1. Background
In spite of dramatic improvements in cardiac surgery processes and anesthesia over the past 50 years, substantial stress due to such major surgical interventions still severely affect clinical outcomes (1). Available literature identified that major surgeries such as on-pump coronary artery bypass graft surgery (CABG) induce profound and sometimes prolonged systemic inflammatory responses comparable with those of the systemic inflammation response syndrome (SIRS) (2, 3). It is well established that surgery related stress responses with any degrees of severity trigger tissue injury and several complex pathways are activated following inflammatory response in which apoptosis contributes to multi organ failure (4). Multiple stimuli are recognized in this process including blood exposure to artificial surfaces, ischemic-reperfusion injury, endotoxemia due to splanchnic hypo perfusion injury and temperature fluctuations, release of inflammatory mediators, and reactive oxygen species (ROS) generation (3). Among the mentioned modulating factors, CPB is known as a major reason to induce inflammatory responses and neutrophil activation. The available data demonstrate that miniaturized CPB, instead of conventional CPB, attenuates inflammation (5). When inflammatory mediators are released, increased endothelial cell permeability, generation of free oxygen radicals, coagulopathy, micro circulate dysfunction, alternation in immunologic function, and tissue damage occur, which result in organ failure (6). Well-established correlation between such inflammatory cascades and the development of adverse clinical outcomes focused interest on searching for novel strategies with more cardio protective properties. Theoretically, any modality with anti-inflammatory effects might lead to better outcomes (7). Several mediators such as tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-6, IL-8, and C-reactive protein (CRP) are identified as oxidative stress markers following major surgeries and different strategies are applied to blunt them with different degrees of success. According to the complex nature of inflammatory reactions and triggering factors, it is not surprising that till now no universal approach with proven cardio protective effects is determined (8-13).
Recently, trace elements are supposed to be promising candidates in this respect. They are essential cofactors in antioxidant (AOX) enzymes function involved in body defense against inflammatory reactions. Therefore, the drop of their concentration, which occurs during CABG surgery, might be the predisposing factor of a systemic inflammatory response (14). Among them, Se is an essential micronutrient for human health with supporting antioxidant defense systems capabilities. It is one of several agents targeting inflammatory pathways investigated in several clinical and experimental studies with success (15-17). In spite of available studies that clinically applied Se in different conditions to achieve tissue protective effects, no study evaluated the relationship between this supplementation and white blood cell (WBC) count in CABG surgery.
2. Objectives
The current study aimed at assessing the anti-inflammatory properties of Se in CABG surgery.
3. Methods
3.1. Setting
The current randomized, double-blind clinical trial was conducted in Dr. Heshmat Hospital from May 2015 to September 2015. This teaching hospital is a specialized and referral center for different types of cardiac surgeries and is affiliated to Guilan University of Medical Sciences (GUMS), Rasht, Iran.
3.2. Study Participants
Patients were visited for a physical examination, a medical history, and also their demographic data. After considering the inclusion criteria, informed consent was obtained from all participants. Baseline laboratory data were recorded and subjects were followed to record the next results.
3.3. Inclusion Criteria
Patients aged 30 - 65 years with ASA (the American Society of Anesthesiologists) class II and III candidates for isolated elective CABG under CPB were selected as the study samples.
3.4. Exclusion Criteria
Trace element supplements during the past month, poor controlled diabetes, thyroid disease, liver or renal dysfunction, pregnancy, immunosuppression therapy and malignant diseases, a history of chemotherapy or radiation exposure, anemia, all conditions with underlying inflammatory (e. g. inflammatory bowel disease, rheumatoid arthritis) autoimmune disease, smoking, primary hematologic disease, ejection fraction < 40% - 45%, and not giving informed consent.
3.5. Sample Size
With a margin of error α = 0.05 and β = 10%, an expected power of 90%, and a Z value of 1.28, it was calculated that a sample of at least 55 patients in each group was required.
3.6. Randomization and Blinding
Based on randomized fixed quadripartite blocks, 114 eligible patients with an equal probability of being assigned to each group were divided into two groups of Se (S) and control (C). The patients and investigators recording the type of injection were blinded, but the responsible anesthesiologist was aware of it.
3.7. Intervention
In the Se group, an intravenous bolus of 600 µg Se (vial 50 µL/mL; Fellbach Germany) diluted with 20 mL sodium chloride 0.9% was slowly administrated just before induction of anesthesia and the group C received the same amount of normal saline as placebo. The measured outcome included leukocyte count as the index of inflammation, at four measurement time points: Preoperatively (T0), and three times after CPB initiation including immediately after the end of CPB (T1), as well as 24 (T2) and 48 hours (T3) after surgery.
3.8. Anesthesia and Surgery
The standard anesthesia and surgical protocol of the hospital was followed. Anesthesia was induced with midazolam 0.05 mg/kg, sufentanil 1 µg/kg, and etomidate 0.2 mg/kg. In order to prevent myoclonus linked to etomidate, a low dose of 0.03 mg/kg was intravenously used prior to the induction dose (18). Cisatracorium 0.2 mg/kg was administrated to achieve enough flaccidity for tracheal intubation. Thereafter, mechanical ventilation was started with intermittent positive pressure with a tidal volume of 8 - 10 mL/kg and positive pressure at the end expiration of 3 - 5 cmH2O. The timing and the type of any treatment within the surgery were according to the patients’ condition. To maintain hemoglobin > 9.5 g/dL and hematocrit > 25%, packed red blood cells were transfused if needed. Within the operation, mean arterial blood pressure was kept at 50 - 90 mmHg, arterial O2 saturation > 95%, End tidal CO2 35 - 40 mmHg, and BIS value of 40 - 60. Heparin was injected, 300 mg/kg into the central vein, and after 3 - 5 minutes an activated clotting time of more than 400 seconds was achieved. At the end of CPB, the effect of heparin was neutralized with protamine chloride at a ratio of 1:1. Patients were admitted to coronary care unit and received routine post operation care at least for 48 hours. When standard clinical criteria were fulfilled, weaning from ventilatory support and tracheal extubation were performed. Cooperation of the patient with stable vital signs, normal electrolytes profile, and arterial blood gas was considered as standard criteria.
3.9. Side Effects
In fact, significant adverse effects of Se such as carcinogenesis, cytotoxicity, genotoxicity hepatotoxicity, gastrointestinal disturbances, hair loss, dizziness, and pulmonary edema are reported following long-term drug administration (1, 19-21). However, the current study subjects were carefully monitored and in case of any adverse effects, not necessarily due to the treatment or clinical signs of Se, overdose happened, the patient was excluded from the study, immediate measurement of Se serum level and symptomatic therapy were performed.
3.10. Statistical Analysis
The data were analyzed using standard statistical methods provided by SPSS version 16 (SPSS Inc, Chicago, II). Chi-square test was performed to compare the categorical variables between the two groups. The Kruskal-Wallis test was applied to verify the hypothesis of the normal distribution of variables followed by parametric tests. Comparison and determination of the parametric data between the two groups were performed using independent t test. The parametric data in five time points were compared by repeated measurement test. All data were expressed as mean ± standard deviation and P values of less than 0.05 were considered significant.
4. Results
In the current study, 120 patients were randomly divided into selenium (S) and control (C) groups. In the group S, three patients needed valve repair during surgery and intra-aortic balloon pump was used for one. In the group C, two could not be extubated within the expected time. Finally, data from 114 patients were analyzed.
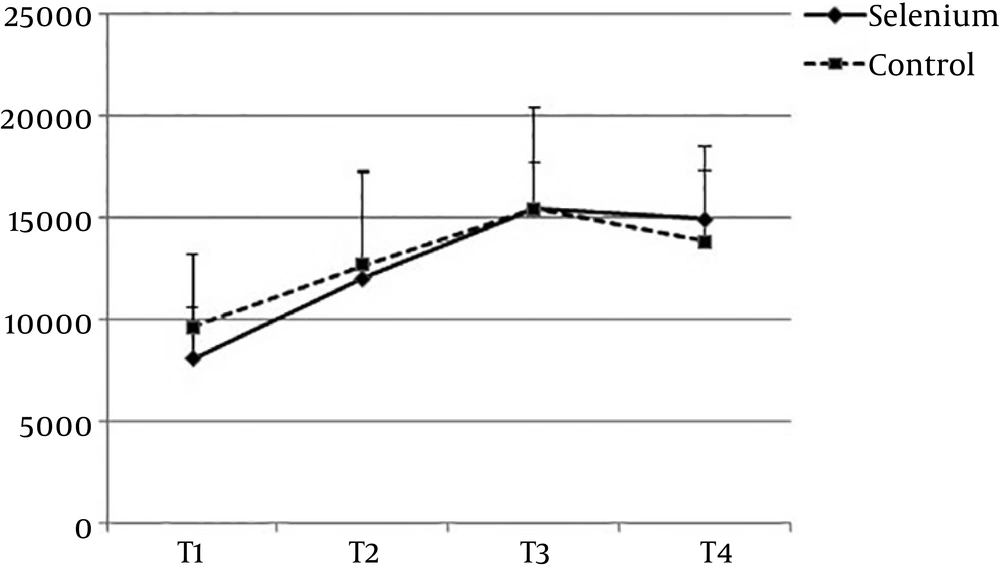
There was no significant difference between the two groups regarding the baseline characteristics (Table 1). Baseline peripheral white blood cell (WBC) count (P = 0.0512) showed no significant differences either (P = 0.0512) (Table 2). There was no significant difference between the two groups at the other time points: T1 (P = 0.571), T2 (P = 0.974), and T3 (P = 0.215) (Figure 1). But the trend of changes was statistically significant in each group (P = 0.0001). However, comparison of the two groups showed no significant difference between them (P = 0.166). None of the patients in the S group experienced adverse effects due to the intervention.
| Variable | Group | ||
|---|---|---|---|
| Placebo (N = 56) | Selenium (N = 58) | P Value | |
| Age, y | 58.84 ± 9.01 | 57.9 ± 8.85 | 0.58 |
| Weight, kg | 72.72 ± 6.98 | 74 ± 12.97 | 0.522 |
| Height, cm | 166.6 ± 5.83 | 167.9 ± 8.82 | 0.356 |
| BMI, kg/m2 | 29.04 ± 2.29 | 28.98 ± 4.05 | 0.876 |
| Gender, % | 0.268 | ||
| Female | 28.3 | 37.6 | |
| Male | 71.7 | 62.4 | |
| Operation time, min | 170.6 ± 17.08 | 173.14 ± 22.01 | 0.584 |
| Pump time, min | 590.8 ± 18.65 | 57.91 ± 13.7 | 0.38 |
| Clamp time, min | 38.07 ± 11.03 | 35.64 ± 9.41 | 0.189 |
| Ejection fraction, % | 49.03 ± 3.65 | 47.63 ± 8.97 | 0.586 |
Baseline Characteristics and Data of Surgerya
5. Discussion
The current study aimed at investigating the effects of Se on inflammation in CABG surgery. This trace element is administrated by different roots and a wide range of doses of 50 up to 4000 µg in clinical studies. No clearly proven evidence suggests that Se induces beneficial effects at the dosage lower than 500 µg. In the current study, concern about the studied high-risk cases with comorbidities, and also scanty available knowledge about pharmacodynamics and safety of Se, the applied dose was 600 µg, which presented just a little above the presumed effective dose (22-25). Experimental and human studies show that during the early phase of CABG, cellular components of hematopoietic system undergo significant alternations (26). According to the data from previous studies, expressing peripheral WBC count was one of the major components and a reliable marker of inflammation, selected as an index in the current study (27-29). Its measurement is easy with no need for especial kits, and can be performed with the equipment available in all laboratories. Therefore, it might help to detect more at risk patients. Strong evidence indicates that leukocytosis is a known risk factor for post-operative recurrent ischemic events one year following surgery and early post-operative events such as stroke (27-29). It is also demonstrated that the patients representing a greater increase in WBC count during CPB are at more risk for excessive bleeding (26). In addition, elevated WBC count was significantly correlated with the recurrence of atrial fibrillation in patients with CABG (29). Neutrophils were activated during inflammatory response stimulating plackets activation, and patients with increased WBC count following surgery, especially neutrophil subtypes, were strongly affected by thrombotic events (26). In fact, neutrophil derived cathepsin G and thrombin stimulate p-selectin expression on plackets, which modulates cellular interaction between plackets and neutrophils in thrombotic and inflammation states (30). In CABG, during the reperfusion period, inflammatory markers are generated and WBC is activated linked to the inflammation-induced tissue damage. In the differential count, a marked elevation in neutrophils, corresponding with band and immature forms are observed (26, 31). It is believed that the mobilization of the marginalized neutrophils and release of new ones from pulmonary and bone marrow lead to a sharp rise of total WBC count. Nevertheless, it should be considered that exact discrimination of an inflammation response due to surgery from the one secondary to infection in this particular population is not easy (26, 28). The complete blood cell count with differential test does not provide useful information to discern two specific causes of leukocytosis, since both conditions are manifested by significant elevation of band forms and immature neutrophils (26, 32); however, there are some guiding differences between them. Firstly, post CABG infection is more expected in patients with underlying conditions such as AIDS, chemotherapy, and hemodialysis (32). Second, studies showed that WBC count gradually rise during the early stages of surgery and after stopping the stimuli a slow recovery starts toward the pre-operative values. As a rule, if the WBC count continuous to increase or occurs after a period of decrease, it represents an infection source rather than an inflammatory reaction. On the other hand, studies show that in differential count of CABG related leukocytosis, neutrophils predominantly rise. Studies indicate that the number of peripheral blood WBC markedly increases at the end of CABG surgery until 24 hours and remain elevated for 48 hours (26, 30). To support them, a dramatic increase was observed in WBC count at T1, which gradually increased at T2 and T3 in both groups. However, despite the evidence of proven Se anti-inflammatory activity, clear documentations of beneficial effects of Se in the studied patients could not be demonstrated. In fact, the current study findings showed that a single bolus administration of Se does not grant myocardial protection in the first 48 hours following CABG surgery. The belief that reduction of surgery stress response may cause several clinical outcomes led to the studies investigating strategies with anti-inflammatory effects in patients with CABG. Giannopoulos et al. suggested a potential role for a perioperative course of colchicine in reducing inflammatory reactions in on-pump CABG (33). Xiong et al. performed a meta-analysis of randomized controlled trials (RCT) to investigate the effects of preoperative statin treatment on outcomes in patients undergoing cardiac surgery. They suggested that this modality could not provide any benefits for clinical outcomes, but may slightly blunt postoperative inflammation according to CRP serum levels (34). Yuan et al. also reported that in patients undergoing isolated CABG surgery, perioperative statin therapy might be promising to prevent postoperative atrial fibrillation (35). Leong et al. based on the belief that the action of antioxidants as a network could be more effective than their single use, evaluated the effects of preoperative coenzyme Q10, lipid acid, Se, orthotic acid, and omega3 administration on oxidative stress in CABG surgery. In contrast to the current study, they reported positive results. It might be due to the differences between the methods. Both cases of elective CABG and/or valve surgery were enrolled in their work and also Se was administrated at least for two weeks before surgery. The weakness of their study could be that the efficacy of each component was not clear (13). Altaei (36) found that administration of Se (140 µg × 3 caps per day), three days before surgery significantly reduced the inflammatory reactions reflected in IL-6 and TNF-α. It should be noted that their Se dosage, timing, and the rout of administration differed from those of the current study. In addition, contrary to the current study, patients with off-pump CABG were not excluded. Strong evidence suggests that the degree of stress response is not the same between patients with On-pump and Off-pump CABG and it is a theory that needs confirmation, maybe these types of interventions are more effective in conditions expressing less stress degrees. Stoppe et al. (37) demonstrated that even high doses of Se could not induce long-term beneficial effects on patients. Their subjects underwent elective CABG surgery, received an intravenous bolus of 2000 µg Se after induction of anesthesia, and 1000 µg/day within the intensive care unit stay. Se serum levels dropped after the first post-operative day and patient’s outcome did not improve in spite of a short term improvement reflected in SOFA (sequential organ failure assessment) scores at the admission time. Sedighinejad et al. (12) developed a study in which patients undergoing elective cardiac surgery received an intravenous bolus of 600 µg Se before induction of anesthesia. They aimed at testing its anti-inflammatory properties reflected by TNF-α, CRP, and IL-6. However, their hypothesis was not strongly confirmed. They presumed a short-term cardio protective role for this supplementation. McDonald et al. (38) demonstrated that patients with CABG and low preoperative selenium concentrations were associated with post-operative atrial fibrillation. The fact that AF is strongly related to inflammation raises the question of whether selenium supplementation in the selected cardiac surgical patients may reduce inflammatory system activation (39). Schmidt et al. reported that high-dose selenium supplementation could suppress oxidative stress and the postoperative inflammation and improve the clinical outcomes, and reduce the need for postoperative vasoactive support (40). Rayman et al. recommended that diet Se plus supplementation should not be over 300 µg/day. In a 10-year follow-up, they found that mortality increased in these individuals compared with the ones that received lower doses (41). The discrepancy among the results of the studies is partly explained by the multiplicity of causes for inflammation, tremendous variability among patients in regard to the severity of stress response, and also that the current knowledge about human pharmacodynamics is not complete. It is largely unknown whether Se distribution is affected by genetic background of patients, their genotype, and phenotype or not (42, 43). The nature of inflammatory reactions may alter in different patient conditions such as gender, comorbidities, and genetic component. Additionally, humans Se intake and Se status are different in the populations (17). Regarding the intraoperative factors, anesthesia techniques and drugs that surgeons experience are iatrogenic trauma, CPB duration, hemodynamic statues, and temperature management (6, 44). There are methodological, demographical, and pharmacological aspects of this new study that may help to explain the unexpected results. Perhaps to achieve significant findings a multimodal strategy should be considered.
5.1. Suggestions
Since this supplementation is safe, cost effective, and easy to administer, further trials leading to achieve more conclusive results are strongly recommended. Ultimately, more powerful prospective clinical trials in future should address the role of Se therapy in patients undergoing cardiac surgery to reduce inflammatory reactions.
5.2. Limitations
The current study had potential limitations. First, it was a single-center trial with a small sample size. Second, the selected time points might not be optimal. Third, the evaluation was just based on WBC count, since there is still little agreement as to which marker is the best predictor of inflammatory reaction, different results might be achieved if other markers were measured. Finally, fear of high risk patients with several co-morbidities and lack of enough data about the safety and presumed adverse effects of Se (20, 21, 45), earlier start of intervention with larger and repeated doses were avoided.
5.3. Conclusion
Se was safe with no drug-related adverse events in the high-risk patients. Despite the body of work indicating cardio protective potential from different groups and models, Se’s promise, as a therapeutic agent to mitigate inflammation, was not translated in this clinical trial.