1. Background
Ulcerative colitis (UC) is a form of inflammatory bowel disease (IBD) that leads to inflammation and ulceration of the colon. It is more prevalent in developed countries and poses significant economic and societal burdens (1). Ulcerative colitis typically manifests through symptoms such as frequent bowel movements, bloody diarrhea, and abdominal pain, which are common in young adults. The severity and type of UC can vary, with symptoms fluctuating over time (2). In certain periods, the symptoms may lessen or disappear, leading to what is referred to as the "silent" phase of the disease. However, at other times, the disease may flare up with more severe symptoms (3, 4).
Uncontrolled inflammation in UC patients increases the risk of developing colon cancer (5-9). A hallmark feature of UC is the infiltration of neutrophils and macrophages into the colon's mucosal tissue. Activated neutrophils in the intestinal mucosa produce reactive oxygen species (ROS) such as superoxide ions, hydroxyl radicals, and hydrogen peroxide (10). These ROS contribute to lipid peroxidation, increase mucus and blood vessel permeability, and further neutrophil infiltration into the mucosal tissue, thereby exacerbating inflammation. Through their impact on cytokine gene expression and enzymes involved in the inflammatory response, ROS contribute to the degradation of the intestinal wall, leading to ulcers, bleeding, and diarrhea. Moreover, oxidative stress reduces antioxidant defenses, worsening the severity of the disease (11).
As a result, the use of antioxidant substances could help manage the disease and prevent its recurrence. Antioxidant compounds neutralize free radicals, thereby preventing cell damage (12). Given the role of inflammation and oxidative stress in UC pathogenesis, plant extracts and compounds with anti-inflammatory and antioxidant properties offer a promising treatment strategy for this condition (13).
Ulmus minor is a deciduous or semi-deciduous tree or shrub that belongs to the Ulmaceae family (14). This species first appeared in the Miocene period, approximately 20 million years ago, in what is now central Asia. According to various sources, the most medicinally effective parts of the tree are its bark and leaves. The Ulmus. minor plant contains several chemical compounds that contribute to its therapeutic properties, including mucilage, tannins, caffeic acid derivatives (particularly chlorogenic acid, or CGA), and sterols such as β-sitosterol and stigmasterol (15).
Tannins and caffeic acid derivatives belong to a larger and more complex group of phenolic compounds, which are by-products of the photosynthesis process. These compounds provide protection against free radical attacks and tissue damage. CGA, in particular, offers multiple therapeutic benefits, such as antioxidant, antibacterial, hepatoprotective, cardioprotective, anti-inflammatory, antipyretic, neuroprotective, anti-obesity, antiviral, antimicrobial, antihypertensive properties, and acts as a free radical scavenger and a central nervous system (CNS) stimulator (16).
2. Objectives
Given these properties, the present study was conducted to evaluate the protective effects of hydroalcoholic and aqueous extracts of Ulmus minor leaves on UC induced by acetic acid in rats.
3. Methods
All chemicals used in this experimental study were supplied by Sigma-Aldrich (GmbH, Munich, Germany). A total of 35 Neranjad Wistar rats, each weighing approximately 220 ± 20 grams, were selected for the study. The rats were housed under standard laboratory conditions, with a 12-hours light/dark cycle, a controlled temperature of 25°C, and free access to water and food. Their diet consisted of concentrated food, provided by the Fars Animal and Poultry Feed Company (17). The animal procedures were approved by the Ethics Committee of Kermanshah University of Medical Sciences (IR.KUMS.REC.1400.535).
3.1. Preparation of Ulmus minor Leaf Extract
First, the leaves of the Ulmus minor tree from the northern regions of Iran (Gorgan) were collected during the flowering season (early spring) and dried after being approved by the official at the Razi University Herbarium. The specimen was registered in the herbarium center with identification number RANK 10649.
For the preparation of the aqueous extract of Ulmus minor leaves, 100 grams of plant powder was placed into a beaker with 300 cc of water and put into a shaker. For the hydroalcoholic extract, 100 grams of plant powder was added to a 70% hydroethanolic solution (1000 cc) in an Erlenmeyer flask and placed into a shaker. After 24 hours, the extracts were collected, and the residues were washed with 300 cc of water or the hydroethanolic solution to account for any lost volume. This process was repeated three times, after which the solvent was evaporated using a rotary evaporator (16, 18).
3.2. Phytochemical Analysis
3.2.1. Total Phenol Content
The total phenol content in both aqueous and hydroalcoholic extracts was measured using the following method (19). Gallic acid was used as the standard phenolic compound, along with the Folin-Ciocalteu reagent. Approximately 0.2 mL of each extract was placed into test tubes, followed by the addition of 1.8 mL distilled water, 1 mL of 10% Folin-Ciocalteu reagent, and 1 mL of 8% Na₂CO₃. The mixture was then allowed to stand for 30 minutes in a dark environment, after which the absorbance was measured at 765 nm. The total phenol content was calculated using a formula derived from the standard gallic acid calibration curve.
3.2.2. Total Flavonoid Content Assessment
The total flavonoid content was measured using the aluminum chloride colorimetric method, with quercetin as the standard (20). First, standard solutions of quercetin in methanol were prepared with concentrations of 3.12, 6.25, 12.5, 25, and 50 mg/L. Then, 0.2 mL of aluminum chloride was added to 0.2 mL of each standard solution or plant extract, and the mixture was thoroughly mixed. The samples were incubated at room temperature in a dark environment for 60 minutes. After incubation, the absorbance of the samples was measured at a wavelength of 420 nm. Finally, the total flavonoid content was determined by referencing a standard calibration curve based on quercetin.
3.2.3. Reducing Ferric Capacity Ferric Reducing Antioxidant Power (FRAP)
To determine the total antioxidant capacity of Ulmus minor extracts, the FRAP assay was employed (21). This method measures the antioxidant power of the plant extract by assessing its ability to reduce ferric ions (Fe³⁺) to ferrous ions (Fe²⁺). The working FRAP reagent was freshly prepared by mixing 25 mL of 300 mM acetate buffer (pH 3.6), 2.5 mL of 10 mM TPTZ (2,4,6-tri(2-pyridyl)-s-triazine) in 40 mM HCl, and 2.5 mL of 20 mM FeCl₃. Then, 50 µL of each extract was added to 1.5 mL of the FRAP reagent. After incubating for 30 minutes, the absorbance of the samples was measured at 593 nm. Finally, the total antioxidant capacity was determined using a standard calibration curve.
3.3. Induction Method of Ulcerative Colitis
In the present study, 35 rats were randomLy divided into seven groups (five rats in each group). Colitis was induced using 3% acetic acid, with 2 mL of the solution injected intrarectally. The study groups were as follows: The first group (healthy) had no disease induction and did not receive any treatment; the second group (control) received water orally (p.o.) for 14 days, with ulcerative colitis induced by 3% acetic acid on day 7; the third group (sulfasalazine group) received sulfasalazine at a dose of 500 mg/kg (p.o.) for 14 days, with colitis induction on the 7th day; the fourth and fifth groups were administered hydroalcoholic extracts of Ulmus minor leaves at 100 mg/kg and 200 mg/kg (p.o.), respectively, for 14 days, with colitis induction on the 7th day; and the sixth and seventh groups received aqueous extracts of Ulmus minor leaves at 100 mg/kg and 200 mg/kg (p.o.), respectively, for 14 days, with colitis induction on the 7th day. Throughout the study, rats were provided with water and standard chow (22).
3.4. Examining the Severity of Macroscopic Damage and Colon Edema
Rats were sacrificed under anesthesia (intraperitoneal injection of ketamine (90 mg/kg) and xylazine (15 mg/kg)) after colitis was induced. A lower abdominal incision was made to open the abdominal cavity and expose the colon. An 8 cm section of the distal colon was excised and opened longitudinally, then rinsed with cold saline solution. After assessing the extent of macroscopic damage, a portion of the colon was fixed in a 10% formalin solution for histological examination. The severity of colonic damage was evaluated using a colon macroscopic scoring method as described by Abdolghaffari et al. (2010) (23, 24).
3.5. Microscopic Examination of Colon Tissue Damage
In the present study, to perform microscopic evaluations, the tissue samples isolated and fixed in 10% formalin were embedded in paraffin. A histologist, blinded to the study groupings, stained the sections with hematoxylin and eosin (H&E). Microscopic scoring was assessed according to the criteria described previously (25).
3.6. Data Analysis
It should be noted that the desired level of significance was set at (P ˂ 0.05). The obtained data were analyzed using SPSS Statistics software (version 22.0) with One-way ANOVA and Tukey's post hoc test. All data were expressed as Mean ± SEM and presented as averages.
4. Results
4.1. Total Phenol and Flavonoid Content
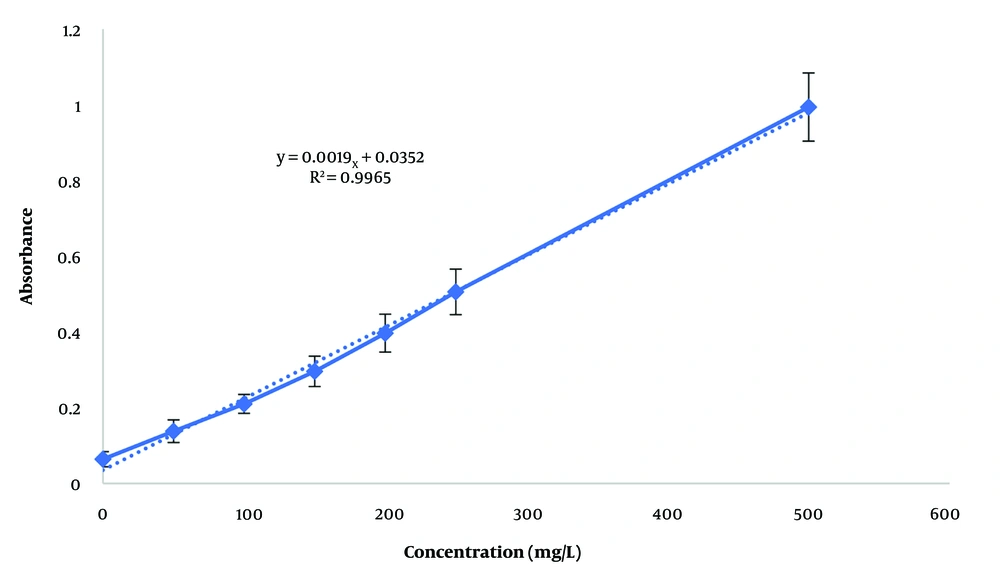
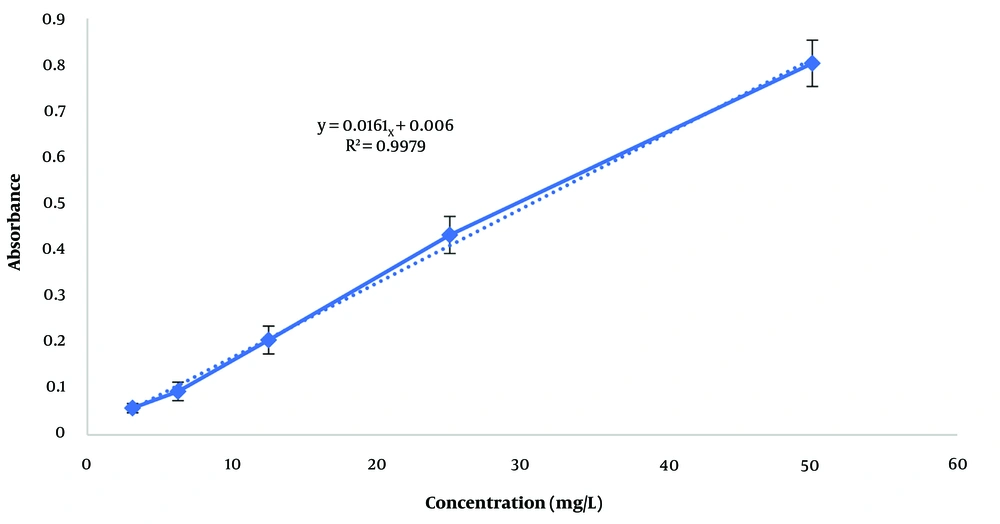
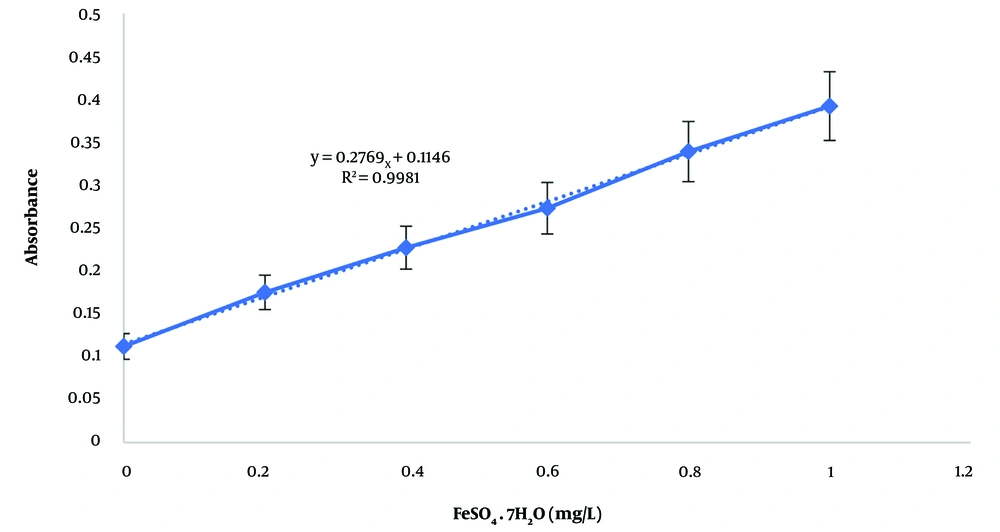
The total phenol content in each extract was determined and expressed in terms of gallic acid, a pure phenolic compound, using the calibration curve (Figure 1) and the Folin-Ciocalteu method. The equation derived from the standard curve of gallic acid, used to calculate the total phenol content, is y = 0.0019x + 0.0352 (R² = 0.9965). The total phenol content in the hydroalcoholic and aqueous extracts was 194.105 ± 12.6 mg/L and 802.105 ± 25.4 mg/L, respectively. Additionally, the total flavonoid content in the hydroalcoholic and aqueous extracts was 48.94 ± 3.5 mg/L and 127.95 ± 11.6 mg/L, respectively, based on the calibration curve of quercetin (Figure 2) with the equation y = 0.0161x + 0.006 (R² = 0.9979).
4.2. Evaluation of Total Antioxidant Capacity
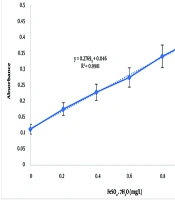
By calculating the antioxidant capacity using the calibration curve with the equation y = 0.2769x + 0.1146 (R² = 0.9981) (Figure 3), it was determined that the aqueous extract exhibited a higher antioxidant capacity than the hydroalcoholic extract. The antioxidant capacity values were 4.93 ± 0.5 mg/L for the aqueous extract and 3.44 ± 0.34 mg/L for the hydroalcoholic extract.
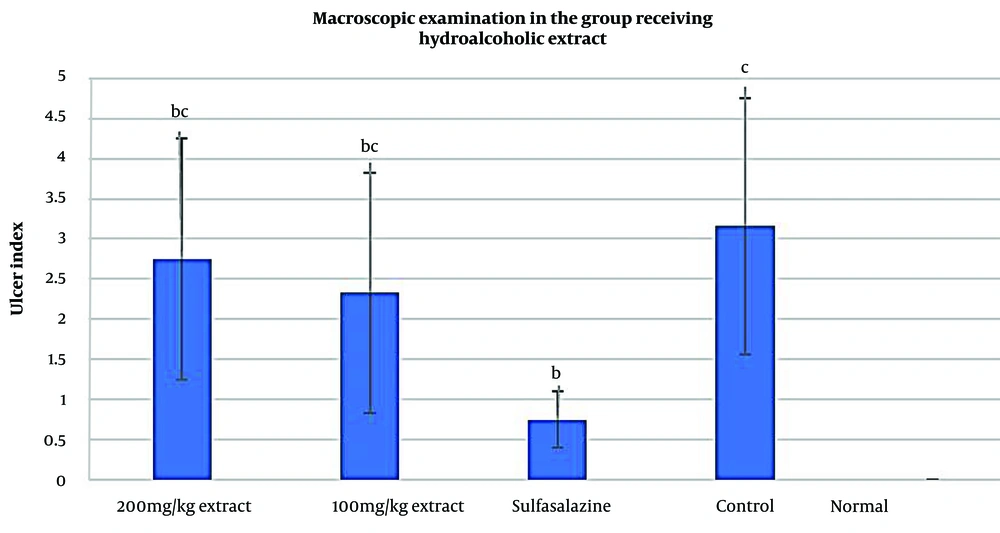
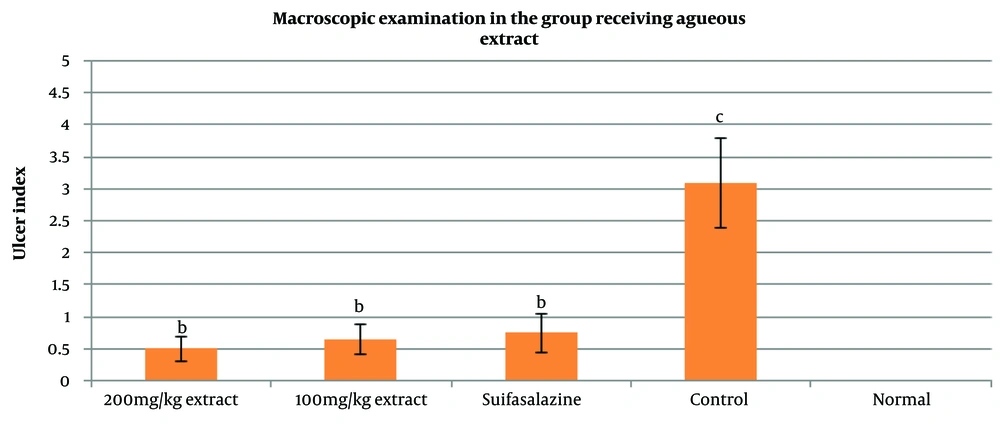
4.3. Macroscopic Effects of Ulcerative Colitis
The macroscopic examinations of colitis in the current study are presented in Figures 4 and 5. In the groups treated with the hydroalcoholic extract, the results showed no tissue damage or wounds in the healthy group (Normal). In contrast, the control group exhibited wounds, adhesions, thickening of the intestinal wall, necrosis, and severe inflammation. Additionally, in the groups receiving the hydroalcoholic extract of Ulmus minor leaves at doses of 100 mg/kg and 200 mg/kg, some improvement was observed, although this improvement was less significant compared to the sulfasalazine group (P < 0.05). However, both groups receiving hydroalcoholic extract showed significant improvement in UC symptoms compared to the control group (P < 0.05).
Regarding the groups treated with the aqueous extract, the findings indicated that both doses of 100 mg/kg and 200 mg/kg had very favorable effects, with significant improvements compared to the control group (P < 0.05). However, no significant difference was found when compared with the sulfasalazine group (P > 0.05). Overall, the results of the macroscopic examinations showed that the aqueous extract of Ulmus minor leaves at a dose of 200 mg/kg had the most significant effect on colon tissue compared to the hydroalcoholic extracts (100 mg/kg and 200 mg/kg) and the aqueous extract at 100 mg/kg, with a statistically significant difference from the control group (P < 0.05).
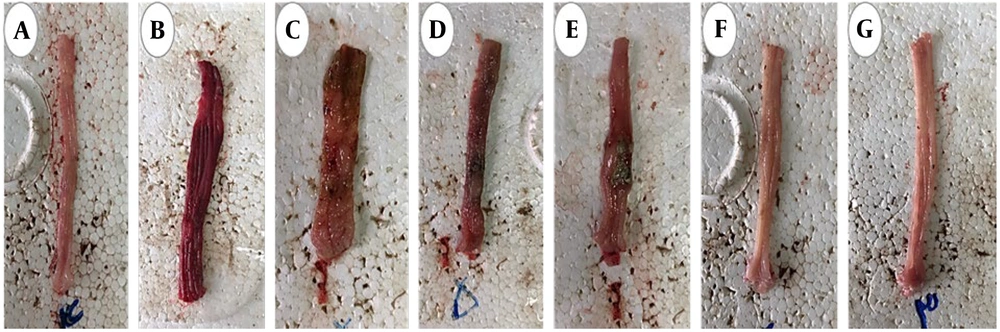
The macroscopic images of colon tissue from the different study groups are shown in Figure 6. As seen, in the groups treated with both aqueous and hydroalcoholic extracts, the symptoms of UC showed improvement.
Images related to intestinal tissue isolated from different studied groups. A, healthy group; B, control group (acetic acid); C, sulfasalazine group, mild wound and inflammation were observed; D, group receiving hydroalcoholic extract (100 mg/kg). There was disruption of secretory glands with moderate inflammation; E, the group receiving hydroalcoholic extract (200 mg/kg), a mild wound was observed and there was no crypt abscess; F, the group receiving aqueous extract (100 mg/kg), mild wound and inflammation were observed and there was no destruction of glands; G, group receiving aqueous extract (200 mg/kg), no wound was observed and no destruction of secretory glands was observed in some areas of gland restoration.
4.4. Microscopic Studies
The colons of all rats in the healthy group had a normal structure, while the group receiving acetic acid exhibited mild, moderate, and severe tissue changes. Histological results, as assessed by a pathologist, revealed that in the control group (ulcerative colitis model), there were signs of active inflammation, severe wounds, necrosis, gland destruction, accumulation of neutrophils and lymphocytes, as well as abscess formation in the mucosa and submucosa. Compared to the control group, the administration of hydroalcoholic and aqueous extracts of Ulmus minor leaves and sulfasalazine significantly reduced pathological lesions, particularly the spread of necrosis and inflammation caused by acetic acid in the colon tissues (P < 0.05).
Histological examinations of the group receiving sulfasalazine, when compared to the control group, showed mild inflammation, partial healing of the secretory glands, and mild wound presence. In the group that received the aqueous extract of Ulmus minor leaves at a dose of 100 mg/kg, wounds and mild inflammation were observed. No gland destruction was noted, and mucosal repair and regeneration of the secretory glands were evident, with no abscess formation (P < 0.05). In the group receiving the aqueous extract at 200 mg/kg, compared to the control group, no wounds or inflammation were observed, there was no destruction of the secretory glands, gland restoration was observed in some areas, and no abscess was present (P < 0.05).
In the group receiving the hydroalcoholic extract at 100 mg/kg, compared to the control group, a mild wound was observed, moderate inflammation persisted, healing was not significant, and there was some disruption of the secretory glands, along with the presence of lymphocytes and neutrophils (P < 0.05). The tissue showed signs of healing in a mild form. In the group treated with the hydroalcoholic extract at 200 mg/kg, a very mild wound was observed, gland restoration was significant, no abscess was found, and very few lymphocytes and neutrophils were present.
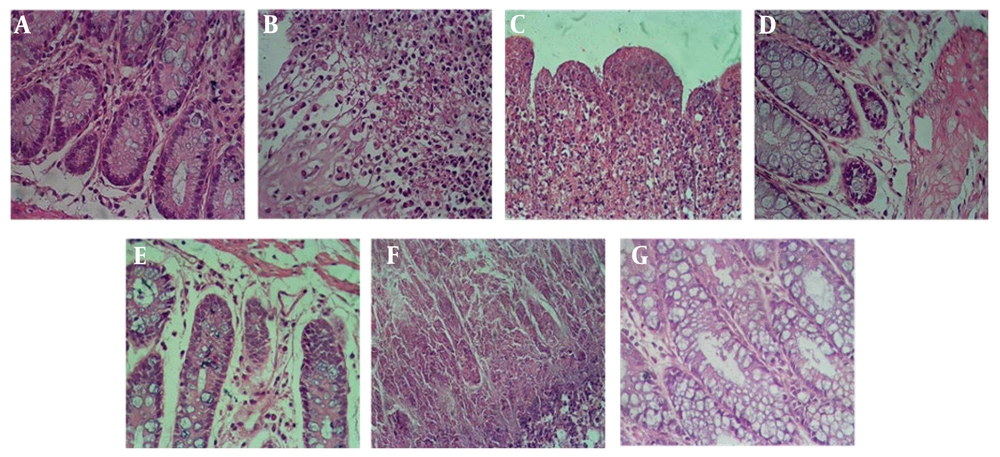
Overall, the control group exhibited the highest score of intestinal lesions, followed by the hydroalcoholic extract groups at 200 mg/kg and 100 mg/kg, the aqueous extract group at 100 mg/kg, and the sulfasalazine group. In the comparison between the groups treated with hydroalcoholic and aqueous extracts of Ulmus minor leaves, the most protective effects were observed with the administration of the aqueous extract at a dose of 200 mg/kg. The obtained results are depicted in (Figure 7).
Histopathology images of intestinal tissue in different studied groups. Images are shown with 400 magnifications. A, healthy group; B, control group (acetic acid); C, sulfasalazine group, D, group receiving hydroalcoholic extract (100 mg/kg); E, group receiving hydroalcoholic extract (200 mg/kg); F, group receiving aqueous extract (100 mg/kg); G, group receiving aqueous extract (200 mg/kg).
5. Discussion
Ulcerative colitis is a type of inflammatory bowel disease characterized by chronic inflammation of the colon and rectum, resulting in long-term mucosal inflammation and ulceration (26, 27). The traditional gold standard treatments for ulcerative colitis include surgery and the administration of drugs such as 5-aminosalicylic acid, corticosteroids, and immunosuppressants (28). A recent study demonstrated that the composition of the fecal microbiota in ulcerative colitis patients differs significantly from that of healthy individuals, suggesting that microbial alterations may play a role in the disease (29). However, some patients do not achieve clinical improvement with conventional drug regimens, posing a significant challenge to the effective management of ulcerative colitis (30).
As a result, alternative treatment options, such as traditional and herbal medicines, have been actively explored, with some already being introduced into clinical practice (31, 32). There is ongoing and effective development of traditional therapies for ulcerative colitis in both preclinical and clinical studies. Numerous herbal medicines have been integrated into traditional medical systems, showing various therapeutic effects on ulcerative colitis, including analgesic, antibacterial, anti-inflammatory, and anti-diarrheal properties, as well as the ability to regulate immune responses, inhibit macrophage/monocyte activity, and adjust inflammatory processes (33, 34).
Phytochemical investigations of plants in the Ulmaceae family have revealed the presence of active compounds such as terpenoids, alkaloids, glycosides, carbohydrates, steroids, sterols, saponins, tannins, proteins, and flavonoids. Key bioactive compounds isolated from these plants, such as β-amyrin, β-sitosterol, holopterin-A, holopterin-B, hederagenin, hexacosanol, beta-D-glucose, friedlin, epifriedlin, 2-aminonaphthoquinone, and 1,4-naphthalenedione, are considered responsible for their medicinal properties (35).
Common compounds identified in elm (Ulmus spp.) plants include mucilage, tannins, caffeic acid derivatives (especially chlorogenic acid, CGA), and sterols such as β-sitosterol and stigmasterol. Mucilage, composed of highly hydratable and gel-forming polysaccharides, contains biologically active compounds such as flavonoids, alkaloids, and terpenoids. For instance, flavonoids act as anti-inflammatory agents by inhibiting the enzymes cyclooxygenase and lipoxygenase, key players in arachidonic acid metabolism. Additionally, flavonoids help modulate oxidative bursts in neutrophils, reducing reactive oxygen species and accelerating wound healing (36). Tannins, another group of natural compounds, exhibit notable anti-inflammatory, antiviral, antimicrobial, immunomodulatory, antitumor, and hepatoprotective activities (37). Caffeic acid, a major representative of hydroxycinnamic acids, has various physiological and pharmacological properties, including antiviral, antioxidant, anti-inflammatory, anticarcinogenic, immunomodulatory, antidiabetic, cardioprotective, antiproliferative, and hepatoprotective effects (38). Moreover, previous studies have shown that sterols, particularly β-sitosterol, can reduce the mRNA expression of pro-inflammatory cytokines, thereby contributing to their anti-inflammatory potential (39).
In this study, it was shown that both groups receiving the hydroalcoholic extract of Ulmus minor at doses of 100 and 200 mg/kg exhibited some improvement, although the improvement was less pronounced compared to the sulfasalazine group. Additionally, the results in both groups receiving the hydroalcoholic extract were significant compared to the control and sulfasalazine groups (P < 0.05). The groups receiving the aqueous extract of Ulmus minor at doses of 100 and 200 mg/kg showed very favorable effects, with a significant difference observed when compared to the control group (P < 0.05). However, there was no significant difference in comparison with the sulfasalazine group.
Overall, the results from the macroscopic examinations indicated that the aqueous extract of Ulmus minor leaves at a dose of 200 mg/kg had the most beneficial effect on colon tissue when compared to the groups treated with hydroalcoholic extracts at doses of 100 and 200 mg/kg and the aqueous extract at 100 mg/kg. This favorable effect, compared to the control group, was statistically significant (P ˂ 0.05).
Supporting these findings, research conducted in 2022 by DʼAngiolo et al. focused on the phytochemical investigation of Ulmus minor subspecies. The results of this study demonstrated that the extract (6.25 - 50 μg/mL) significantly inhibited the release of nitric oxide (NO) and the expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) in macrophages. These data highlight the anti-inflammatory properties of several compounds isolated from Ulmus minor, suggesting their potential use in nutrition (35). In a 2007 study by Lee et al., the immunomodulating effects of Ulmus minor bark extract were investigated. The study focused on the proliferation of spleen cells and the capacity to produce cytokines, including interleukin (IL)-1beta, IL-6, and tumor necrosis factor (TNF)-alpha, in rat peritoneal cells. The results showed that spleen cell proliferation increased when treated with Ulmus minor bark extract in combination with lipopolysaccharide or concanavalin A at a concentration of 500 mg/kg body weight, compared to the control group. Furthermore, the highest production of IL-6 and TNF-alpha was observed at a concentration of 500 mg/kg body weight. In conclusion, this study demonstrates that Ulmusdavidiana var. japonica (a variety of Ulmus minor bark) extract can enhance immune properties such as spleen cell proliferation and cytokine production in activated macrophages, and it may offer protective effects in mice (40).
Administration of hydroalcoholic and aqueous extracts of Ulmus minor leaves, along with sulfasalazine, led to a significant reduction in pathological lesions, particularly in the necrosis and inflammation caused by acetic acid in the colon tissues. Histological examinations of the groups treated with sulfasalazine showed mild inflammation, partial healing of secretory glands, and mild wounds. In the group receiving the aqueous extract of Ulmus minor leaves at a dose of 100 mg/kg, mild wounds and inflammation were observed, along with the preservation of glandular structures and mucosal restoration. Secretory gland regeneration was also noted.
A 2017 study by You-Suk et al. investigated whether administration of Ulmus minor bark could improve immune competence and mitigate radiation-induced intestinal damage in irradiated mice. The results showed that radiation exposure reduced T cell proliferation in the spleen and decreased interleukin-1β and interleukin-6 levels from macrophages by week 2. Supplementation with Ulmus minor bark extract at a low concentration (50 mg/kg body weight; EB-50) increased T and B cell proliferation in irradiated mice. Histological analysis revealed that Ulmus minor-50 bark treatment mitigated intestinal damage caused by radiation, resulting in lower histological grades in comparison to the irradiated control group at week 4. The extract also decreased glutathione levels by day 5 and week 2, and reduced myeloperoxidase activity by week 4, suggesting that Ulmus minor bark may alleviate radiation-induced intestinal inflammation by improving immune modulation and reducing oxidative stress. These findings confirm the results of the present study, highlighting the potential protective effects of Ulmus minor bark (41).
5.1. Conclusions
Both groups receiving hydroalcoholic extracts of Ulmus minor leaves at doses of 100 mg/kg and 200 mg/kg showed slight improvements, although the improvement rate was lower compared to the sulfasalazine group but higher than the control group (P < 0.05). In the groups receiving aqueous extract, both 100 mg/kg and 200 mg/kg doses produced favorable effects, with significant differences observed compared to the control group (P < 0.05). However, no significant difference was found when compared to the sulfasalazine group. Overall, macroscopic examinations indicated that the aqueous extract of Ulmus minor leaves at 200 mg/kg had the best protective effect on colon tissue. The results showed that treatment with hydroalcoholic extracts at doses of 100 mg/kg and 200 mg/kg, and aqueous extract at 100 mg/kg, had favorable effects compared to the control group, with a significant difference (P < 0.05).