1. Background
The benign proliferation of nasal and paraaanasal sinus mucosa leads to nasal polyposis (NP) (1). Nasosinusal polyposis is a common type of chronic inflammation of the nose and paranasal sinuses (i.e., chronic rhino sinusitis, CRS) (2). The prevalence of NP is 1 - 4%, and around 20% of CRS patients have NP (3). Also, NP is more common in patients with asthma and aspirin-exacerbated respiratory diseases. The recurrence rate of NP is high, especially in the case of eosinophilic NP. Eosinophils are activated in several allergic conditions and regulate local inflammatory responses (1, 4). Eosinophils are partially activated by exposure to proinflammatory mediators, including GM-CSF (granulocyte-macrophage colony-stimulating factor), interleukin 3 (IL-3), and IL-5 (5). A recent study reported that eosinophilic infiltration in CRS and NP affects the expression of intercellular adhesion molecule-1 and indices of NP-associated mucosal remodeling (6). In addition, another study revealed a lower expression of apoptotic mediators (caspases 3, 9, and p53) in the mucosa of patients with nasal polyps compared to the mucosa of healthy individuals (7).
Apoptosis is an essential process that maintains cell numbers in various tissues (8). In inflammatory eosinophilic diseases such as polyps, there are changes in the rate of eosinophils’ apoptosis and their survival (8). Researchers showed that IL-3, IL-5, IL-6, and GM-CSF could delay apoptosis in eosinophils (9), and GM-CSF could control the expression of apoptosis-related proteins (10). In addition, IL-5 and IL-6, as pro-survival factors, can include apoptosis (11). The B cell leukemia-2 (Bcl-2) family of proteins comprises proapoptotic proteins (such as Bax and Bad) and anti-apoptotic members (such as Bcl-xL, Bcl-2) and have critical roles in the regulation of the mitochondria-mediated pathway of apoptosis (12).
It seems that anti-inflammatory and proapoptotic therapeutic strategies can be promising in NP. Frequent recurrences and patient dissatisfaction with conventional treatments of CRS and NP have diverted researchers' attention toward new drugs, such as herbal medicines, with higher effectiveness and minimal side effects (13). Medicinal plants have been traditionally used to treat diseases in many countries as safe and cost-effective agents (14). Many plant-derived materials have been suggested to be effective in treating inflammation and, therefore, can be considered a valuable source for developing new therapeutic agents (15).
Among these herbs, Sambucus Ebulus L. (SE), which belongs to the Caprifoliaceae family, is a well-known plant known as dwarf elder (13, 15). This plant species is widely distributed in Europe, Northwest Africa, and Asia, particularly in the north of Iran) (14). This plant is known as an anti-inflammatory herbal medicine in traditional medicine in Europe and Asia (16). SE fruits, in particular, have been traditionally used as an expectorant, diuretic, antiseptic, and wound-healing agent (14). It was reported that SE extract, while having no toxic effects on normal cells (17), could act as an immunostimulant by inducing the expression of IL-6, TNFα (tumor necrosis factor-α), Ccl2 (monocyte chemoattractant protein-1), COX2 (cyclooxygenase-2), and iNOS (nitric oxide synthase) (15).
Investigations on the phytochemical constituents of SE fruits showed high amounts of polyphenolic compounds, such as phenolic acids, anthocyanins, and flavonoids (13, 17). Recently, the health benefits of natural polyphenols have been reported in many studies (13, 15). Other pharmacological properties like antioxidant, anti-inflammatory, and antimicrobial have also been reported for this herb (16). The anticancer activity of these compounds can be exerted by mechanisms such as the inhibition of cell cycle progression and apoptosis induction, as well as regulating the activity of the enzymes involved in tumor cell proliferation (18). The number of studies focusing on SE biological properties is increasing, but the exact molecular mechanisms of action of this herb are not sufficiently known (15). Also, the effects of this plant on recurrent NP and relevant molecular mechanisms remain unknown.
2. Objectives
Regarding the possible therapeutic effects of SE on chronic inflammation, this study aimed to investigate the total phenolic, flavonoid, and anthocyanin contents of this plant, as well as the antioxidant, anti-inflammatory, and apoptotic-modulating activities of SE extract on tissues from patients with recurrent NP.
3. Methods
3.1. Plant Material and Extraction
Fruits were collected from the Someh Sara region, Guilan province, Iran, in September 2017. The voucher specimen was deposited in the Herbarium of the School of Pharmacy, Guilan University of Medical Sciences. The fruits were dried at room temperature for two weeks and powdered in a mixer grinder. Then the powder (200 g) was extracted with ethanol (70%, 1000 mL) using the percolation method for 24 h (19). Extraction was repeated three times. The solvent was evaporated using a rotary vacuum evaporator (Heidolph, Germany) at 45°C, yielding 50 g dried extract. The extract was dissolved in phosphate-buffered saline (PBS) under sterile conditions. The working solution was prepared by diluting DMEM-F12 to prepare different doses of the extract.
3.2. Determination of Total Phenolic Content
The Folin-Ciocalteu method was applied to measure the total phenolic content (TPC) of the whole extract using a method previously reported (20). The absorbance was measured at 765 nm using a UV/Vis spectrophotometer (LAMBDA 25, PerkinElmer). All the experiments were done in triplicate. Gallic acid (GA) at different concentrations was used as the standard. Lastly, TPC was expressed as mg of gallic acid equivalents (GAE)/g of the extract (20).
3.3. Determination of Total Anthocyanin Content
The total anthocyanin content (TAC) of the SE extract was measured using the pH differential method described by Wrolstad and Giusti (21). The extract (1 mg. mL-1) was dissolved in potassium chloride buffer (KCl, 0.025 M, pH = 1.0) and sodium acetate trihydrate (CH3COONa. 3H2O, 0.4 M, pH = 4.5) at a pre-determined dilution factor. The absorbance of samples was measured at 510 and 700 nm using a UV/Vis spectrophotometer. The blank included deionized water. The absorbance (A) of the sample was then calculated using the following formula:
Monomeric anthocyanin pigment content in the extract was calculated using the following formula:
In this formula, cyanidin-3-glucoside molecular weight (MW = 449.2) and molar absorptivity (ɛ = 26,900) were used as constants. Finally, TAC was reported as mg cyanidine-3-glycoside equivalents (C3GE)/g extract.
3.4. Determination of Total Flavonoid Content
Total flavonoid content (TFC) was measured using the Dowd method. The absorbance of the solution was read at 415 nm using a UV/Vis spectrophotometer. All the experiments were repeated three times. Quercetin was used as the standard flavonoid at different concentrations to plot the calibration curve. Finally, TFC was expressed as mg of quercetin equivalents (QE)/g extract.
3.5. Investigation of Radical Scavenging Activity
The 2,2'-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging test was performed to determine the radical scavenging activity of the extract. In this assay, 2 mL of freshly prepared DPPH methanol solution (40 μg. mL-1) was mixed with 1 mL of the sample. The absorbance was measured after 30 min at λmax 517 nm using a UV/Vis spectrophotometer. The blank comprised the DPPH solution (2 mL) and distilled water (1 mL). The control included 1 mL of the sample and 2 mL of distilled water. All the experiments were repeated three times. The radical scavenging percentage was calculated using the following formula:
The radical scavenging activity was expressed as IC50 by plotting the percentage of inhibition against the sample concentration.
3.6. Inclusion and Exclusion Criteria
All patients signed an informed consent form. Inclusion criteria were as follows: (1) fulfilling nasal polyp’s diagnostic criteria (2), (2) concurrent chronic sinusitis with NP, (3) being a candidate for endoscopic sinus surgery, and 4) histological confirmation of eosinophilic nasal polyp after surgery. Exclusion criteria were as follows: (1) non-eosinophilic polyps, (2) concurrent infectious conditions, (3) concurrent treatment with corticosteroids (oral/topical) for four weeks before enrollment in the study, and (4) the presence of nasal septal deformity or anatomical problems.
3.7. Tissue Culture Condition and Treatment
Nasal polyp tissue samples were collected from 20 patients in our otorhinolaryngology clinic. All patients signed an informed consent form. The tissue samples were taken from the middle meatus of the nose at the beginning of the surgery and immediately transferred to the laboratory in a PBS solution in a dry ice box. Then, the tissues were washed several times with a solution containing PBS, penicillin-streptomycin antibiotics (1% pen/strep), and an antifungal drug (5 μg/mL, fungizone).
After washing, each patient’s tissue was divided into several pieces (2 mm3) by a surgical blade in a sterile dish under a microscope loop. The air-liquid interface method was used to culture polyp tissues (22). Polyp tissues from each patient were used as both the control and experimental parts for molecular studies and the TUNEL test. Nasal polyp tissues were randomly placed into the wells of a six-well plate containing 3 mL of the culture solution (DMEM-F12, 1% penicillin-streptomycin, 10% FBS, and purified SE fruit extract). The experimental groups were as follows: Group 1 (control) received the culture solution without SE fruit extract, and groups 2, 3, and 4 received 50, 315, and 1000 μg/mL of the plant extract, respectively. In the next step, the tissues were placed in a wet incubator at a temperature of 37°C, 95% oxygen, and 0.5% carbon dioxide for 24 hours.
3.8. Analysis of NP Tissue Apoptosis
In this study, a TUNEL staining kit (Roche-Applied-Science, Mannheim, Germany: In Situ Cell Death) was used to quantify the number of apoptotic cells, according to the manufacturer’s instructions. The paraffin embedding polyp tissue was deparaffinized (heated at 60°C and then kept in xylene for 30 minutes). The slides were subsequently hydrated in 100%, 90%, 80%, and 70% alcohol solutions. In the next step, the tissue slides were washed with PBS and then incubated with proteinase K for 30 min at 37°C. Next, the slides were incubated with the permeabilizing solution provided by the kit at room temperature for 10 min, followed by washing with PBS twice. Then, 50 µL of the TUNEL reaction agent was poured on each tissue sample. The slides were incubated for 60 min at room temperature in a humidified dark chamber and were again washed with PBS and incubated for another 30 min with 50 μL of the TUNEL-enzyme and TUNEL-label solutions, which were used in a ratio of 1: 99 to stain positive control samples. As the negative control, the tissue sections were treated the same way but without the addition of the TUNEL-label solution. The slides were observed under a fluorescent microscope (Zeiss LSM 5 fluorescent microscope). In this protocol, the TUNEL-enzyme and TUNEL-label solutions were used in a ratio of 1: 9 to stain positive control samples. The slides were then stained with 4’, 6-diamidino-2-phenylindole (DAPI), and apoptotic cells were identified as green colored (TUNEL) and condensed cells with blue colors (DAPI). The slides were visualized under a fluorescent microscope (Zeiss LSM 5 fluorescent microscope). Five microscopic fields (magnification 400X) were observed in each group to count apoptotic cells. We considered a surface equal to 1 mm2 in each group. The mean survival of the NP tissue was calculated using Image J software, and the percentage of apoptotic cells was determined by dividing the number of apoptotic cells by the total number of cells.
3.9. Bax and Bad Expression Analysis by Real-time PCR
Real-time PCR was used to determine BAD and BAX gene expressions. Total RNA extraction was performed using the Innu PREP RNA mini kit (Germany) according to the manufacturer’s protocol. Genomic DNA was removed using a DNase I and RNase-free kit (Sinacolon, Iran). Appropriate cDNA was generated using a 2× RT-PCR Pre-Mix kit (BioFACT, Korea) following the manufacturer’s instructions. The reaction was performed in a volume of 15 μL (1 μL of cDNA (45 ng/μL), 60 nmol/L of forward and reverse primers, 7.5 μL of real-time master mix (ALpliqon, Denmark), and 5.5 µL distilled water) using a real-time machine (Rotor-Gene 6000, Corbett Life Science). PCR amplification was conducted using specific primers (Sinclon) for GAPDH, BAX, and BAD (Table 1) and the Applied Biosystems Thermal Cycler (USA). The PCR cycling condition is shown in Table 1. The expression of the BAX and BAD genes was quantified by the SYBR-green method (Step One Plus™ real-time PCR).
| Genes | Primers and Sequences (5’ to 3’) | PCR Program |
|---|---|---|
| GAPDH | 55°C× 2 min, 94°C× 5 min, 40 cycles (94°C× 20s, 56.8°C or 56°C × 30s, 72 × 35s) and 72°C× 5 min | |
| Forward | ACCCACTCCTCCACCTTTGA | |
| Reverse | CTGTTGCTGTAGCCAAATTCGT | |
| BAX | 55°C× 2 min, 94°C× 5 min, 40 cycles (94°C× 20s, 56.8°C× 30s, 72 × 35s) and 72°C× 5 min | |
| Forward | GTCGCCCTTTTCTACTTTGCC | |
| Reverse | CTCCCGCCACAAAGATGGTCA | |
| BAD | 55°C× 2 min, 94°C× 5 min, 40 cycles (94°C× 20s, 56°C× 30s, 72 × 35s) and 72°C× 5 min | |
| Forward | CCAGATCCCAGAGTTTGAGCC | |
| Reverse | CCATCCCTTCGTCGTCCTCC |
Primer Sequences and PCR Cycling Program
3.10. IL-5 and GM-CSF ELISA Assay
The culture media of the tissues treated with different doses of SE fruit extract were collected after 24 h and stored at - 80°C. We used a human IL-5 kit (Estbiopharm, USA) for measuring the level of IL-5 and a human GM-CSF ELISA MAXTM kit (D\deluxe set, Biolegend, USA) for GM-CSF following the manufacturer’s instructions. The optical density of the samples was measured, and a standard curve was created to calculate the unknown concentrations of IL-5 and GM-CSF. ELISA tests were run in duplicate.
3.11. Statistical Analysis
Data were analyzed by SPSS 22 Software. The results were presented as mean ± SD. The Kolmogorov-Smirnov test was used to check data normality, and one-way ANOVA was utilized for data analysis at a significant level of P < 0.05.
4. Results
In this study, 20 patients (10 females and 10 males, mean age ± SD: 32.65 ± 7.09 years) who fulfill our eligibility criteria were selected. Nasal polyp tissues with ≥ 40% eosinophils, confirmed by Hematoxylin and Eosin (H&E) staining, were included in the study.
4.1. Phytochemical Analysis and Antioxidant Activity
Phytochemical analysis of SE fruit extract was performed to determine total phenolic, flavonoid, and anthocyanin contents. The TPC of the SE extract was determined using a standard curve and gallic acid as the reference standard (y = 0.0192x - 0.0267, R2 = 0.996). Also, the TFC of the extract was reported according to a standard curve for quercetin as a reference flavonoid (y = 0.0188x - 0.0304, R² = 0.995). The TAC of SE extract was determined by the pH differential method and obtained as 0.56 ± 0.01 mg C3GE/g extract. Also, the DPPH radical scavenging activity of the extract was obtained as 190.78 ± 0.55 µg.mL-1 (Table 2).
4.2. TUNEL Assay
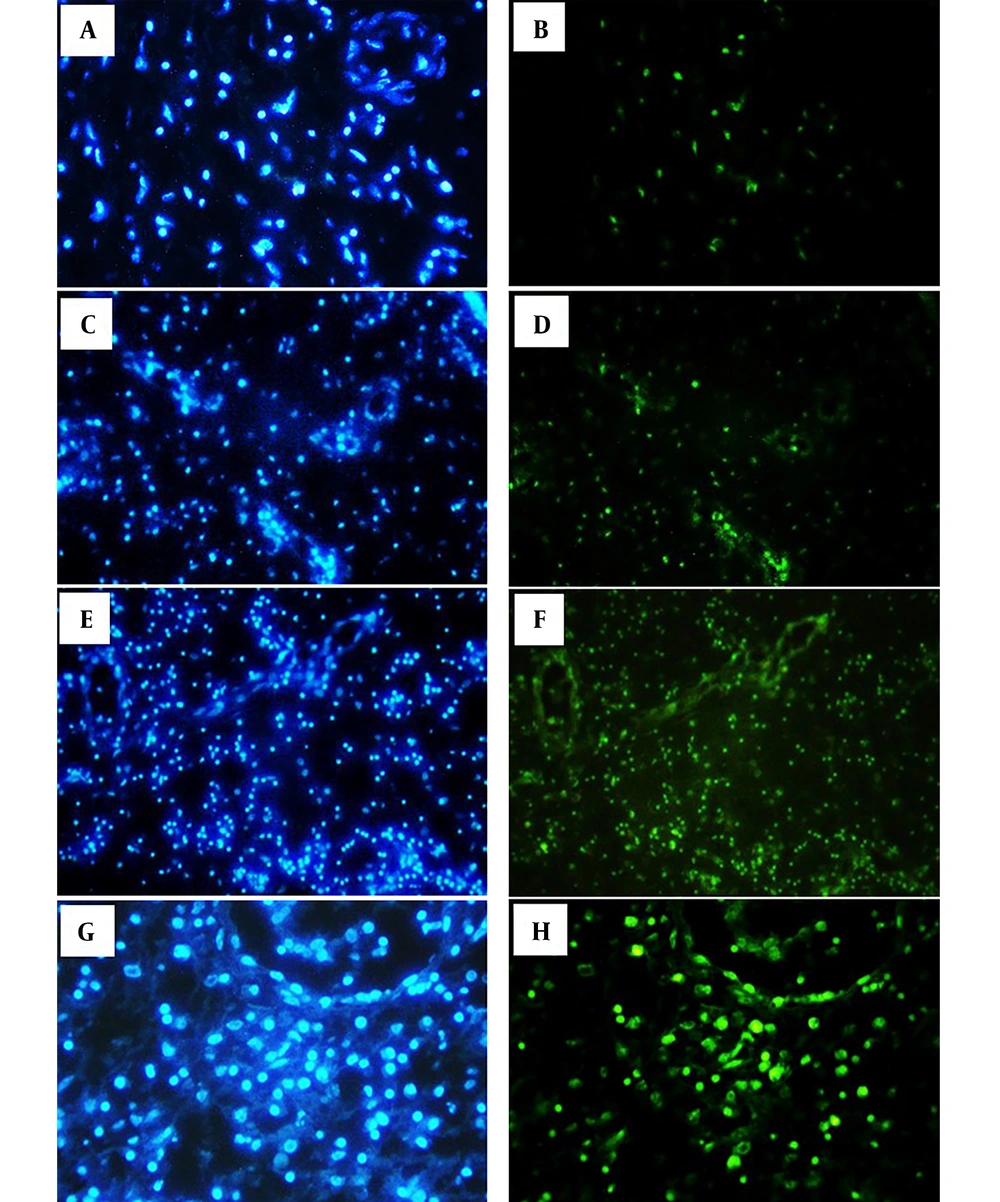
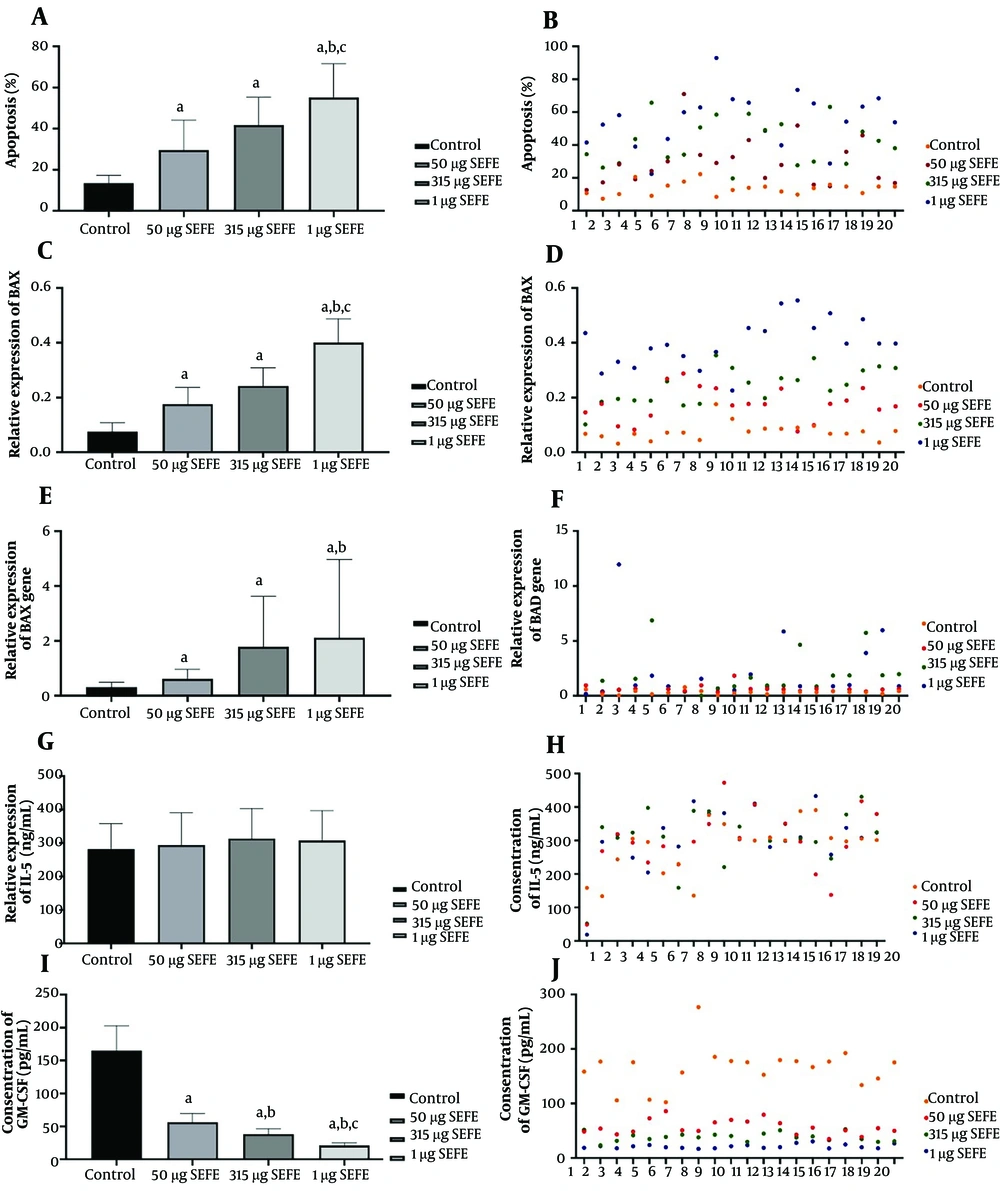
The data showed a significant increase in apoptosis after treatment with different doses of the SE extract compared to the control group (Figure 1). Green spots in Figure 2 show the presence of apoptotic cells. The mean survival of the NP tissue in the control group was 86.66 ± 5.2% compared to 71.20 ± 1.6% in the group treated with 50 μg/mL of the SE fruit extract. In addition, the survival of the tissues treated with 315 and 1000 μg/mL were 55.0% and 35%, respectively (P < 0.05). Moreover, the increase in apoptotic cells in the NP tissues treated with SE fruit extract was dose-dependent and significant at high doses (P = 0.004).
The left column shows bright blue nuclei stained with DAPI, and the right column shows bright green nuclei stained with TUNEL, which are considered apoptotic cells in the nasal polyp tissue. A and B show the control group. C and D (50 μg/mL SE); E and F (315 μg/mL SE); G and H (1000 μg/mL SE). SE: Sambucus Ebulus.
4.3. BAX and BAD Gene Expression
We found that the expression of the BAX and BAD genes increased in the NP tissues treated with SE fruit extract at the doses of 50, 315, and 1000 µg/mL (P < 0.0001). Comparison of BAX gene expression showed upregulation in low, high, and medium doses of the SE extract, indicating significant differences between the control group and group 2 (315 µg/mL, P = 0.009), as well as between the control group and group 3 (1000 µg/mL, P < 0.0001), and between group 2 and group 3 (i.e., 315 vs. 1000 µg/mL, P < 0.0001) (Figure 2). There was no significant difference in BAD gene expression between experimental groups 1 and 2 (P = 0.069) or between groups 2 and 3 (P = 0.991). However, the BAD gene expression showed a significant difference between group 1 and group 3 (P = 0.033) (Figure 2), indicating the prominent overexpression of the BAD gene in the highest concentration of SE extract, inducing apoptosis in the nasal tissue.
4.4. IL-5 and GM-CSF Levels
The results exhibited that GM-CSF levels significantly and dose-dependently decreased after treatment with different doses of the extract compared to the control group (P < 0.0001, Figure 2). This observation suggested that the use of the SE extract (especially at high concentrations) might reduce the survival and migration of eosinophils to the polyp tissue. Nevertheless, there was no statistically significant difference in IL-5 levels between the experimental and control groups (P > 0.05, Figure 2).
A and B show apoptosis percentage. C, D, E, and F show BAX and BAD gene expression. G, H, I, and J indicate IL-5 and GM-CSF concentrations in different study groups (treatment with different doses of SE extract). “a" indicates a significant difference in comparison with control (P < 0.01); “b" significant difference in comparison with 50 μg/mL. SE extract (P < 0.001), and “c"; significant difference in comparison with 315 mg/mL of SE extract (P < 0.001). SE: Sambucus Ebulus.
5. Discussion
The extract of SE has been reported to be an effective anti-inflammatory herbal medicine (14, 23). However, the effect of SE extract on recurrent NP and its molecular mechanisms are still unknown. Thus, in this study, we investigated the effects of SE extract on eosinophil apoptosis and inflammatory cytokines. Our data demonstrated statistically significant changes in NP tissues after treatment with SE fruit extract. The results of this study showed that SE whole extract was a good source of phenolic compounds. The TPC and TFC of the hydroalcoholic extract (ethanol 70%) of SE were obtained as 38.44 (mg GAE/g extract) and 8.62 ± 0.12 (mg QE/g extract), respectively. Plant-derived polyphenolic compounds have revealed anti-inflammatory and immune-modulatory actions in vitro and in vivo (14, 17). Recent findings have highlighted the beneficial role of these natural compounds in the treatment of several acute and chronic diseases (14, 17). Also, it has been demonstrated that bioactive polyphenols can modify the expression of numerous proinflammatory genes (such as cytokines, nitric oxide synthase, cyclooxygenase, and lipoxygenase) (16). These compounds are considered natural antioxidants with potent ability to scavenge ROS (reactive oxygen species) and can regulate inflammatory signaling pathways (24). In a study, Ebrahimzadeh et al. quantified the TPC and TFC of the methanolic fraction of SE as 93.97 ± 4.7 (mg GAE /g extract) and 9.94 ± 0.34 (mg QE/g extract), respectively (25). It can be suggested that the type of extraction solvent can have a greater influence on TPC than on TFC. Moreover, Jimenez et al. showed that the TAC of the ripe fruit extract of SE ranged from 0.35 ± 0.01 to 2.18 ± 0.03 mg C3GE/g, which engulfs the respective value obtained in our study (0.56 ± 0.01 mg C3GE/g extract) (26).
So far, different secondary metabolites, including hydroxycinnamic acids (e.g., caffeic acid, ferulic acid, and chlorogenic acid), catechin, epicatechin, and flavonols (such as quercetin-3-O-rutinoside) have been identified as the constituents of SE fruits (13, 17). Also, anthocyanins, including cyanidin-3-O-galactoside and cyanidin-3-O-arabinoside, have been identified (15). In our study, the IC50 of SE extract, according to the DPPH radical scavenging assay, was obtained as 190.78 ± 0.55 µg.mL-1. Previously, the in vitro antioxidant activity (i.e., free radical scavenging power) of several Sambucus species, such as S. nigra, S. australis, and S. canadensi, has been investigated (16). For example, the fruits of elderberry (Sambucus nigra) showed considerable antioxidant activity with an IC50 of 62.56 ± 1.12 µg.mL-1 (13). It has been reported that berries are rich sources of antioxidants (14). Many studies have shown that oxidative stress plays a main role in the development of chronic inflammatory diseases and cancer (17, 27). It has been suggested that the anti-inflammatory and anti-apoptotic activities of SE extract can be partly due to its antioxidant ingredients.
The β common chain of cytokines such as GM-CSF, IL-5, and IL3 regulate the inflammatory responses that contribute to the rapid elimination of pathogens and also promote chronic inflammation (28, 29). Indeed, β common cytokines are the pleiotropic controllers of inflammatory conditions (29). Among other activities, IL-5 particularly affects the differentiation and proliferation of eosinophils (9), and GM-CSF also increases the survival of these cells by affecting their differentiation and maturation (28). It seems that the cooperation of these β common cytokines can extend the survival and count of eosinophils in the polyp tissue (9). Altogether, the important role of these cytokines in regulating inflammation has made them interesting targets for the treatment of chronic inflammation and cancer (29).
Our results showed a significant decrease in GM-CSF levels after treatment with different doses of SE fruit extract in comparison to the control group (P < 0.0001). This data suggested that high concentrations of SE extract, partly through depressing GM-CSF production, may reduce the survival and migration of eosinophils to the polyp tissue. Some studies have assessed the effects of herbal extracts on GM-CSF production. Sakthivel and Guruvayoorappan showed that the extract of Acacia ferruginea significantly reduced the levels of proinflammatory cytokines, including GM-CSF (30). Also, Daniela et al. reported that Leontopodium alpinum extract inhibited the release of GM-CSF from primary human keratinocytes (PHKs) (31).
By increasing the dose of the SE extract in our study, the percentage of apoptotic cells in the NP tissue significantly increased. Some studies showed that the rate of apoptosis was lower in the eosinophils infiltrating into polyp tissues than in the cells of healthy individuals (32). Nasal polyps are recurrent protrusions of the mucosal membranes of the nasal sinuses into nasal cavities, obstructing the sinus entrance (32, 33). Infiltration by eosinophils, lymphocytes, and other inflammatory cells induces the production of proinflammatory cytokines, further maintaining and promoting allergic response (1). These results suggest that an increase in apoptosis in polyp eosinophils may be a potential mechanism recruited by SE extract for inhibiting nasal polyp proliferation. In this regard, our results are consistent with those of previous reports. Notably, mitochondria are known as the major organelles involved in the intrinsic cell death pathway mediated via cytochrome C (34). Also, Bcl-2 family molecules influence apoptosis through modulating cytochrome C, the caspase pathway, and P53-mediated apoptosis (12). The members of the Bcl-2 family (such as BAX and BAD) increase mitochondrial membrane permeability and promote the release of cytochrome C from mitochondria, a phenomenon that can be used as a therapeutic strategy for nasal polyps (34). BAX can form a complex with Bcl-2, and the Bax/Bcl-2 ratio determines the fate of the cell, and shifting of this ratio in favor of Bax can activate caspase‑9, triggering mitochondria-dependent apoptosis (8, 12).
We found that Bax gene expression and apoptotic cell death in the polyp tissue significantly increased after treatment with the SE extract. Omrani et al. showed that this extract could reduce the size and invasion of breast tumors in nude mice with no toxicity on other cells, reporting Bax gene upregulation after the treatment of breast cancer cells with the SE extract (17). Another study suggested that ursolic acid could induce mitochondria-dependent apoptotic cell death via apoptosis-inducing factor and endonuclease G in human lung cancer cells (27). Indeed, researchers showed that extrinsic Bax protein expression through gene transfer increased apoptosis induction in nasal polyp fibroblasts (32). According to our results, we can suggest that one possible mechanism of apoptosis in the polyp tissue treated with SE extract may encompass the combined effects of GM-CSF signaling and an unbalanced Bax/Bcl-2 ratio (11, 12, 32).
5.1. Conclusions
The results of our study showed that SE fruit extract might have positive effects on nasal polyps by inducing apoptosis and reducing inflammatory cytokines. So, SE fruit extract is possibly a viable choice to reduce the proliferation of NP via activating proapoptotic genes. Total phenolic content, flavonoids, and anthocyanins were quantified, showing that SE fruit extract was a good source of antioxidants, which could play a main role in the inhibition of apoptosis and inflammation. These findings provide new insights into the anti-proliferative properties of SE fruit extract, which deserve investigation in future trials focusing on the treatment of CRS, especially NP.