1. Background
Inflammation serves as the body's defense mechanism against invasive infections. Numerous studies have indicated that inflammation plays a role in the pathophysiology of a wide range of diseases, including cancer, cardiovascular disease, and other severe and disabling conditions (1). The balance or regulation of the inflammatory cycle is maintained by anti-protease and anti-inflammatory interleukins (IL-1, IL-4, IL-10, IL-11, and IL-13). The manifestations of inflammation include edema, leukocyte infiltration, and granuloma formation, with many inflammatory responses triggered by various implicated mediators (2).
Mast cells significantly influence neuroinflammatory and allergy conditions exacerbated by stress. They are a major source of histamine, which includes IL-6, a vasoactive and pro-arrhythmogenic compound (3). Known for releasing their cytoplasmic granules, mast cells are key immunological players during inflammation. Pro-inflammatory cytokines such as IL-1β, IL-6, and tumor necrosis factor-alpha (TNF-α) contribute to inflammation and pathological pain. Upon activation, mast cells can release different types of pro-inflammatory mediators, including histamine, TNF-α, tryptase, and IL-6 cytokines, leading to increased inflammation through heightened blood vessel permeability or tissue damage (4).
Cromolyn sodium (CS), a mast cell stabilizer, is commonly used to treat allergic conditions. Originally developed for allergic asthma, it has shown potential in managing mastocytosis, allergic skin diseases, and intestinal allergies. Previous research on CS’s pharmaceutical effects has shown it suppresses mediators released from mast cells triggered by specific antigens, potentially reducing the activation of other cell types (5-7). Cromolyn is effective in treating asthma, systemic mastocytosis, aphthous ulcers, and food allergies (8). Furthermore, cromolyn has been found to significantly inhibit Aβ aggregation in vitro and is structurally similar to fisetin (9, 10). However, substances such as quercetin have been identified as more effective than sodium cromolyn in inhibiting mast cell histamine release (11). Carrageenan-induced paw edema, an acute form of inflammation, is commonly used to screen new anti-inflammatory drugs (12, 13). The early phase after carrageenan injection into the hind paw, noticeable around one hour, involves the release of histamine, bradykinin, serotonin, and, to a lesser extent, prostaglandins by cyclooxygenase enzymes (COX). The delayed phase, occurring beyond 1 hour, is characterized by neutrophil infiltration and continued prostaglandin production (14).
Subcutaneous injection of a saline solution containing carrageenan leads to rapid swelling in rats; this swelling reaches its peak between 3 - 5 hours after injection and subsides 24 hours later. In contrast, administering a saline solution with Freund's complete adjuvant subcutaneously in rats results in a more prolonged effect, with edema lasting at least seven days and peaking 24 hours later (14). These models of inflammation can be used to evaluate the production of inflammatory mediators at inflammation sites, the anti-inflammatory properties of drugs, such as nonsteroidal anti-inflammatory drugs (NSAIDs), and the effectiveness of potential analgesics in reversing skin hypersensitivity. Inflammation induced by carrageenan is a suitable method for identifying potent anti-inflammatory drugs due to its strong predictive value for anti-inflammatory drugs that act as mediators of acute inflammation.
Many NSAIDs, like indomethacin, are known to cause gastrointestinal side effects, including stomach upsets. Therefore, anti-inflammatory drugs, such as sodium cromolyn, which do not produce such side effects (15), can be considered a viable alternative.
2. Objectives
This study aims to demonstrate the potential anti-inflammatory activity of CS and its impact on IL-6 levels using a carrageenan-induced acute inflammation model.
3. Methods
3.1. Drugs and Chemicals Used
Lambda carrageenan was obtained from Sigma, Switzerland. Indomethacin and sodium cromolyn were sourced from Merck, Germany. Ketamine hydrochloride 10% vial (500 mg/10 mL) was acquired from Daroodar Company in Iran. ELISA Rat IL-6 Cytokine assay standard kits were purchased from eBioscience, Switzerland. A Plethysmometer model 7140 (made in Italy) was used to measure the volume of the rats' paws. An analytical scale (Sartorius 2434 model, made in Germany, with a sensitivity of 0.01 mg) was utilized for precise drug weighing, and a single-bottomed scale (DAHUS model, made in the United States) was used for weighing rats. An incubator (XB032 model) and ELISA reader (BIOTEK ELX800TS model) were employed to determine the IL-6 levels in the samples, while a simple centrifuge without refrigeration and a rotation speed of 2500 rpm was used to separate the plasma for IL-6 cytokine measurement. A homogenizer (SR 30 model) was utilized to homogenize the rat paw tissue.
3.2. Animals
Male Wistar rats weighing between 130 - 170 g were utilized in this study. They were fed standard pellet chow and water. The rats were divided into six groups, with each group comprising six rats (n = 6). Group A served as the control group, receiving an intraperitoneal (IP) injection of the vehicle (carrageenan control), followed by 100 microliters of physiological saline subcutaneously injected into the sole of the right foot 30 minutes later. Group B, the negative control (control-saline), received an IP injection of an equivalent volume of the CS carrier (saline), followed by a subcutaneous injection of 100 microliters of 1% carrageenan (volume/weight) as an inflammatory agent into the sole of the right foot 30 minutes later. Groups C, D, and E received CS doses of 25 mg/kg, 50 mg/kg, and 100 mg/kg, respectively. Group F served as the positive control, receiving indomethacin at 5 mg/kg (Table 1). The decision to start the study with a dose of 25 mg/kg was based on previous studies on the anti-inflammatory and antihistaminic effects of cromolyn and the existence of a direct relationship between the mast cell stabilizing mechanism and the anti-inflammatory effect (16, 17). All experimental procedures adhered to the guidelines of the Institutional Animal Ethics Committee (IEAC) and each rat was utilized for only one test. The study was conducted at Ahvaz Jundishapur University of Medical Sciences, verified through its Behsan system, with certification codes available at http://www.BEHSAN.ajums.ac.ir.
| Groups | Intraperitoneal Injection | Intraplantar Injection |
|---|---|---|
| A: Control (carrageenan control) | Saline | Saline |
| B: Negative control (saline) | Saline | Carrageenan |
| C: Cromolyn 25 mg/kg | Cromolyn 25 mg/kg | Carrageenan |
| D: Cromolyn 50 mg/kg | Cromolyn 50 mg/kg | Carrageenan |
| E: Cromolyn 100 mg/kg | Cromolyn 100 mg/kg | Carrageenan |
| F: Positive control (indomethacin 5 mg/kg) | Indomethacin 5 mg/kg | Carrageenan |
Animals Were Divided Into Six Groups (n = 6 Per Group)
3.3. Evaluation of Anti-inflammatory Activity
The anti-inflammatory effects were evaluated across six groups. Group A (vehicle, carrageenan control) received 1 mL/kg IP saline. Group B (negative control, control-saline) received 1 mL/kg IP saline. Group C received 25 mg/kg IP CS dissolved in normal saline. Group D received 50 mg/kg IP CS dissolved in physiological saline. Group E received 100 mg/kg IP CS dissolved in normal saline. Group F (positive control) received an IP injection of indomethacin (5 mg/kg body weight). Except for group A, all test groups received 0.1 mL of carrageenan (1%) IP 30 minutes later (Table 1).
3.4. Carrageenan-Induced Acute Inflammatory Model
The carrageenan-induced paw edema rat model was employed to assess the anti-inflammatory efficacy. This classic model of edema formation and hyperalgesia was used to evaluate anti-inflammatory drugs in this study. Thirty minutes post-drug administration, each animal received an intraplantar (IPL) injection of 0.1 mL of carrageenan into the right hind paw (18). Edema was measured immediately before and at 0.5, 1, 2, and 3 hours after carrageenan administration. The paw edema volume was calculated as the difference (mL) between the final and initial volumes, measured using a Ugo Basile plethysmometer (Italy) (3). Edema was induced by IPL administration of 100 μL of 1% freshly prepared carrageenan solution in normal saline into the right hind paws of each rat in groups B, C/D/E, and F, except for group A. These animals were treated with a single dose of the vehicle, sodium cromolyn, or indomethacin, respectively, 30 minutes before the carrageenan injection. Paw volume (in milliliters) was recorded prior to carrageenan injection, referred to as “0 hours,” and then at 0.5, 1, 2, and 3 hours after the injection.
Where Vt represents the rat paw volume (mL) at 0.5, 1, 2, or 3 hours after the injection of carrageenan, and V0 is the rat paw volume (mL) before the injection of carrageenan. The mean and standard deviation of the percentage of relative paw edema observed at 0, 0.5, 1, 2, and 3 hours after the carrageenan injection are documented in Table 2.
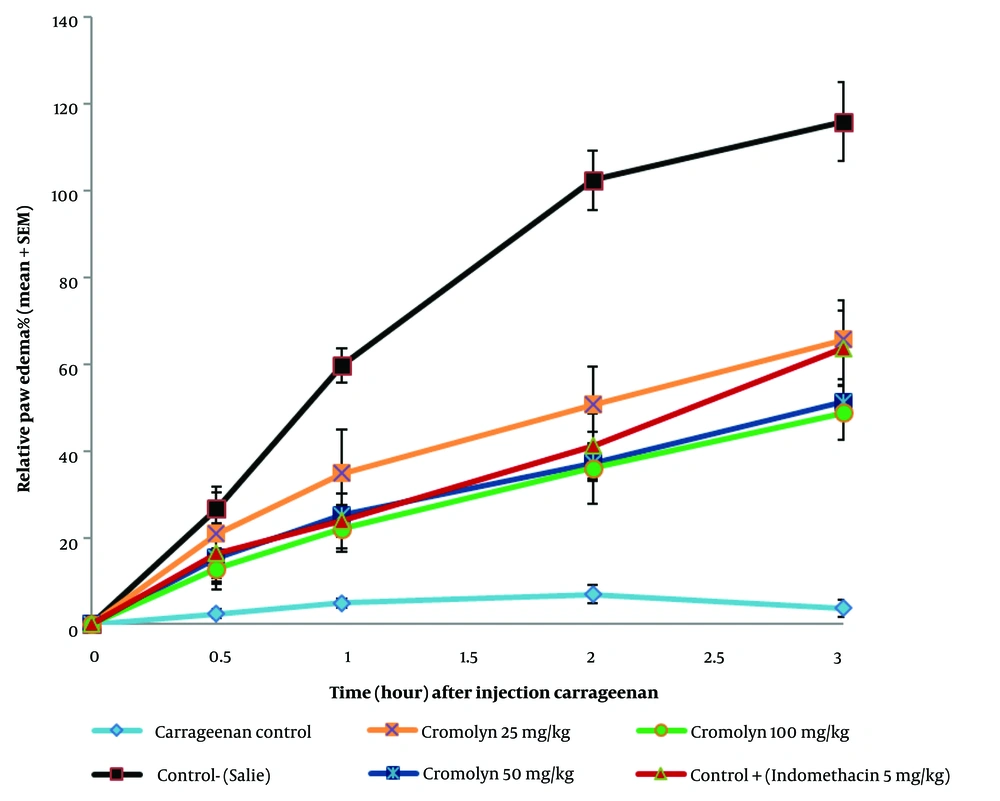
| Time, Hour | Carrageen a Control | Negative Control-(Saline) | Positive Control (Indomethacin 5 mg/kg) | Cromolyn 25 mg/kg | Cromolyn 50 mg/kg | Cromolyn 100 mg/kg |
|---|---|---|---|---|---|---|
| 0.5 | 2.45 ± 1.01 | 26.61 ± 3.9 | 16.39 ± 6.93 | 20.84 ± 10.93 | 15.38 ± 4.61 | 12.78 ± 4.79 |
| 1 | 4.88 ± 1.01 | 59.65 ± 3.88 | 23.84 ± 6.35 | 34.87 ± 10.05 | 25.22 ± 5.01 | 22.09 ± 5.36 |
| 2 | 6.929 ± 2.149 | 102.37 ± 6.83 | 41.13 ± 7.5 | 50.59 ± 8.85 | 37.22 ± 4.27 | 36.05 ± 8.35 |
| 3 | 3.76 ± 1.96 | 115.87 ± 9.01 | 63.71 ± 8.54 | 65.60 ± 9.05 | 51.35 ± 3.6 | 48.76 ± 6.24 |
Percentage of the Relative Paw Edema in all Six Groups of Animals (n = 6 Per Group) a
3.5. Sample Collection
All animals were anesthetized and euthanized with ketamine three hours following the carrageenan injection. Blood and tissue samples were collected, allowed to clot at room temperature for 60 minutes, and then centrifuged at 2500 rpm for 15 minutes, after which the serum was stored in new tubes for cytokine analysis. The hindlimb tissues of all animals were homogenized in the same saline solution using a homogenizer, centrifuged at 2500 rpm for 15 minutes, and the supernatant was preserved in a new tube. Serum and foot tissue samples were also collected and stored at -20°C.
3.6. Cytokine Assay
IL-6 levels were measured in picograms per milliliter (pg/mL) using an ELISA Reader (Lisa Plus, Germany), following the manufacturer's instructions (Table 3). The ELISA test procedure typically involves the following steps, applicable across various IL-6 measurement kits (19, 20):
- Coating: Adsorbing an antigen or antibody onto solid surfaces.
- Adding samples.
- Incubation: Allowing sufficient time for the reaction to occur.
- Washing with an ELISA washer to separate bound/reacted reagents from free/unbound ones.
- Adding enzyme-linked agents.
- Incubating the reactants again.
- Washing again using the ELISA washer.
- Adding an enzyme substrate to detect the reactants.
- Another incubation period.
- Terminating the enzymatic reaction and measuring the optical density with the ELISA reader.
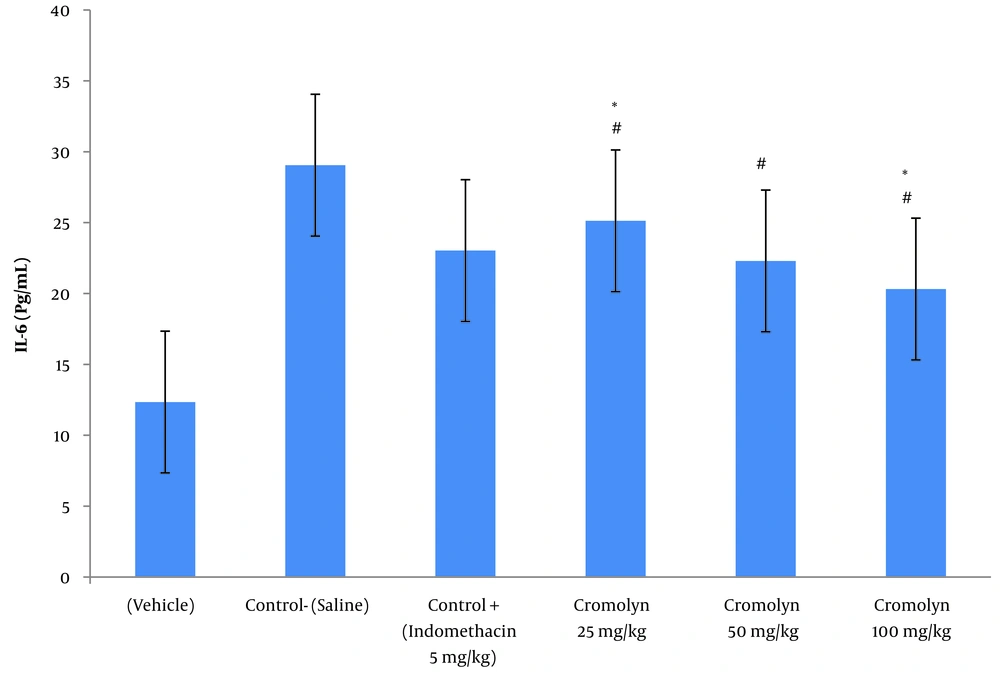
| Groups | N | Mean ± Standard Deviation | Standard Error of Mean |
|---|---|---|---|
| Carrageenan control (vehicle) | 6 | 12 ± 3.391 | 1.385 |
| Negative control (saline) | 6 | 29 ± 3.874 | 1.581 |
| Positive control (indomethacin 5 mg/kg) | 6 | 23 ± 3.633 | 1.483 |
| Cromolyn 25 mg/kg | 6 | 25 ± 2.292 | 0.936 |
| Cromolyn 50 mg/kg | 6 | 22.30 ± 3.472 | 1.418 |
| Cromolyn 100 mg/kg | 6 | 20.32 ± 3.357 | 1.371 |
IL-6 Serum Concentration (pg/mL) at the Time of Peak Inflammation (t = 3) in All Six Groups of Animals (n = 6)
This research was conducted in compliance with the ethical approval code IR.AJUMS.ABHC.REC.1397.058.
3.7. Statistical Analysis
Data are presented as mean ± SEM for 6 animals per group. Statistical analyses were performed using one-way analysis of variance (ANOVA), followed by a Student-Newman-Keuls post hoc test for multiple comparisons. A P-value of less than 0.05 was deemed to indicate statistical significance.
4. Results and Discussion
In this study, animals were divided into six groups, each consisting of six rats. As detailed in Table 1, every rat received both IP and IPL injections. Different doses of CS were administered in groups C, D, and E. Moreover, there were both positive and negative control groups, which received 5 mg/kg of indomethacin and saline, respectively. Notably, group A served as the carrageenan control, receiving saline for both IP and IPL injections.
The mean ± SD of the percentage of relative paw edema across all six animal groups is presented in Table 2, and the percentage of relative paw edema ± SEM at 0, 0.5, 1, 2, and 3 hours after carrageenan injection is depicted in Figure 1. The group receiving 100 mg/kg of sodium cromolyn exhibited the lowest mean ± SD of the percentage of relative paw edema at 0.5, 1, 2, and 3 hours, whereas the highest was observed in the group receiving 25 mg/kg of sodium cromolyn.
Changes in the percentage of the relative paw edema at t = 0, 0.5, 1, 2, 3 hours (n = 6) (significant at P < 0.05). Edema was induced by injecting 0.1 mL of 1% solution of carrageenan (w/v) into the sub-plantar surface of the right paw. Data are expressed as mean ± standard error of six rats per group. Group A: Carrageenan control (vehicle); group B: Negative control (control-saline); group C: Cromolyn 25 mg/kg; group D: Cromolyn 50 mg/kg; group E: Cromolyn 100 mg/kg; group F: Positive control (indomethacin 5 mg/kg).
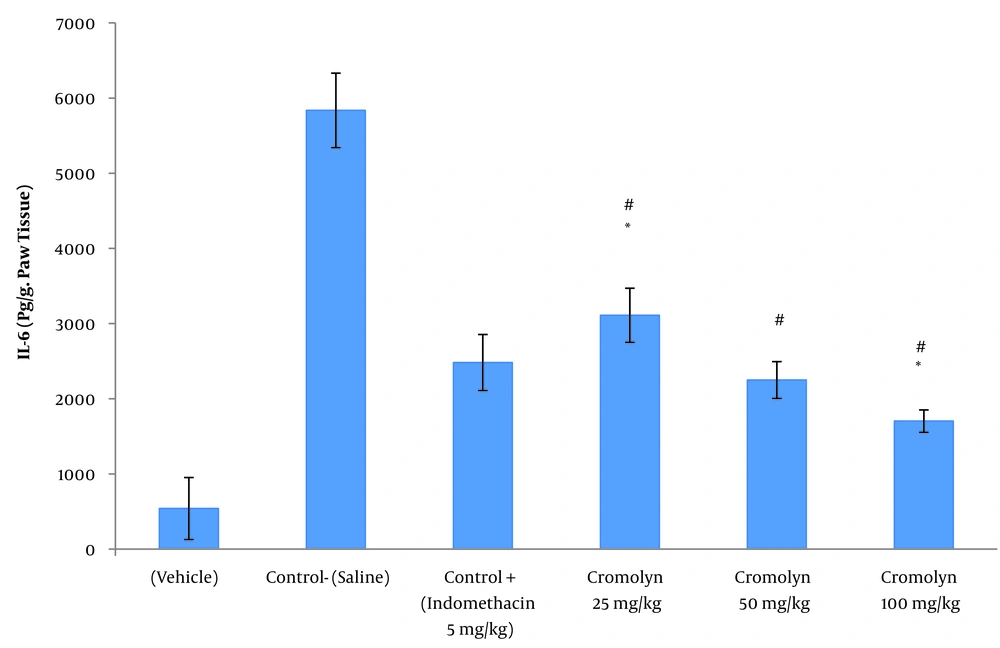
Tables 3 and 4 illustrate the mean ± SD of serum IL-6 concentration (pg/mL) and paw tissue IL-6 concentration (pg/g.paw tissue) at the peak of inflammation (t = 3), respectively. Among the groups treated with sodium cromolyn, the highest and lowest concentrations of IL-6 were found in group C (receiving 25 mg/kg of sodium cromolyn) and group E (receiving 100 mg/kg of sodium cromolyn), respectively. However, the most significant increases in serum and paw tissue IL-6 levels were observed in group B, the negative control (control-saline), with values of 29 ± 3.87 and 5649 ± 495.41, respectively. Conversely, treatment with sodium cromolyn significantly reduced IL-6 levels in groups C, D, and E at P < 0.05. Additionally, the groups treated with sodium cromolyn and the negative control (control-saline) showed significant differences. The 50 mg/kg dose of cromolyn and the positive control dose (5 mg/kg of indomethacin) did not significantly differ in both serum and paw tissue IL-6 levels (P < 0.05) (Figures 2 and 3).
| Groups | N | Mean ± Standard Deviation | Standard Error of Mean |
|---|---|---|---|
| Carrageenan control (vehicle) | 6 | 608 ± 148.042 | 60.438 |
| Negative control (control-saline) | 6 | 5649 ± 495.417 | 202.253 |
| Positive control (indomethacin 5 mg/kg) | 6 | 2482 ± 372.798 | 152.194 |
| Cromolyn 25 mg/kg | 6 | 3110.67 ± 359.795 | 146.886 |
| Cromolyn 50 mg/kg | 6 | 2249.50 ± 244.526 | 99.827 |
| Cromolyn 100 mg/kg | 6 | 1702 ± 148.649 | 60.686 |
IL-6 Paw Tissue Concentration (pg/g.paw tissue) at the Time of Peak Inflammation (t = 3) in All Six Groups of Animals (n = 6)
IL-6 paw tissue concentration (pg/g.paw tissue) at the time of peak inflammation (t = 3 h). Group A: Vehicle; group B (control-saline): Carrageenan (cgn); group C: Positive control (cgn + indomethacin 5 mg/kg); group D: cgn + cromolyn 25 mg/kg; group E: cgn + cromolyn 50 mg/kg; group F: cgn + cromolyn 100 mg/kg; group. Data are expressed as mean ± standard error of six rats per group (significant at P < 0.05). # Compared to the control-saline group (group B) (P < 0.05). * Compared to the positive control group (indomethacin 5 mg/kg) group (P < 0.05).
IL-6 serum concentration (pg/g.paw tissue) at the time of peak inflammation (t = 3 h). Group A: Vehicle; group B (control-saline): Carrageenan (cgn); group C: Positive control (cgn + indomethacin 5 mg/kg); group D: cgn + cromolyn 25 mg/kg; group E: cgn + cromolyn 50 mg/kg; group F: cgn + cromolyn 100 mg/kg; Data are expressed as mean ± standard error of six rats per group (significant at P < 0.05). # Compared to the control- saline group (group B) (P < 0.05). * Compared to the control + (indomethacin 5 mg/kg) group (P < 0.05).
This study demonstrated that cromolyn, at doses of 50 mg/kg and 100 mg/kg, exhibited remarkable anti-inflammatory efficacy in this model, significantly reducing rat paw volume in groups D and E. According to this model, inflammation triggers the release of prostaglandins, leukotrienes, histamine, bradykinin, TNF-α, cytokines, and other inflammatory mediators (21, 22). Carrageenan injection into the hind paw induced progressive edema that peaked after three hours. This model is widely utilized to evaluate the anti-inflammatory effects of various drugs. The induced edema involves a two-phase system. The first phase occurs within an hour of carrageenan injection, resulting from the release of serotonin, histamine, and pro-inflammatory cytokines from mast cells. The subsequent phase is characterized by an increase in prostaglandin release at the site of inflammation, with kinins acting as an intermediary mediator. Additionally, this second phase is responsive to steroidal and NSAIDs, which are clinically effective (23).
Cromolyn may act as an anti-inflammatory agent by blocking histamine and pro-inflammatory mediators, playing a crucial role in inhibiting inflammatory pathways. Mediators like mast cell histamine and pro-inflammatory cytokines are pivotal in the inflammatory process. Therefore, targeting histamine load and pro-inflammatory signaling pathways can effectively prevent carrageenan-induced inflammation (24). Intraperitoneal administration of cromolyn significantly downregulated pro-inflammatory cytokines while upregulating anti-inflammatory cytokines (25). This approach underlies the frequent use of the carrageenan-induced rat paw edema model in previous studies to evaluate the anti-edematous effects of drugs, serving as a useful tool for assessing anti-inflammatory medications. Kolaczkowska et al. discovered that in Balb/c mice, cromolyn's blockage of zymosan-induced mast cell degranulation led to a substantial reduction, though not complete, of peritoneal histamine concentrations within 30 minutes of inflammation (26). Furthermore, cromolyn was shown to alleviate inflammation by reducing the neutrophil percentage and FeNO levels (27). However, Weng et al. found that cromolyn was less effective than Quercetin in preventing the release of IL-8 and TNF from LAD2 mast cells activated by substance P (SP). Additionally, Quercetin decreased the secretion of IL-6 from human cord blood-derived cultured mast cells (hCBMCs) in a dose-dependent manner. Notably, Quercetin proved effective as a preventive measure, whereas Cromolyn needed to be added simultaneously with the trigger to remain effective (28).
Recent studies have questioned the effectiveness of CS in stabilizing both mouse (29) and human (28) cultured mast cells. Despite this, earlier research on the pharmacological actions of CS demonstrated its ability to inhibit mediator release through mast cells (5). This agent was found to enhance healing and reduce inflammatory responses in the nose, trachea, and lungs by modulating the expression of IL-6, TNF-α, TLR3, and TRIF in a rat model of influenza (30). Currently, CS is considered to assist individuals with COVID-19 by reducing inflammation and cytokine storms (31, 32).
Prostaglandins play a critical role in inflammation, whereas anti-inflammatory cytokines such as IL-10, IL-4, IL-6, and IL-13 inhibit the production of prostaglandins and cyclooxygenase-2. The synthesis of increased prostaglandins is mediated by COX-2. Our findings align with those of multiple previous studies (25, 33, 34). Research indicates that IL-10 is a potent inhibitor of macrophage activation, blocking the production of TNF-α, IL-1, IL-8, and granulocyte-macrophage colony-stimulating factor (GM-CSF) by human monocytes (35). The introduction of IL-10 to human monocytes modulates IL-8 production. The comprehensive effect of IL-10 on inflammatory cells may play a significant role in regulating the body's response to IL-1. According to Hashimoto et al., IL-10 specifically blocks GM-CSF-induced signaling events to prevent monocyte survival dependent on GM-CSF, but IL-10 must be administered within 48 hours of GM-CSF stimulation to exert an inhibitory effect (36). Wang et al. (37) discovered that cromolyn significantly reduced a broad range of inflammatory mediators, including cytokines such as IL-1, IL-6, and IL-8. In a notable clinical trial, patients with early Alzheimer's disease (AD) are treated with low-dose ibuprofen and cromolyn (25). Recent advancements in understanding immune-mediated mechanisms in metabolic contexts reveal that cromolyn plays a role in modulating immunity by either enhancing or suppressing immune responses. It has been shown that inhibiting macrophage activity and stabilizing mast cells with CS can alter the immune system, inducing varied cytokine patterns and adhesion molecule release (35, 37). Wang et al.'s study involved a low dose of NSAIDs (ibuprofen) and a fluorinated analog of cromolyn in AD treatment. This study introduced specific doses of cromolyn to prevent the development of inflammatory diseases involving interleukins. Unlike Wang et al.'s study, which focused on cromolyn's anti-inflammatory effect on the neurodegenerative disease Alzheimer's, this research aimed to explore cromolyn's anti-inflammatory impact on peripheral tissues. The findings suggest that CS inhibits the initial stage of carrageenan-induced paw edema, demonstrating NSAID-like properties. Developing mast cell inhibitors may aid in treating inflammatory and neurodegenerative diseases, as well as allergy disorders, as shown by this and Wang et al.'s study (37).
4.1. Conclusions
Cromolyn sodium has very low bioavailability (less than two percent) when administered orally. Hence, oral use is not recommended. It is usually administered via inhalation in humans, targeting primarily the lungs, and the inhaled form is used to alleviate allergy symptoms and shortness of breath. However, in this study, higher doses were administered intraperitoneally in rats to relieve inflammation symptoms and achieve systemic drug distribution in all organs except the brain. While an injectable form of cromolyn has not yet been produced or used, if its effectiveness and efficacy in reducing inflammation and inflammatory cytokines are well confirmed, considering its limited side effects and high patient tolerance, this form could be a suitable and safe alternative to NSAIDs, particularly in cases of digestive issues caused by NSAIDs. In summary, this study demonstrated that cromolyn possesses anti-inflammatory properties, making it a potential candidate for inclusion in the pharmacological treatment of inflammatory diseases, such as rheumatoid arthritis and inflammatory bowel disease, due to its lack of serious side effects.