetic acid (AA)-induced colitis rats. Data were analyzed using one-way analysis of variance, followed by Tukey's post-hoc test. ** P < 0.01, *** P < 0.001 vs. colitis group; ### P < 0.001 vs. control group. AV, A. vulgaris.
4.5. A. vulgaris Alleviated Microscopic Alterations in the Rat Model of Colitis
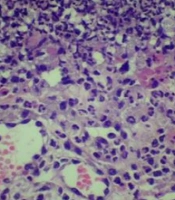
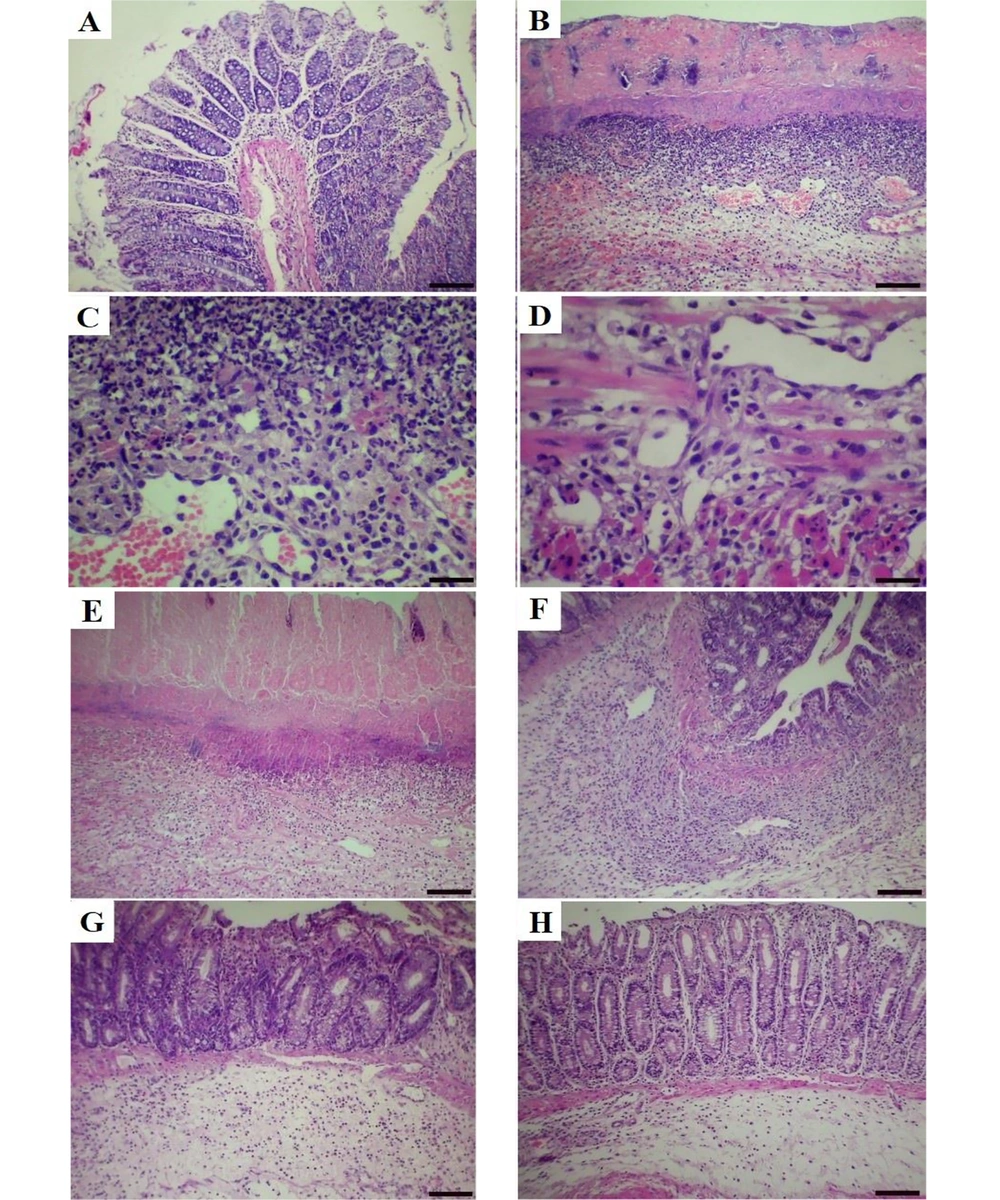
As depicted in Table 1, the colon of all the animals in the control group showed a typical architecture, including normal colonic mucosa lined with simple columnar epithelium and muscularis mucosae, submucosa with infiltration of a few inflammatory cells, muscularis, and serosa layers (Figure 5A). In contrast, the colon of rats in the groups that received acetic acid showed mild, moderate, and severe tissue changes. The main histopathologic findings in the colon were focal ulceration, necrosis, loss of goblet cells, crypt disarray, submucosal edema, mucosal and submucosal mono- and polymorphonuclear cells infiltration with crypt abscess. The extent and severity of the lesions were more prominent in the acetic acid group (Figure 5B-D). Treatment with the A. vulgaris hydroalcoholic extract and sulfasalazine significantly reduced acetic acid-induced pathological lesions in the colon tissues. The administration of sulfasalazine and A. vulgaris at a dose of 200 mg/kg showed less focal necrosis, hemorrhage, ulceration, and inflammatory reaction in the mucosa, submucosa, and muscularis layers, reduction in submucosal edema and loss of goblet cell compared to the group treated with A. vulgaris at doses of 100 and 50 mg/kg (Figure 5E-H). Regarding the pathological scores, the highest score of colon lesions was observed in the acetic acid control group, followed by A. vulgaris extract at 50, 100, and 200 mg/kg and sulfasalazine groups, respectively (Table 1). In the comparison between the groups treated with A. vulgaris extract, the most therapeutic effects were observed with the administration of 200 mg/kg of the extract.
| Groups | Histopathological Scores | Inflammatory Response Scores | ||
|---|---|---|---|---|
| Mean ± Standard Error | Median (Min - Max) | Mean ± Standard Error | Median (Min - Max) | |
| Control | 0.00 ± 0.00 | 0 (0 - 1) | 0.20 ± 0.10 | 0 (0 - 1) |
| Colitis | 11.86 ± 0.33 # | 12 (9 - 14) | 2.80 ± 0.10 # | 3 (2 - 3) |
| Sulfasalazine | 3.93 ± 0.18 *# | 4 (3 - 5) | 1.00 ± 0.13 *# | 1 (0 - 2) |
| A. vulgaris (50 mg/kg) | 8.60 ± 0.25 *# | 9 (7 - 10) | 2.20 ± 0.10 *# | 2 (2 - 3) |
| A. vulgaris (100 mg/kg) | 5.93 ± 0.20 *# | 6 (5 - 8) | 1.73 ± 0.11 *# | 2 (1 - 2) |
| A. vulgaris (200 mg/kg) | 4.40 ± 0.19 *# | 4 (3 - 6) | 1.20 ± 0.14 *# | 1 (0 - 2) |
Histopathological and Inflammatory Response Scores of Colons in Different Groups a
Histopathological lesion in the colon tissue of normal and acetic acid groups (hematoxylin-eosin (H&E) staining). A, normal group, normal architecture of colon tissue (scale bar = 150 µm); B, acetic acid group, focal ulceration, severe necrosis and crypt destruction in the mucosal layer (scale bar = 150 µm); C, acetic acid group, severe infiltration of mono- and polymorph nuclear cells in the submucosal layer (scale bar = 70 µm); D, necrosis of muscle tissue and infiltration of inflammatory cells in the muscularis layer (scale bar = 70 µm); E, A. vulgaris (50 mg/kg) group, moderate to severe crypt destruction and necrosis with moderate inflammation; F, A. vulgaris (100 mg/kg) group, moderate crypt destruction and necrosis with moderate inflammation; G, A. vulgaris (200 mg/kg) group, mild crypt destruction, and necrosis with mild inflammation; and H, sulfasalazine group, mild crypt destruction with minimal mucosal inflammation.
5. Discussion
This study showed the anti-inflammatory effects of the hydro-alcoholic extract of A. vulgaris in experimentally induced acute colitis, characterized by ameliorated colonic lesions, improved body weight, reduced MDA, NO, TNF-α levels, increased SOD activity, and GSH levels in the serum.
Several experimental studies indicated that colitis is triggered by inflammation and oxidative stress (15, 34). Excessive free radical production and decreased antioxidant capacity can affect cellular integrity (35), activation of various transcription factors, and expression of some genes involved in inflammatory pathways that directly and indirectly damage intestinal epithelial cells and lead to cell death (36), demonstrating that flavonoid derivatives in herbs have anti-inflammatory effects, along with marked antioxidant activity and inhibition of enzymes involved in the production of eicosanoids (37, 38). In our study, treatment with A. vulgaris (as a potent flavonoid) promoted the serum SOD activity and GSH level, significantly reduced serum TNF-α, NO levels, and lipid peroxidation (MDA), and alleviated colonic damage (39). These results suggest that the anti-inflammatory and antioxidant effects of A. vulgaris extract may be due to the presence of flavonoids (40). Few experimental models of UC were performed on other species of the genus Artemisia. In this regard, Shin et al. reported that Artemisia argyi extract treatment for 10 days reduced the expression of inflammation-related proteins and genes in the colon and serum, increased antioxidant capacities, and relieved symptoms of UC and body weight in the dextran sodium sulfate (DSS)-induced colitis mouse model (41). Another study showed that treatment of two flavonoid compounds from Artemisia asiatica (eupatilin and quercetin) for 4 days before and after inducing colitis in rats reduced the NO production and malondialdehyde levels and increased glutathione levels in the colon. Moreover, this study displayed attenuation in the morphology of the lesions (42). Kalhor et al. demonstrated that Artemisia dracunculus L. administration for 10 consecutive days improved total antioxidant capacity and suppression of pro-inflammatory cytokines in the AA-induced colitis Wistar rat model (43). Furthermore, several studies reported the beneficial effects of Artemisia in models of various inflammatory disorders in the liver (37) and lung (44) tissues. It seems that alleviated damage and improved function of the colon in UC animals by A. vulgaris treatment is mediated, at least in part, through the modulation of oxidative stress and inflammatory mediators. Possible mechanisms for this finding are attributed to the inhibition of the expression of genes relevant to the inflammatory process, including COX-2, iNOS, and nuclear factor-kappa B (NF-kB), which reduces the release of pro-inflammatory factors in the tissue (23, 45). Additionally, evidence shows that Artemisia extract enhances the expression of enzymes involved in the antioxidant system via activation of the Nrf2 (25). Thus, the A. vulgaris extract can be an effective treatment for colitis. We suggest that future studies assess the confirmatory factors of these changes in the chronic UC model and confirm their subclinical effects in UC patients.
5.1 Conclusions
Our findings revealed that A. vulgaris extract had protective effects in AA-induced colitis rats in a dose-dependent manner by improving body weight loss and colonic histopathology, decreasing NO and TNF-α levels, and increasing serum antioxidant levels. Thus, it could be considered a potential therapeutic agent against UC.