1. Background
Constipation is one of the most common digestive complaints, affecting up to 16% of the general population (1). Constipation can cause significant problems for individuals and greatly reduce their quality of life. In addition, constipation imposes high economic costs on the individual and society, leading to substantial healthcare resource expenditure for its diagnosis and treatment (2-4). As conventional treatments have been satisfactory for only about one-third of patients (5), finding effective treatment strategies remains of utmost importance.
Constipation has various possible causes, with impaired gastrointestinal movements being the most common cause. Ample evidence demonstrates that alteration of gut microbiota may affect intestinal motility. Intestinal dysbiosis, which refers to an imbalance in gut microbiota characterized by a decrease in beneficial microorganisms and an increase in potentially pathogenic ones, may result in constipation. These changes can affect the metabolic environment of the large bowel by impairing bacterial fermentation metabolites, thereby reducing colon secretory function and transit time (6-8). This can be particularly attractive to gastrointestinal researchers.
The World Health Organization has defined probiotics as live bacteria or yeasts that are available in specific foods or supplements (9). Probiotics could potentially influence an individual's health by modulating the gut microbial flora if administered in appropriate doses (10). In cases with a disturbance in the content of intestinal flora, probiotics can be suggested as a supportive treatment (11). Moreover, improvement in constipation symptoms, including softening the stool, increasing colonic transit, and subsequently, more frequent defecation, have been observed after probiotics administration (12). Prebiotics, which are indigestible compounds, serve as food for probiotics. Ongoing evidence has revealed the role of prebiotics in the treatment of functional constipation (FC) (13). The combination of probiotics and prebiotic fibers, known as synbiotics, may manifest synergistic effects on improving human health and preventing diet-related diseases (14).
2. Objectives
According to the fact that combination therapy with different strains of probiotics is more effective than single therapy (15), the present study was designed to evaluate the effect of a commercially available product in Iran. The efficacy of this product, which contains both prebiotics and multispecies probiotics, was assessed on FC in adults. In this study, a 4-week experiment was conducted to evaluate the efficacy of the supplement in both men and women who were not taking any drugs or agents known to influence FC.
3. Methods
3.1. Study Subjects
Patients (aged more than 18 years) with FC were screened against Rome IV criteria to be included in the current study. This randomized controlled trial was performed from January to June 2021 at gastroenterology clinics in Sari, Iran. According to Rome IV (16), patients must have 2 or more of the following criteria for the last 3 months with symptom onset at least 6 months prior to diagnosis during more than 25% times of defecations: (a) Straining, (b) lumpy or hard stools, (c) sensation of incomplete evacuation, (d) sensation of anorectal obstruction or blockage, (e) manual maneuvers, and (f) fewer than 3 defecations per week. Moreover, patients fulfilling the Rome IV criteria are not allowed to meet the criteria for irritable bowel syndrome (IBS), and loose stools must rarely be present without the use of laxatives.
Exclusion criteria included the following: Patients with constipation secondary to diseases (such as metabolic disorders, hypothyroidism, hypercalcemia, and malignancy), medication (such as opium, alcohol, antidepressants, iron supplements, statins, cholestyramine, COX-2 inhibitors, anticholinergics, sulfasalazine, steroid inhalers, opioid analgesics, using laxative within 2 last weeks, and oral steroid one month before or during the study), diseases (such as cancer, IBS, depression, and systemic diseases such as diabetes mellitus, hypothyroidism, and inflammatory bowel disease (IBD)), previous surgery (bowel resection, prostate, and lumbar), pregnancy and breastfeeding, and patients with alarm symptoms (weight loss and anemia).
The current clinical trial followed the ethical principles of the Declaration of Helsinki and Good Clinical Practice guidelines. All patients signed informed consent before initiating treatments, and their medical and nutritional histories were thoroughly collected and recorded prior to the intervention. The study has been conducted in accordance with the CONSORT statement.
The protocol of this study was approved by the Human Research Ethics Committee of Imam Hospital at the Mazandaran University of Medical Sciences (IR.MAZUMS.IMAMHOSPITAL.REC.1399.067) and registered on www.irct.ir as IRCT20200915048726N1.
The sample size was determined considering α = 5%, corresponding to a confidence level of 95% and a power (1 – beta) of 90%. The following formula was used based on the mean and standard deviation (SD), resulting in a sample size of 84 individuals for both the control and treatment groups. Additionally, accounting for a 10% probability of sample dropouts, finally, a total of 187 patients suffering from constipation were included to screen for eligibility.
3.2. Randomization and Masking
This clinical trial with parallel control and interventional groups was performed on 115 patients. Each patient was assigned a unique code using a computer-generated numerical sequence. To minimize confounding effects, all participants were randomly allocated (1: 1) into groups by a balanced block randomization scheme (block sizes of 4). Envelopes containing psyllium sachet or synbiotic capsules related to the control or case group were prepared by researcher physicians and coded according to the patients' unique codes. Corresponding coded closed envelopes were assigned to each patient at the time of their first visit to eliminate selection bias in the randomization process. Also, the intake instructions for the drugs were placed into the envelopes. The randomization resulted in 58 in the psyllium + synbiotic arm (case group) and 57 subjects receiving psyllium alone (control group).
Patients' codes were disclosed to physicians and outcome appraisers following randomization but remained confidential to statisticians. The codes were decoded by the principal researcher at the end of the analysis to interpret the results.
3.3. Drugs Administration
Psyllium was prescribed as a standard treatment for constipation. During the first visit, patients in both groups were instructed to take one sachet of psyllium (containing 10 - 15 mg), mixed in 250 mL water or juice, daily for the entire 4 weeks. The case group was requested to take synbiotics twice daily (one half an hour before breakfast and one before dinner), in addition to psyllium, for 4 weeks; furthermore, for a longer follow-up, they were instructed to continue taking two daily doses of synbiotic capsules alone for another 2 weeks. They were advised to keep the capsules in a refrigerator. All patients were advised not to change their routine diet or physical activity, avoid using chemical or herbal laxatives, refrain from consuming other probiotic-containing products or supplements, and avoid using digital assistance or enema during the treatment period.
3.4. Baseline Data
A questionnaire included five domains of demographic data: Stool consistency, stool volume, bloating severity, and constipation intensity was considered for each patient. Prior to the interventions, the questions were asked, and one researcher recorded data.
3.4.1. Evacuation Categorization
The stool consistency based on the Bristol stool form was scored with 7 items, including 1-nuts-like, 2-lumpy, and sausage, 3-sausage with cracks, 4-smooth snake, 5-soft blobs, 6-fluffy pieces or mushy, 7-watery with no solid pieces that appear upon defecation. Types 1 and 2 indicated constipation. The ideal stool, which was easily defecated while not containing excess liquid, was defined with types 3 and 4. Type 5 indicated a lack of dietary fiber, and types 6 and 7 defined diarrhea (17). Bloating severity was graded into 3 classes: Mild, moderate, and severe, scored from 1 to 3. The overall score was reported as the mean score of all patients. Additionally, patients were asked about stool volume according to three classifications of amount (1- low, 2- moderate, and 3- high), and the frequency of each grade was calculated as a percentage (%).
3.4.2. Constipation Intensity Categorization
To assess the severity of constipation, the Wexner Constipation Scoring System (WCSS) with 8 characteristics was asked and recorded for each patient. This system consists of the following symptoms: Frequency of bowel movements, difficulty or painfulness of evacuation, incomplete evacuation sensation, abdominal pain, length of time per attempt, unsuccessful efforts for evacuation per 24 hours, duration of constipation, and type of assistance for defecation. All items were rated on a 5-point Likert scale with a minimum score of 0 (absence of symptom) and a maximum score of 4 (very severe symptom), except for the 8th item, which was defined with scores of 0 (without assistance), 1 (laxative), and 2 (digital assistance/enema). The overall score (0 - 30) was the sum of all 8 items, which represented the overall assessment of the severity of constipation for each patient. A decrease in the score meant a decrease in the severity of constipation. Wexner scores of 1 - 10, 11 - 20, and 21 - 30 were considered as mild, moderate, and severe constipation, respectively (18).
3.5. Clinical Response Evaluation
All patients were given self-report daily forms related to data on stool evacuation, constipation severity, and bloating severity, the same as questions completed by the researcher at the first visit. Sufficient information to complete the questionnaires accurately was also provided. The only difference in the questions was about stool volume, which instructed to answer three states: Unchanged, decreased, or increased. Then, the final result was presented as the percentage of those who reported a bulky stool.
The clinical responses to treatments were evaluated through patient follow-up. The evaluation sessions were scheduled biweekly, and patients were contacted by phone by the same researcher to answer a questionnaire. The purpose was to verify the intake of psyllium and synbiotics and the adherence to the treatment, as well as to inquire about any adverse events and issues related to data recording. At the end of the follow-up period, data on the patients' impressions of changes in their constipation status were collected. The final scores for each domain (categorizations of evacuation and constipation intensity) were calculated based on the mean scores of questionnaires regarding both treatments. The clinical response to treatments (improvement of symptoms) was evaluated by considering the final scores of the domains, which were represented using a 1 to 4 Likert scale as very effective, effective, partially effective, and not effective. The first three degrees were considered indicative of the treatment's efficiency for final evaluation.
3.6. Characteristics of Supplements
The content of the synbiotic mixture (GeriLact) is shown in Box 1. This compound is a multispecies (probiotic + prebiotic) formulation that contains high amounts (109 colony forming units (CFU)) of 7 safe and beneficial bacterial strains plus fructooligosaccharides (FOS) as a prebiotic. This gluten-free product is manufactured in capsule form by Zist Takhmir Co., Tehran, Iran (19).
| Synbiotic Content |
|---|
| Microbial content a |
| Lactobacillus rhamnosus NCIMB 30188 |
| Lactobacillus casei NCIMB1 30185 |
| Bifidobacterium breve NCIMB 30180 |
| Lactobacillus acidophilus NCIMB 30184 |
| Bifidobacterium longum NCIMB 30182 |
| Streptococcus thermophilus NCIMB 30189 |
| Lactobacillus bulgaricus NCIMB 30186 |
| FOS |
Abbreviation: FOS, Fructooligosaccharide.
a All 109CFU
3.7. Statistical Analysis
Data from this study were analyzed using SPSS software (version 25; SPSS Inc., Chicago, IL, USA). According to the Kolmogorov-Smirnov test, all quantitative parameters had a normal distribution. For descriptive statistics, the mean ± SD of the variables was calculated. Bonferroni´s correction was used for multiple comparisons between groups in each session (baseline, 2nd, and fourth weeks). An analysis of variance (ANOVA) for repeated measurements and generalized estimating equations (GEE) were used to compare the results from the beginning to the end of the treatment in each group. All participants were included in the intention-to-treat (ITT) analysis. Those patients who followed the whole protocol until the end of the treatments were included in the per-protocol (PP) analysis. In the current study, the P-value < 0.05 was considered statistically significant.
4. Results
4.1. Patients
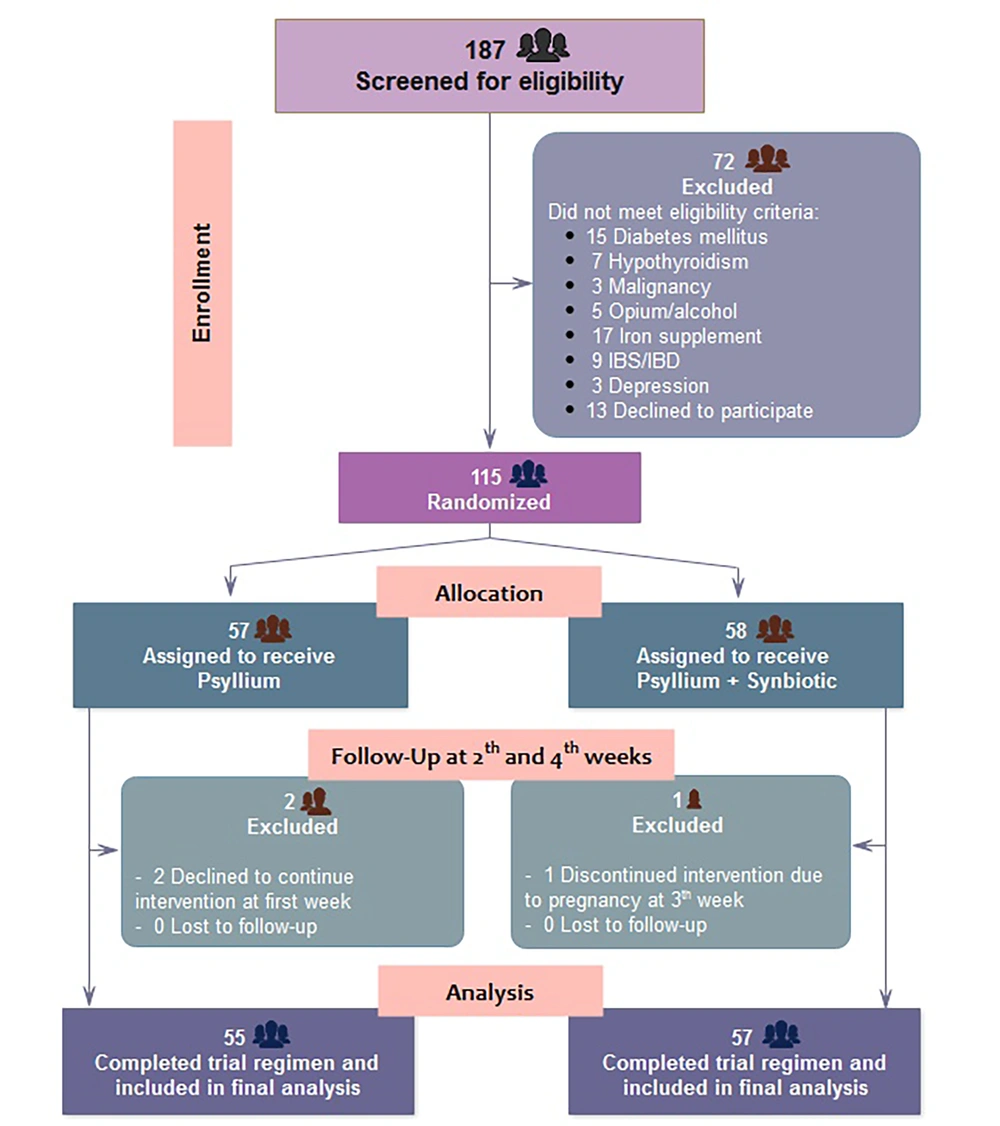
Out of 187 patients assessed for eligibility, a total of 72 individuals who did not meet the inclusion criteria were excluded. Therefore, 115 were enrolled and randomly assigned to receive psyllium alone (n = 57) or psyllium + synbiotic (n = 58). After excluding 2 patients who did not receive at least one dose of the study drugs and 1 who discontinued intervention before completing the study due to pregnancy, 112 patients entered the final analysis population (Figure 1).
Consort diagram of the population participating in the study. One hundred thirteen eligible patients were enrolled in the randomized trial. Analysis differed based on patients who were enrolled (ITT: Intention to treat) or completed the study (PP: Per-protocol) in the synbiotic-containing group because one patient was excluded due to pregnancy at the 4th-week of follow-up.
As shown in Table 1, there was no significant difference in gender between the two groups (P = 0.109). The mean age of patients in the control and case groups was 41.36 ± 15.41 and 43.45 ± 14.92, respectively (P = 0.467). But in both groups, males had a significantly higher mean age than females (P = 0.001 and P = 0.003, respectively). Body mass index (BMI) was not significantly different between the two groups (P = 0.798). Additionally, a higher BMI than the normal range (18.5 - 24.9) was observed in 54.5% of patients in the psyllium group and 67.2% of patients in the psyllium + synbiotic group (P = 0.167). We also found no significant difference in BMI between male and female participants in any of the groups (P = 0.127 and P = 0.883, respectively). Our results also showed no statistically significant difference in the distribution of patients regarding their education level (P = 0.662), occupation (P = 0.458), and place of residence (P = 0.885) between the two groups (data not shown).
a Values are presented as mean ± SD or No. (%).
b Comparison between the two groups by chi-square tests or independent t-tests.
c Comparison between males and females by independent t-test.
4.2. Evacuation Assessment
As shown in Table 2, treatment with psyllium alone for 4 weeks did not result in a significant increase in fecal consistency or volume, nor did it lead to a reduction in bloating intensity. However, 4 weeks of administration of psyllium + synbiotic caused a significant improvement in all three variables. The results demonstrated that psyllium + synbiotic had a notable increase in stool consistency from the second week in comparison to psyllium alone. (P = 0.015). However, a significant improvement in stool bulk or bloating was found from the fourth week between the two groups (P = 0.004 and P < 0.0001, respectively).
| Evacuation Categorization | Groups | P-Value b | |
|---|---|---|---|
| Psyllium | Psyllium + Synbiotic | ||
| Stool consistency | |||
| Baseline | 0.82 ± 2.35 | 0.84 ± 2.09 | 0.101 |
| 2nd week | 0.95 ± 2.65 | 0.76 ± 3.05 | 0.015 c |
| 4th week | 1.18 ± 2.83 | 1.06 ± 3.76 | 0.0001 c |
| P-value d | 0.135 | < 0.001 c | |
| Bulky stool | |||
| Baseline | 15 (27.3) | 6 (10.3) | 0.029 c |
| 2nd week | 25 (45.5) | 20 (34.5) | 0.236 |
| 4th week | 26 (47.3) | 43 (74.1) | 0.004 c |
| P-value d | 0.06 | < 0.001 c | |
| Bloating severity | |||
| Baseline | 2.40 ± 0.74 | 2.45 ± 0.71 | 0.722 |
| 2nd week | 2.29 ± 0.72 | 2 ± 0.68 | 0.028 c |
| 4th week | 2.27 ± 0.73 | 1.64 ± 0.77 | < 0.001 c |
| P-value d | 0.611 | < 0.001 c | |
a Values are presented as mean ± SD or No. (%).
b Comparison between the two groups by independent t-test or chi-Square tests.
c Significant values (< 0.05).
d Comparison between baseline, 2nd, and fourth weeks by repeated measures ANOVA or generalized estimating equations.
4.3. Constipation Intensity Assessment
Characteristics regarding constipation intensity are tabulated in Table 3. We investigated whether treatments were effective over time. The evaluation of 8 constipation severity indexes from the baseline to the fourth week showed that treatment with psyllium alone added no significant improvement in any of the indexes, except for those who needed significantly less assistance for evacuation compared to before the treatment (P = 0.003). In contrast, synbiotic administration resulted in significant improvements in all constipation indexes at the end of the study.
| Constipation Severity Categorization | Groups | P-Value b | |
|---|---|---|---|
| Psyllium | Psyllium + Synbiotic | ||
| Frequency of bowel movement | |||
| Baseline | 1.95 ± 1.04 | 1.98 ± 1.07 | 0.851 |
| 2nd week | 1.73 ± 1.08 | 1.33 ± 0.94 | 0.039 c |
| 4th week | 1.53 ± 1.20 | 1.0 ± 0.92 | 0.01 c |
| P-value d | 0.145 | < 0.001 c | |
| Time: Lavatory per attempt (min) | |||
| Baseline | 1.58 ± 0.94 | 1.52 ± 0.82 | 0.698 |
| 2nd week | 1.38 ± 0.91 | 1.05 ± 0.71 | 0.034 c |
| 4th week | 1.36 ± 0.99 | 0.79 ± 0.64 | < 0.001 c |
| P-value d | 0.409 | < 0.001 c | |
| Difficulty: Painful evacuation effort | |||
| Baseline | 2.04 ± 1.48 | 2.28 ± 1.52 | 0.101 |
| 2nd week | 1.76 ± 1.41 | 1.50 ± 1.22 | 0.015 c |
| 4th week | 1.69 ± 1.46 | 1.09 ± 1.11 | < 0.001 c |
| P-value d | 0.423 | < 0.001 c | |
| Abdominal pain | |||
| Baseline | 2.15 ± 1.54 | 2.22 ± 1.51 | 0.785 |
| 2nd week | 1.98 ± 1.50 | 1.71 ± 1.26 | 0.294 |
| 4th week | 1.76 ± 1.51 | 1.17 ± 1.17 | 0.022 c |
| P-value d | 0.419 | < 0.001 c | |
| Completeness: Feeling incomplete evacuation | |||
| Baseline | 1.95 ± 1.58 | 2.76 ± 1.44 | 0.005 c |
| 2nd week | 1.85 ± 1.51 | 1.90 ± 1.37 | 0.877 |
| 4th week | 1.76 ± 1.51 | 1.38 ± 1.40 | 0.165 |
| P-value d | 0.824 | < 0.001 c | |
| Failure: Unsuccessful evacuation efforts/24 h | |||
| Baseline | 1.0 ± 0.67 | 1.16 ± 0.62 | 0.202 |
| 2nd week | 1.0 ± 0.72 | 1.02 ± 0.48 | 0.882 |
| 4th week | 0.98 ± 0.71 | 0.90 ± 0.45 | 0.448 |
| P-value d | 0.988 | < 0.001 c | |
| Assistance: Type of assistance | |||
| Baseline | 1.04 ± 0.72 | 1.17 ± 0.70 | 0.312 |
| 2nd week | 0.75 ± 0.8 | 0.22 ± 0.53 | < 0.001 c |
| 4th week | 0.52 ± 0.77 | 0.15 ± 0.49 | < 0.001 c |
| P-value d | 0.003 | < 0.001 c | |
| History: Duration of constipation (y) | |||
| Baseline | 1.87 ± 0.90 | 2.0 ± 1.04 | 0.489 |
| Total score | |||
| Baseline | 13.56 ± 4.48 | 15.09 ± 4.18 | 0.312 |
| 2nd week | 12.33 ± 5.19 | 10.72 ± 4.18 | 0.065 |
| 4th week | 11.49 ± 5.72 | 8.41 ± 4.1 | 0.002 c |
| P-value d | 0.109 | < 0.001 c | |
a Values are presented as mean ± SD.
b Comparison between the two groups by independent t-test.
c Significant values (< 0.05).
d Comparison between baseline, 2nd, and 4th weeks by repeated measures ANOVA.
No significant difference was observed in constipation severity indexes at baseline between the two groups, but the patients in the psyllium group reported less incomplete evacuation than the other group (P = 0.005). At the 2nd-week assessment, patients receiving synbiotics exhibited a significant reduction in the frequency of bowel movements, time in lavatory per attempt, difficulty or painful evacuation effort, and need for assistance.
The overall score obtained from the total scores of 8 indexes showed that the initial constipation severity observed in both the psyllium and psyllium + synbiotic groups was moderate, considering WCSS (13.56 and 15.09, respectively). Interestingly, psyllium administration led to a reduction in the severity of constipation, although this difference was not statistically significant (13.56 ± 4.48 vs. 11.49 ± 5.72, P = 0.109). In contrast, the total score in the synbiotic-receiving patients decreased significantly from 15.09 ± 4.18 at baseline to 8.41 ± 4.1 in the fourth week of the study (P < 0.001). Comparing the endpoint of the two therapies, the improvement of all constipation severity indexes was still in favor of synbiotic-containing treatment.
4.4. Effectiveness of Treatments
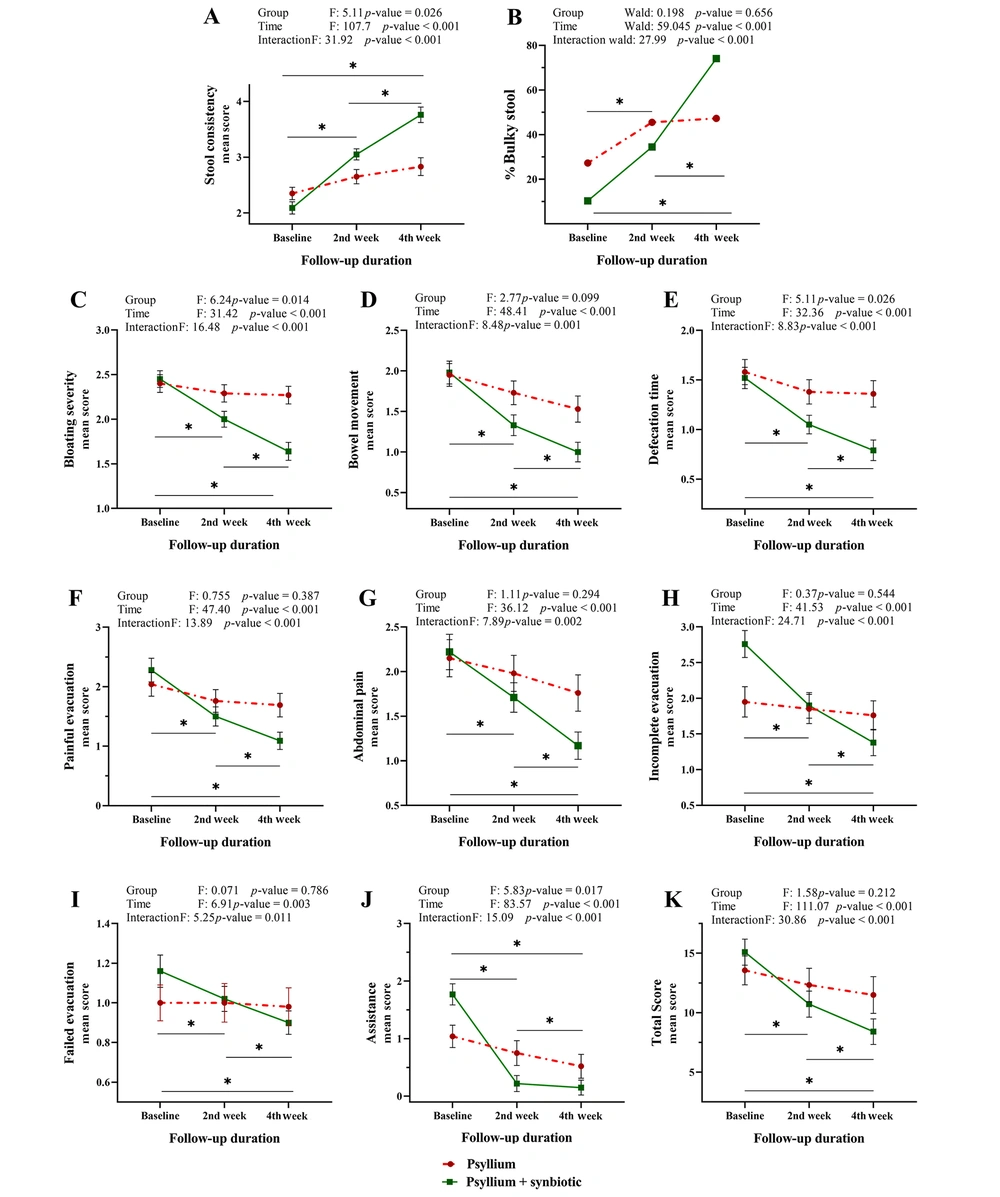
We further aimed to determine whether the treatment response rate was affected by the type of treatments, intake duration, or both. Our analysis (Appendix 1) revealed that while significant differences between groups were not seen for some indexes, the duration of treatment had an influential role in intervention effectiveness. Furthermore, the significance of the combined effect of treatment groups and time indicated that the positive effect of synbiotics on the final improvement of evacuation and constipation (in all indexes) was influenced by the duration of administration (Figure 2). In summary, treatment along with synbiotics for 4 weeks yielded the best outcomes.
The interaction effect of time and type of treatment on related indexes of constipation. Patients treated with synbiotics achieved a significant improvement in constipation. However, significant changes were not observed in the psyllium-alone receiving group. The main effects and interactions of the treatment group and administration duration on the rate of treatment response were evaluated by repeated measures ANOVA. Line graphs illustrate means as symbols and SD at the whiskers. Intersecting lines on the plot indicate an interaction. Following a significant repeated measures ANOVA, a Bonferroni post-hoc test was applied to adjust for multiple comparisons: the time intervals were compared pairwise in the synbiotic-containing group, *P < 0.05, **P < 0.01 shown as a line.
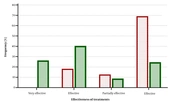
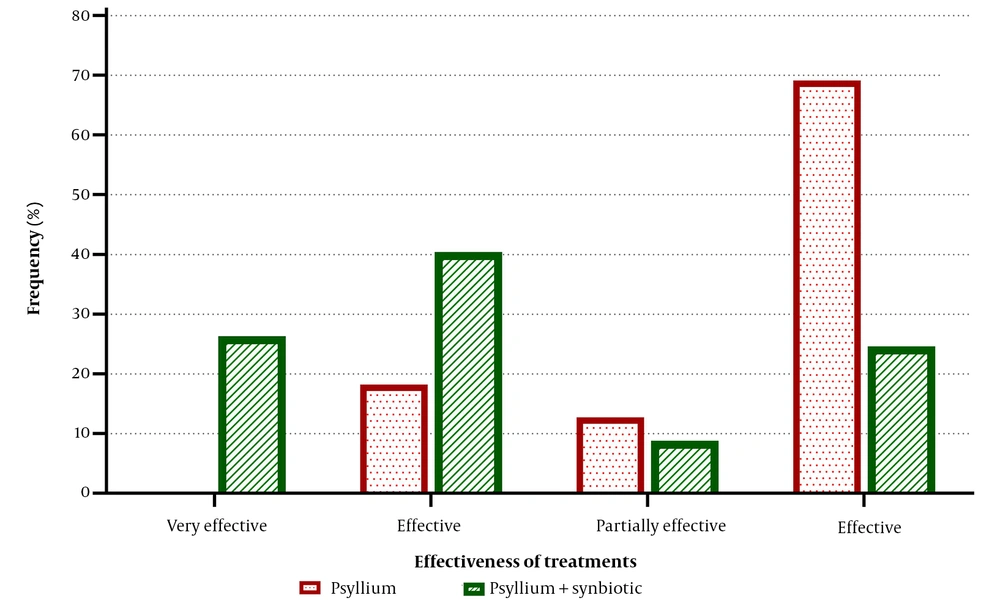
The final assessment of the treatment response revealed that the psyllium + synbiotic intervention was 75.86% effective according to ITT analysis and 75.44% effective based on PP analysis. In contrast, the psyllium alone was effective in 34.48% and 30.91% of cases based on the two analyses, respectively. A statistically significant difference was found between the two groups in terms of their effectiveness in treating FC (Figure 3, P < 0.001).
The effectiveness of treatments. The evaluation was performed at the end of the treatments. Of the synbiotic-containing group, 75.44% considered the treatment to be very effective, effective, or partially effective, compared to only 30.91% in the psyllium-alone group. Conversely, patients in whom the treatment was not effective were more common in the psyllium group than in the synbiotic + psyllium group (69.09% vs. 24.56%). These data are presented based on the per-protocol (PP) analysis, and the comparison was performed using the chi-Square test (P < 0.001).
Concerning the results above regarding the effect of treatment duration, it was postulated that longer use of synbiotics might further improve the outcomes. The finding revealed that continuing sole treatment with synbiotics for up to 6 weeks yielded better improvements in all indexes, regardless of the non-significance of some observed differences (Appendix 2).
Considering the influence of confounding variables, we found that the age, BMI, education level, occupation, and urban or rural residency of the patients enrolled did not affect the efficacy of the treatments. No correlation was found between these factors and treatment effectiveness in any groups (data not shown). No adverse effects were noted in either of the groups (P = 0.924). Psyllium was administrated in powder form, which was found to be less tolerable than the synbiotic administered in capsule form (P = 0.026). Moreover, patients were informed about the potential problems of the therapies (e.g., bloating, stomach pain, nausea, etc.) before the trial started. They were also in contact with the researcher, but there were no reports of problems during the intervention period.
5. Discussion
The present study revealed that administering a multispecies synbiotic supplement for 4 weeks resulted in significant improvements in the clinical manifestations of patients suffering from constipation. We achieved a 75.44% efficacy in treating FC with synbiotics (P < 0.001), whereas only 30.91% of patients receiving psyllium alone exhibited improvement (P = 0.109). This may indicate the superiority of the synbiotic supplement compared to psyllium alone. Notably, this is the first documented outcome of a commercial Iranian synbiotic mixture containing a combination of seven beneficial bacterial species and a prebiotic without gluten among Iranian adult volunteers with FC.
Conflicting effects of probiotics have been reported in previous studies, as described below. Del Piano et al. conducted a study in which the administration of mixed Lactobacillus plantarum LP01 and Bifidobacterium breve BR03 or Bifidobacterium animalis subspecies lactis BS01 resulted in significant relief of evacuation disorders and hard stools in both of their studied groups (20). Mazlyn et al. showed that L. casei strain Shirota did not significantly alleviate constipation severity and frequency compared with placebo (21). In another clinical trial, supplementation of Bifidobacterium animalis subsp. lactis HN019 did not improve bowel transit time and quality of life in adults with FC (22).
It appears that using single- or two-strain probiotics in previous studies has been a limitation in achieving improvements in all aspects of constipation, as a mixture of prebiotic and multispecies probiotics used in this study yielded opposite results. We found a statistically significant improvement in stool consistency, stool frequency, and a reduction in bloating intensity with the current synbiotic. Moreover, significant improvements in Wexner constipation severity scores were observed. Patients receiving psyllium + synbiotic experienced a significant decrease in Wexner's total score from baseline to the fourth week of the study (from 15.09 to 8.41, P = 0.002), representing an improvement in constipation severity and quality of life. However, administration of psyllium alone did not show a significant decrease in total score (from 13.56 to 11.49, P = 0.109). However, a recent study by Cheng et al. reported conflicting results regarding the effect of psyllium (23). They observed high satisfaction rates for psyllium (71.4% of cases) and psyllium + lactitol (69.8% of cases) in terms of bowel movement frequency, increased stool volume, and moisture content. It should not be overlooked that neither of their two treatment groups differed significantly from the placebo group, with an efficacy rate of 75%.
Given the evidence mentioned, comparing the current study to previous ones is challenging due to variations in sample size, evaluation methods, and wide differences in the type, dosage, and duration of prescribed probiotics. The present study went beyond previous studies by continuing synbiotic treatment for up to 6 weeks, which is longer than the typical 2- to 4-week period seen in other studies (20, 21, 24-27). This unique approach adds novelty to the study and provides an opportunity to assess the long-term efficacy of synbiotics in managing FC. Importantly, despite the improvement in FC symptoms during the first 4 weeks of synbiotic treatment, a significant further improvement was observed in the patient's condition in the sixth week (from 8.41 to 6.63, P = 0.009), highlighting the potential effectiveness of this treatment. Therefore, the strength of the present study can be attributed to its longer-term treatment and follow-up, which could serve as a valuable model for future investigations.
Previous studies have reported good tolerance and the absence of side effects associated with probiotics (28, 29). Similarly, we observed no side effects of synbiotics and psyllium, which may confirm the safety of these therapeutic compounds in constipation (23, 24). Furthermore, the synbiotic was administered in capsule form, which may have contributed to greater compliance compared to psyllium powder.
The main limitation of the present study was the small sample size in the treatment groups, which was due to the coinciding COVID-19 pandemic at the time of the study. However, despite this limitation, the number of participants in this study exceeded that of recent studies investigating constipation improvement (23, 24). It should be noted that as we specifically investigated the effects of a particular supplement, the present findings may not be generalizable to other supplements. Furthermore, the varying amounts of pre-and probiotics present in different commercial supplements may limit the comparability of our results to those of previous studies.
5.1. Conclusions
Our results suggest that mixtures of different strains of probiotics with prebiotics may be more effective than either alone in the treatment of FC, as improvements were observed in all aspects of this condition. Our use of a multispecies synbiotic improved stool consistency, stool volume, and all constipation severity indexes according to the WCSS, with a significantly lower overall Wexner score in the fourth week of treatment compared to psyllium alone. Administration of this supplement could be considered a suitable therapeutic approach for FC due to its high efficacy, good tolerability, lack of side effects, and compatibility. However, further studies with more participants may be needed to confirm the effectiveness of this supplement in improving FC.