1. Background
Blastocystis hominies belongs to the stramenopiles, which are evolutionarily a complex and heterogeneous assemblage of heterotrophic and photosynthetic protozoa (1). Four original forms have been introduced for this parasite. In addition to having multiple forms, in many cases this parasite exists with more than one form in an environment (2). The main route of transmission is fecal contamination of water and food (2). This parasite exists in the large intestine of human and other vertebrates. It is probably the most common intestinal protozoa in humans around the world and particularly in Iran (3, 4). Its prevalence in developed countries is between 1.5 - 20% and in developing countries it can reach to 30 - 50% (5, 6). Blastocystis hominis has been noted as an emerging pathogen by numerous studies (2). According to the recent in vivo and in vitro studies, Blastocystis infection is associated with different types of gastrointestinal disorders (called blastocystosis), in particular irritable bowel syndrome (IBS), rectal bleeding, fecal leukocytes, eosinophilia, mucosal inflammation and skin rashes (5). It can remain in the human gastrointestinal tract from a few weeks to a few years until the patient receives a suitable treatment (7). The results of studies in 2013 have shown that Blastocystis subtype 3 by disrupting the P53 and interferon gamma gene can facilitate the proliferation of colon cancer cells and cancer cell metastasis (8). Considering that the most dominant subtype of Blastocystis hominis in Iran is subtype 3, this parasite has high clinical importance in Iran (4, 9-11). The clinical symptoms can be observed in both healthy people and those with immune system disorders and the treatment is performed when the diarrhea is persistent and no pathogen other than Blastocystis is detected in stool samples (12). In this case, metronidazole is considered as first-line therapy for Blastocystis infection (13). Although some of these drugs have been demonstrated to be effective at the global level, treatment failure has also been reported (14). Despite striking advances in the medical field, currently there are no vaccines available against any of the major human parasitic infections. Chemotherapy is the only option for both clinical management and control of the infection. However, the efficacy of conventional medications due to their high cost, drug-resistance, side effects and their toxicity is not optimal and therefore there is a need to develop alternative antimicrobial agents which are safe; such as the use of medicinal herbs (15, 16). Sea cucumbers are echinoderms, belonging to the class Holothuroidea which during the evolutionary period appeared in the oceans around 540 million years ago (17, 18). They spread on or near the ocean floor around the world. Sea cucumbers are marine invertebrates that have attracted much attention by researchers worldwide in recent decades. The nutritional value of this animal and its potential health benefits and its therapeutic usage has been proved by scientific research (Figure 1) (19). Some of the important biological features of the sea cucumber include anti-cancer, anti-clotting, antihypertensive, angiotensin-converting enzyme inhibitor, anti-inflammatory, anti-allergic, anti-microbial, anti-thrombotic, anti-tumor, anti-virus, anti-fungal, anti-Leishmania, hemolytic, cytostatic, regulator of the immune system, beneficial effect on arthritic disorders, wound healing, antihistamine, analgesic and antianaphylaxis (20-29). In recent years, the tendency of unicellular eukaryotes such as Leishmania donovani, Trichomonas vaginalis, Malaria, Toxoplasma, and Blastocystis hominis to induction of programmed cell death (PCD) has been well documented (30). Apoptosis in Blastocystis involves mitochondria and capsize cascade activation which are associated with DNA fragmentation in the parasite. It has been indicated that PCD in Blastocystis is a complicated process and is regulated by several mediators (31). According to the anti-cancer and cytostatic features of sea cucumber extract and its effects on fungal and microbes, it is likely that apoptosis pathways may become activated in Blastocystis hominis following the exposure to this extract.
2. Objectives
The aim of this study was to assess the effect of sea cucumber extract on inducing apoptosis in Blastocystis hominis cysts using flow cytometry and DNA fragmentation tests.
3. Methods
3.1. Preparation of Methanol Extract of Sea Cucumber
Sea cucumbers from the species Holothurian lecospilota were collected from the Persian Gulf coast (city of Bandar Lengeh) by local fishermen, frozen and were transferred to Laboratory of Medicinal Chemistry, School of Pharmacy, Jundishapur University of Medical Sciences in Ahvaz for extraction of sea cucumber body wall (30).
3.2. Microscopic Examination of Stool
Stool samples were collected from patients referred to health centers and then examined by direct wet mount microscopic examination using both normal saline and Lugol’s iodine preparation. Also trichrome staining technique was used for more identification. The positive samples for Blastocystis hominis were selected for in vitro culture.
3.3. Parasite Culture
The parasites were cultured in RPMI1640 (Roswell Park Memorial Institute) containing 20% fecal calf serum (FCS) or fetal bovine serum (FBS). About 2 mg of stool was passed through the gas and transferred to twenty 1.5 mL micro-tubes and then 1 mL RPMI medium containing 20% FBS was added to all micro-tubes. Each micro tube was sub-cultured into fresh medium every 48 hours in order to isolate the parasite and to eliminate fecal remainders. The samples were incubated at 37°C (32, 33).
3.4. MTT Assay
MTT colorimetric assay was performed for investigating the cytotoxicity effect of pharmaceutical compounds on cell growth and proliferation and for determining IC50 of these compounds. The cytotoxicity assay is the first step in apoptosis assessment. Using this method cell death can be evaluated. To determine IC50, MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) assay kit was used according to the manufacture’s instruction (Sigma-Aldrich, UK) (15). To prepare MTT solution, 5 mg of MTT powder was dissolved in 1 ml of sterile PBS solution. Then, 100 µl of sea cucumber extracts with concentrations of 1 µg/mL, 2 µg/mL, 4 µg/mL, 8 µg/mL, 16 µg/mL, 32 µg/mL, 64 µg/mL, 128 µg/mL and 256 µg/mL were added to each well of 96 well plates. Then 100 µL of parasites (1.5 × 105 per mL) was added to each well. In addition, 100 µL of the parasites exposed to different concentrations of metronidazole (0.5 mg/mL, 1 mg/ mL, 1.5 mg/mL, 2 mg/mL) were used as positive control and 100 µL of parasite without exposure to extract was considered as a negative control. All experiments were performed in triplicate well for each condition. The plates were incubated at 37°C and after 24, 48 and 72 hours, 20 µL MTT was added to each well and the plates were incubated at 37°C for 5 hours in the dark place. In order to solubilize Formosan crystals (yellow color) 1% DMSO (dimethyl sulfide) was added to each well and purple color developed (34). Eventually, the IC50 or the concentration of sea cucumber extract that inhibits the growth of 50% of the parasites was determined by the following formula (14).
Where AT represents the absorbance of the exposed parasite, AC represents the absorbance of the unexposed parasite and AB represents the absorbance of the blank.
3.5. Metronidazole Preparation
Metronidazole was prepared by adding 10 mL of distilled water to 600 mg of metronidazole and the solution was diluted to the final concentration of 60 mg/mL and then different concentrations (0.5 mg/mL, 1 mg/mL, 1.5 mg/mL, and 2 mg/mL) were prepared from the stock and kept in dark containers at 4°C (35).
Morphological changes in parasites exposed to methanol extracts of sea cucumber compared to the control group:
In order to observe changes in parasites that were exposed to different concentrations of sea cucumber extract at different times (24 , 48, and 72 hours), slides were prepared from each group and were observed under optical microscope lens with X40 and X100 magnifications. The morphological changes in the nucleus and cytoplasm of parasites exposed to the extract were evaluated at least in 10 microscopic fields and finally were compared to the morphology of parasites in the control group (36).
3.6. Flow Cytometry Technique
To differentiate apoptosis from necrosis at cellular level, Annexing-V-FLUOS Staining Kit with Cat. No. 11,858,777,001 (Roche, Germany) was used. In brief, 100 µL of parasite (cell concentration: 106/mL) was added to 100 µL of methanol extract of sea cucumber with IC50 of 72 hours and incubated at 37°C. Then the parasite-extract suspension was washed with buffer and centrifuged at 200 g for 5 minutes. Then cells were incubated with Annexing-v-Fluorescein solution in a HEPES buffer containing presidium iodide (PI) that was prepared according to the kit instruction. Finally, the intensity of Annexing-V FITC absorbed by cells was assessed using FACSCalibur and the data were analyzed using cell Quest pro software.
3.7. DNA Fragmentation Test
DNA of the samples was extracted using Apoptotic DNA Ladder Kit with Cat.No.11 835 246 001 (Roche, Germany). In brief, 100 µL of the parasites (5 × 106) was added to 100 μL of methanol extracts with concentrations 21 µg/mL, 56 µg /mL, 219 µg/mL and incubated at 37°C for 72 hours. Then DNA was extracted from parasite using DNA Apoptotic Kit and was electrophoresed on 1.5% agarose gel with voltage 80 for 1.5 hours. Finally, DNA bands were observed under UV trans-illuminator and images were analyzed under the Geldoc system (37).
4. Results
4.1. IC50 Calculation
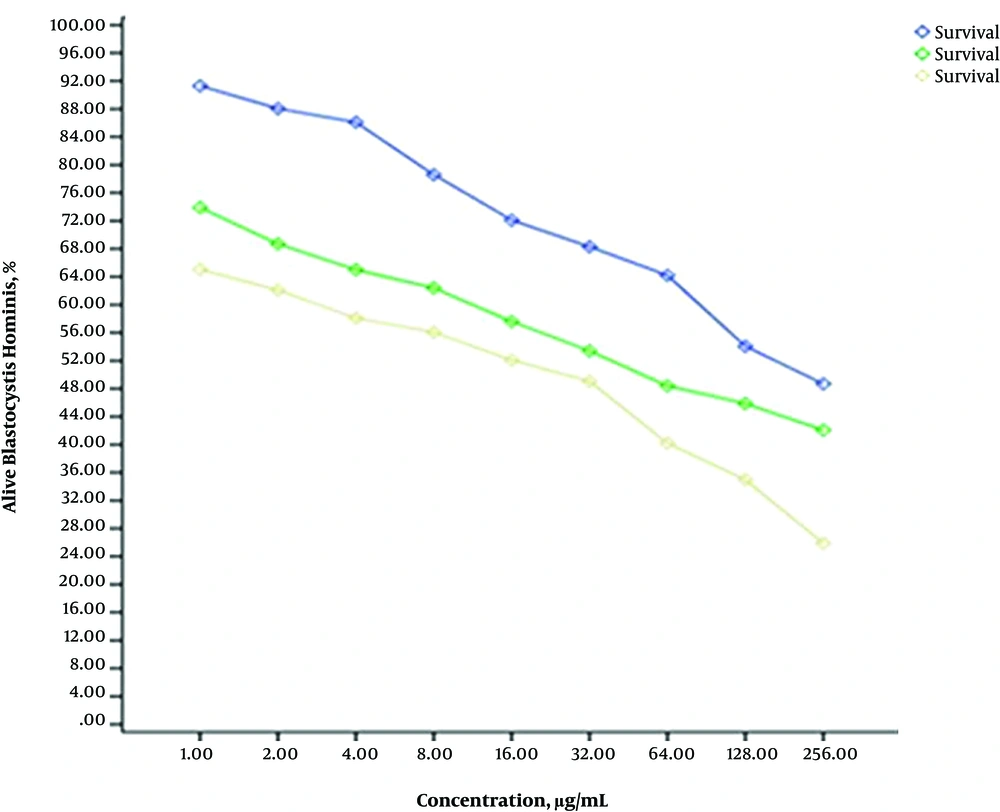
Cell viability of experimental groups (treated with extract) were measured compared to the control group by MTT assay. The intensity of color is an indicator of cell vitality and cell number (The darker the solution, the greater the number of viable cells). The absorbance was read at 570 nm using ELISA reader. The IC50 of the sea cucumber methanol extract after 24, 48 and 72 hours exposure were 219 µg/mL, 56 µg/mL and 21 µg/mL, respectively. The IC50 value in positive control group (parasites exposed to metronidazole) after 24, 48, and 72 hours was calculated 156 µg/mL, 125 µg/mL, and 97 µg/mL, respectively. Statistical analysis MTT test using the program was done using CurveExpert (Figure 2).
4.2. Morphological Changes of the Parasite
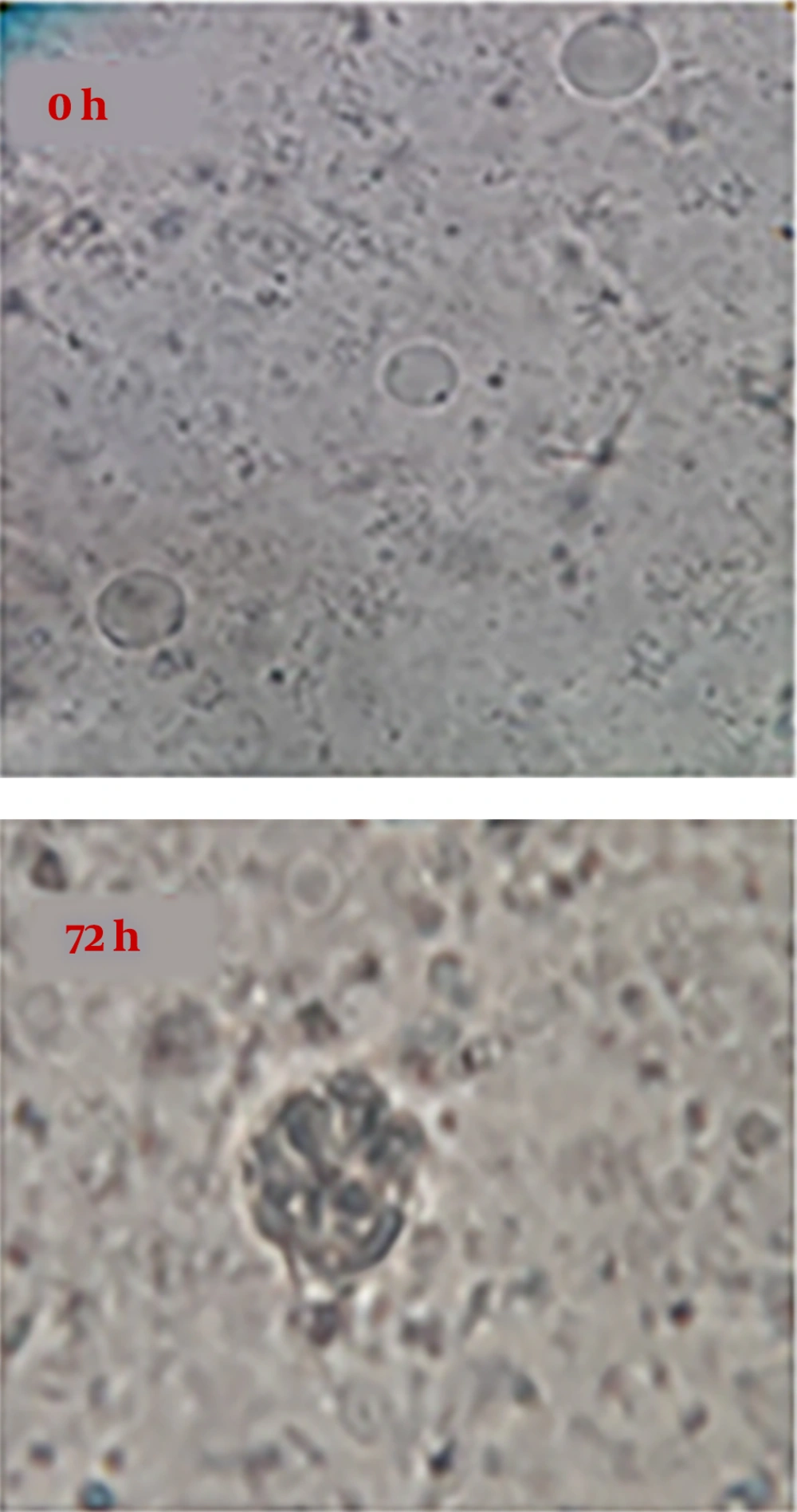
It was observed using an optical microscope that IC50 level of sea cucumber methanol extract causes some changes such as decreased size of parasite, shrinkage of cytoplasm, and some changes in nucleus. So that after 24 hours of exposure, the number of parasites decreased significantly and this trend continued after 48 and 72 hours (Figure 3).
4.3. The Detection of Outer Membrane Phosphatidylserine of Apoptotic Cell by Flow Cytometry
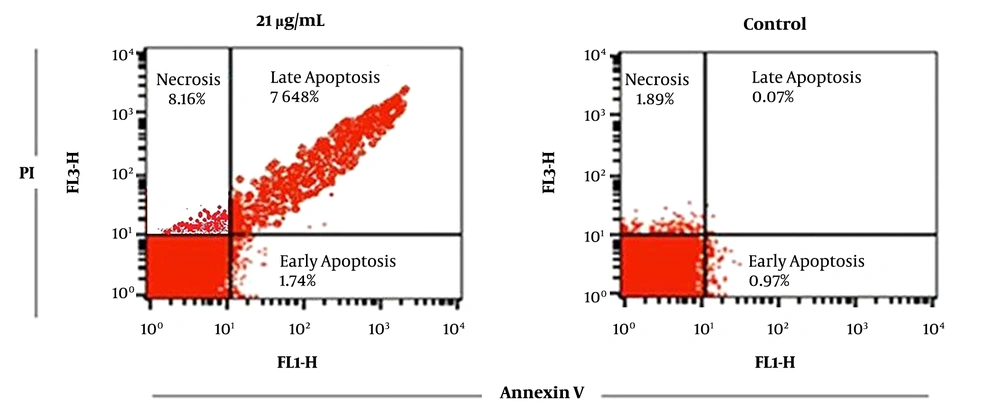
Apoptosis assessment was performed using dual staining (Annexing-V/PI). The used fluorochrome was fluorescein isothiocyanate (FITC) with green light that was detected in FL1 and propidium iodide (PI), which is a DNA-specific dye was detected and evaluated in orange channel (FL3). The percentages of early and late apoptosis after 72 hours incubation of parasites with IC50 concentration (21 µg/mL) were 1.74% and 76.48%, respectively. However, in the control group, the percentage of early and late apoptosis was 0.9% and 0.07% of the cells. More details have been shown in Figure 4.
4.4. DNA Fragmentation in Parasites Exposed to Methanol Extracts and Control Group
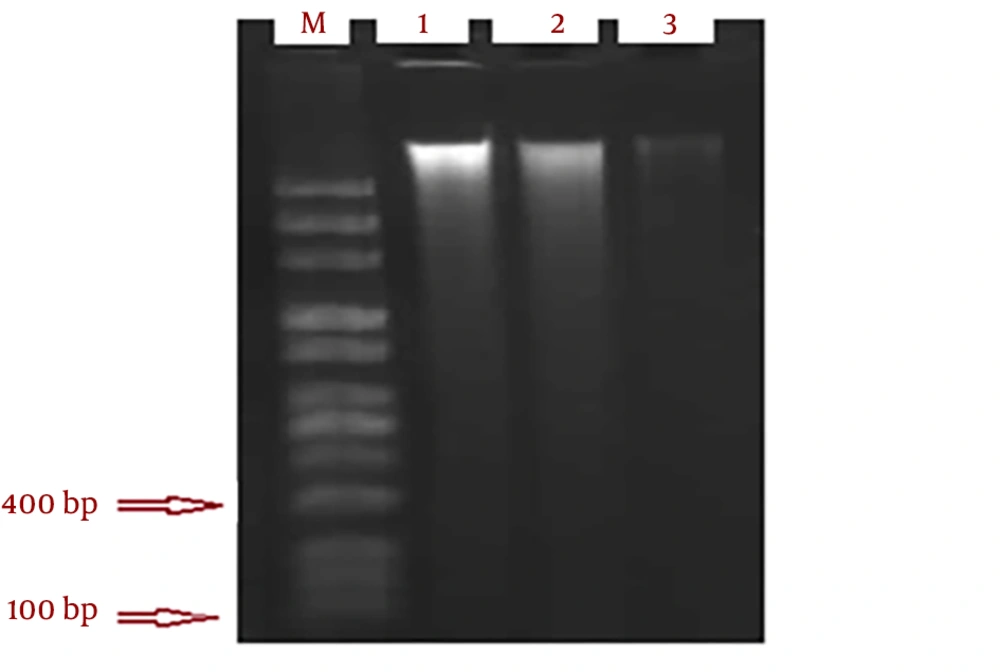
The smear pattern of genomic DNA was observed after 72 hours at concentrations 21, 56 and 219 µg/mL of methanol extract of sea cucumber (Figure 5). Random fracture of DNA normally occurs in necrosis (35). Apoptosis in some cell lines occurs without nucleosome DNA fragmentation (38).
5. Discussion
This study showed that Blastocystis hominis is sensitive to methanol extract of H. Leucospilota at different concentrations and different times. The induction of apoptosis by methanol extract of sea cucumber in other cells has also been confirmed (30, 39, 40). This pioneering study is the first report of apoptosis induction by methanol extract of H. Leucospilota in Blastocystis hominis. The important medicinal ingredients in the sea cucumber consist of triterpene glycosides, chondroitin sulfate, sterols, omega-6, omega-3, fatty acids, glycosaminoglycans, lectins, sulfated polysaccharides, bioactive peptides including gelatin proteins, collagen and mucopolysaccharides (41-45). Sea cucumber is a rich source of glycosides especially triterpene glycosides, the anti-fungal and anti-tumor activities of which have been proved. It has been shown that three new compounds of sea cucumber from species Holothurian leucospilota (e.g. Ganglioside, HLG-1, HLG-2 and HLG-3) are able to stimulate the growth of nerve cells in laboratory mouse. Furthermore, recent studies have reported the anti-cancer effects of a newly discovered compound called philinopside in sea cucumber (19). Bioactive sphingolipid derived from sea cucumbers called (Frondoside A) induces apoptosis through mitochondrial pathways (19). Moreover, the anti-microbial property of this extract has been attributed to steroidal sapogenins. In addition, anti-fungal activity of new triterpene glycosides has been discovered in holothurin A and holothurin B.
Sea cucumber contains a variety of anti-tumor substances. These active components play a key role in various stages of tumor development, tumor invasion and metastasis. Philinopside A has been shown to have a significant anti-tumor activity both in vitro and in vivo. In a study conducted on the effect of acid mucopolysaccharides from “Stichopus japonicas” on HepG2 cell line in China, it was shown that this sea cucumber extract induces apoptosis and inhibits the proliferation of HepG2 cells; such that the sea cucumber extract can be used as a potential anti-tumor component for the treatment of hepatocellular carcinoma (40). According to a study in Tokyo, when Caco-2 cells of colon adenocarcinoma were exposed to hot water extract of sea cucumber (Stichopus japonicas), certain morphological changes happened in cells treated with the extract that based on the researcher’s conclusion are due to apoptosis induction (46). It has also been reported that alcoholic extracts and n-butanol fractions of sea cucumber (Actinopyga lecanora) can significantly inhibit the activity of Leishmania donovani both in vitro and in vivo (25). In another study, it has been demonstrated that hydro-alcoholic extract of H. leucospilota from the body wall of sea cucumbers, coelomic fluid, and cuvierian organ of sea cucumber H. leucospilota at a concentration of 1000 - 2000 μg/mL have bacteriostatic effect while methanol, chloroform and N-hexane extracts of sea cucumber have no considerable anti-bacterial and anti-fungal effects (47). In a study, using MTT assay, IC50 values of the methanol extract of sea cucumber H. leucospilota against Leishmania major promastigotes after 24, 48 and 72 hours exposure were 2000 μg/mL, 300 μg/mL and 85 μg/mL, respectively. Besides, the absence of DNA fragmentation and the occurrence of apoptosis by flow cytometry were shown. In comparison to the present study, the methanol extract of sea cucumber showed a higher IC50 value to Blastocystis hominis. (39). Furthermore, the inhibitory function of different concentrations of curcumin and silver nanoparticles (nCur and NAG) compared to the metronidazole (MTZ) on Blastocystis hominis was evaluated in vitro. Using criteria of growth inhibition rate, after nAg at a concentration of 150 μg/mL, nCur at a concentration of 1000 μg/mL showed the highest cytotoxicity effect compared to metronidazole (48).
The results of a study on 6 ethanolic extracts prepared from different plants On Blastocystis hominis in Ghana were as follows:
Mallotus oppositifolius, IC50, 24 hours 27.8 µg/mL; Vemonia colorata, IC50, 24 hours 117.9 µg/mL; Zanthoxylum zanthoxyloides, cortex IC50, 24 hours 255.6 µg/mL; Clausena anisata, IC50, 24 hours 314.0 µg/mL; Z. zanthoxyloides, radix IC50, 24 hours 335.7 µg/mL and Eythrina senegalensis, IC50, 24 hours 527.6 µg/mL and metronidazole,IC50 24 hours 7.4 µg/mL.
Among these plants, antimicrobial activity was only observed in Clausena anisata at a concentration of 800 μg/mL (49). Recently, it has been revealed that garlic at concentrations 0.1 mg/mL and 0.01 mg/mL can inhibit the growth of Blastocystis hominis (50). In addition, the ethanolic extract of Saw Palmetto, thyme and pumpkin, and grape seed with a concentration of 5 mg/mL was shown to inhibit the growth of Blastocystis hominis after 24 and 48 hours (51). In another study the lowest and highest concentration of verjuice that can inhibit the growth of Blastocystis hominis was reported as 16 mg/mL and 40 mg/mL (36). As in aforementioned reports, methanol extract of sea cucumber has more inhibitory effect on Blastocystis hominis. The limitation of this study is that due to financial and time constraints were not able to identify subtypes of Blastocystis hominis and the impact of sea cucumber extract to be assessed on each subtype.
5.1. Conclusion
This study showed that methanol extract of sea cucumber can induce apoptosis in Blastocystis hominis. Hence, further studies are recommended to determine the efficacy of this extract in vivo in order to achieve appropriate and effective drug combinations for the treatment of Blastocystis hominis infection. Considering the obtained results, in the future studies different fractions of sea cucumber can be separated and the effectiveness of these fractions can be evaluated on Blastocystis hominis.