1. Background
The human body relies on the skin as a fundamental constituent – the largest organ that assumes the pivotal role of the primary barrier safeguarding against the external environment. This organ effectively regulates homeostasis, functioning as a repository of essential nutrients, a defensive mechanism, and a responsive agent to injuries. Whenever the skin is damaged by physical, chemical, or mechanical factors, it leads to a wound that hinders its regular functions and strength. Therefore, prompt restoration of the skin structure and functions is vital for protecting the body against invaders. The healing process of the wound starts immediately after the skin injury for the organism's homeostasis (1, 2). A coordinated interplay of different cellular and molecular events is necessary for effective wound repair.
Among the numerous signaling pathways involved, the Wnt signaling pathway has emerged as a critical regulator in multiple aspects of wound healing. The Wnt signaling pathway controls cellular processes during development, tissue homeostasis, and regeneration (3, 4). When Wnt ligands bind to Frizzled receptors on a cell's surface, it activates the Wnt pathway, which triggers intracellular signaling cascades. These signaling pathways eventually affect the expression of specific target genes responsible for cell proliferation, migration, and differentiation (4). In wound healing, the Wnt pathway has been shown to play a crucial role in various stages of the process (5). During the inflammatory phase, the Wnt pathway regulates immune cell recruitment and inflammatory cytokine production, which is essential for clearing pathogens and initiating tissue repair. In the proliferative phase, the Wnt pathway promotes fibroblast migration, extracellular matrix synthesis, and angiogenesis, facilitating granulation tissue formation (6). Finally, the Wnt pathway regulates tissue remodeling and scar formation in the remodeling phase (7, 8).
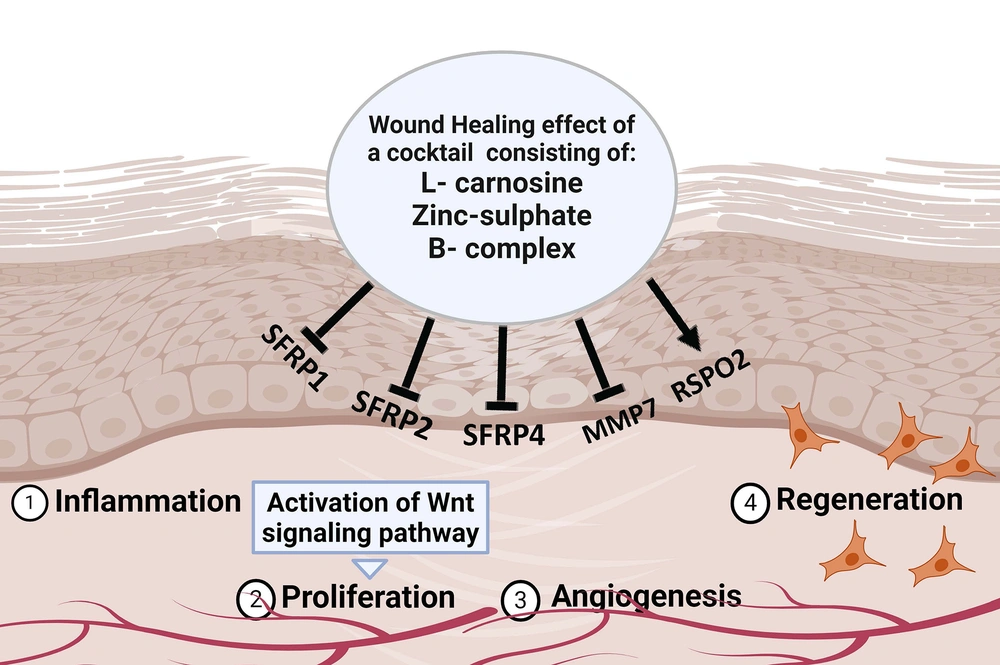
Several genes have been identified within the Wnt pathway as key regulators of wound healing. As shown in Figure 1, one such group of genes are SFRP1, SFRP2, SFRP4, MMP7, and RSPO2. The precise interplay between these genes within the Wnt pathway is important for orchestrating the complex wound-healing processes. Alterations in their expression levels might influence the process of wound repair. During the wound healing process, a range of soluble elements could affect the regulation of the Wnt pathway by changing the expression of related genes, eventually impacting fibroblasts' behavior and accelerating wound healing (9).
L-carnosine is an endogenous dipeptide recognized for its anti-oxidant and anti-inflammatory properties, making it a promising candidate for wound healing applications. Some studies have demonstrated that L-carnosine can promote tissue repair, enhance collagen synthesis, and modulate inflammatory response (10). It was shown that L-carnosine, through some signaling pathways, including Wnt pathways, could activate the fibroblast cells, resulting in wound healing. Zinc sulfate has been used in wound healing for its anti-oxidant effect (11). Zinc plays a multifaceted part in wound healing, involving its necessity in collagen and protein production, cellular growth, and immune capability. All these factors are central to tissue rejuvenation and mending (12). B-complex vitamins, including vitamins B1, B6, B9 (folic acid), and B12, are essential for cellular metabolism and energy production. Deficiencies in B vitamins have been linked to delayed wound healing; however, supplementation has been shown to enhance wound closure rates and promote tissue regeneration (13).
In recent years, there have been many advancements in medical devices designed to treat chronic wounds and promote skin tissue regeneration. These devices include wound dressings (14), wearable wound monitors (15), negative pressure wound therapy devices (16), and surgical sutures (17). They often contain a variety of natural and synthetic polymers, as well as bioactive molecules, such as chemical drugs, silver, growth factors, stem cells, and plant compounds. However, no single wound dressing is able to effectively treat all types of wounds due to varying rates of exudate production (18). Therefore, further research is required to explore the possibilities of new bioactive agents to enhance the regeneration of skin tissues.
In summary, the current study introduces an approach by examining the wound-healing potential of a cocktail comprising L-carnosine, zinc sulfate, and B-complex vitamins. By focusing on the modulation of the Wnt signaling pathway and the expression of key genes involved in wound healing, this study contributes valuable insights that might pave the way for innovative therapeutic interventions in skin tissue regeneration. This study highlights the potential of synergistic combinations of bioactive agents for enhanced wound healing outcomes.
2. Methods
This research study was conducted in compliance with ethical guidelines. The Birjand University of Medical Sciences Ethics Committee in Iran issued the approved ethics ID IR.BUMS.REC.1402.256.
2.1. Cell Culture and Maintenance
The human dermal fibroblast (HDF) (ATCC PCS-201-041) used in this study was provided by the National Cell Bank of Iran (Pasteur Institute, Tehran, Iran). The cells were cultured and maintained in a controlled environment at a temperature of 37°C in a 5% CO2 atmosphere using Dulbecco's Modified Eagle Medium (DMEM) (High Glucose, Glutamax, Bioidea, Iran). The growth medium was supplemented with 10% (v/v) fetal bovine serum (FBS) (Gibco, USA) and 100 IU/mL penicillin-streptomycin (Bioidea, Iran) to enhance the survival of cells.
2.2. Cocktail Preparation
Based on the MTT results of our previous study (not published yet) on HDF cells, we have identified the optimum dosage of each component for the highest HDF cell proliferation rate, compared to the control group. This dosage includes 6.25 mg/mL L-carnosine (Sigma-Aldrich, USA), 12.5 μg Zinc-sulphate (Sigma-Aldrich, USA), and B-complex (Merck, Germany) at a concentration of 25 µg/mL B1 and B6, 0.5 µg/mL B12, and 0.15 µg/mL B9. These amounts were selected to prepare the cocktail.
2.3. Wound Healing Effects of the Cocktail Through the Scratch Assay
The scratch test was conducted to assess the effectiveness of the prepared cocktail in the proliferation of HDF cells. Human dermal fibroblast cells were seeded in 12-well plates at a density of 4 × 105 cells per well. After a 24-hour treatment, a straight scratch was created using a 200 μL yellow pipette tip. The wound healing was observed using a microscope at 0, 6, 12, and 24 hours after treatment with the cocktail. Photographs were taken to capture the boundary areas of the scratches at different time points. The ImageJ software (version 1.52) was employed to analyze the scratch area. Specifically, the ratio between the initial open space at 0 hour and the remaining space after 24 hours was calculated. The experiment was performed three times with triplicate samples within each individual experiment (19).
2.4. Quantitative Real-time Polymerase Chain Reaction Analysis
For the ribonucleic acid (RNA) extraction, the HDF cells were cultured in a 6-cm cell culture plate at a density of 15 × 105 cells per well. After 24 hours of incubation, the cell culture plates were subjected to scratching to simulate a wound. One scratched plate was treated with a particular concentration of prepared cocktail; however, the other was left as the control group. The total RNA was extracted from both groups using a Trizol-based RNA extraction method (Sinaclone-RNX-Plus, Iran), and the complementary deoxyribonucleic acid (cDNA) synthesis was performed using the cDNA synthesis kit (Parstous, Iran). The quantitative real-time polymerase chain reaction (RT-PCR) for SFRP1, SFRP2, SFRP4, MMP7, and RSPO2 was carried out using the specific primers (Table 1). Gene amplification was performed in the ABI Step One™ Real-time PCR System (Applied Biosystems, Foster City, CA). The GAPDH gene was used to normalize the relative expression for interested genes calculated by the ΔΔCT method and SYBR Green kit (Parstous, Iran). All experiments were performed in triplicates (20).
| Primer | Sequence |
|---|---|
| SFRP1 | F: CTTCTACTGGCCCGAGATGCT |
| R: ATGGCCTCAGATTTCAACTCGT | |
| SFRP2 | F: AGCCCGACTTCTCCTACAAGC |
| R: CTTCATGACCAGCGGGATCCA | |
| SFRP4 | F: ACAAATTCTTCTTGCCAGTGTC |
| R: GCCTCTCTTCCCACTGTATG | |
| RSPO2 | F: CTTGTAGCAGAAATAATCGCACATGT |
| R: TGCCTCATTGTCATCTTGCATC | |
| MMP7 | F: GAATGTTAAACTCCCGCGTC |
| R: CGATCCACTGTAATATGCGGTA |
2.5. Statistical Analysis
All statistical calculations were carried out in GraphPad Prism (version 9.4.1) using analysis of variance (ANOVA) with all paired-wise multiple comparison procedures (Tukey). The values are presented as means ± standard deviation (SD), and a p-value < 0.05 was considered statistically significant (20).
3. Results
3.1. Wound-Healing Effects of Cocktail on HDF Cells
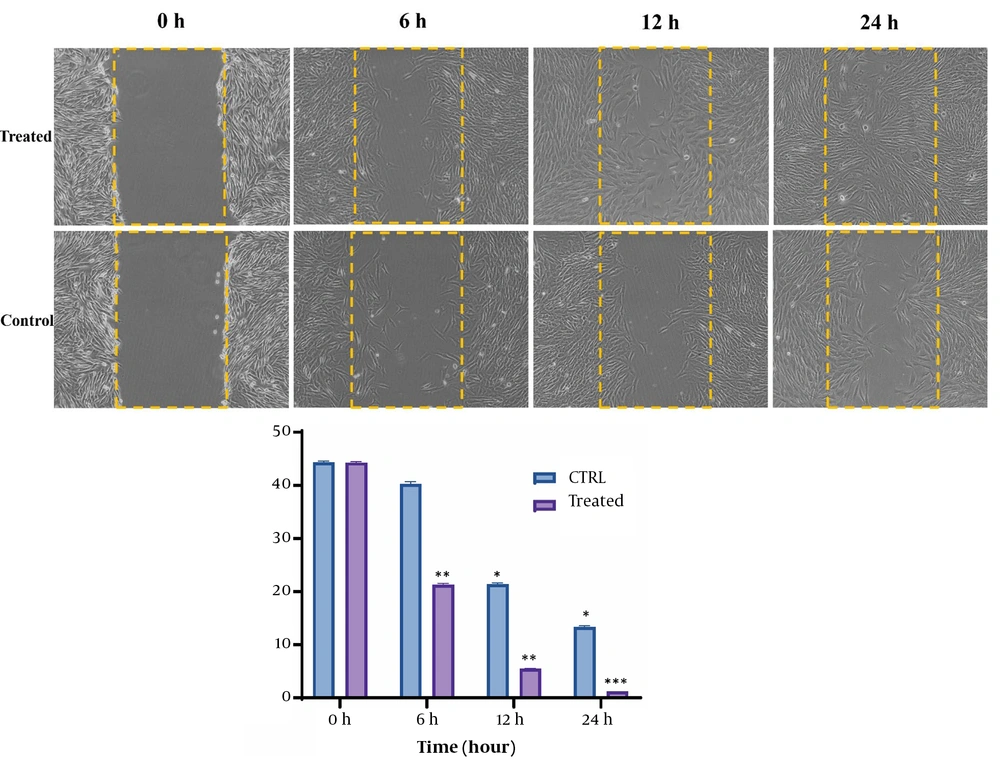
The scratch test was conducted to evaluate the wound-healing effects of the cocktail on the HDF cells. The effect of the cocktail on HDF cell migration and proliferation was investigated under the established conditions. As shown in Figure 2, a significant difference was observed in the treated group, compared to the control group, after 0, 6, 12, and 24 hours. After 6 hours, when the extent of wound closure was 21.3% for the treated group, the extent of closure for the control group was 40%. After 12 hours of treatment, the extent of scratch closure decreased to 4.4%; nevertheless, the control group decreased to 21.4%. Interestingly, 24 hours following the same treatment, the scratch was almost completely closed, compared to 13% of the scratches for the control group.
Scratch assay showing the effects of prepared cocktail on the wound healing process of HDF cells. Under 100% confluence, a scratch was made on wells using a sterile yellow-colored pipette tip. Subsequently, a determined concentration of the cocktail was added to the cells. Pictures were taken at 0, 6, 12, and 24 hours after treatment, and the amount of migration was calculated. P < 0.001, P < 0.01, and P < 0.05, respectively ***, **, * (HDF: Human dermal fibroblast).
3.2. Change in the Expression of Genes Involved in the Wnt Pathway by Cocktail
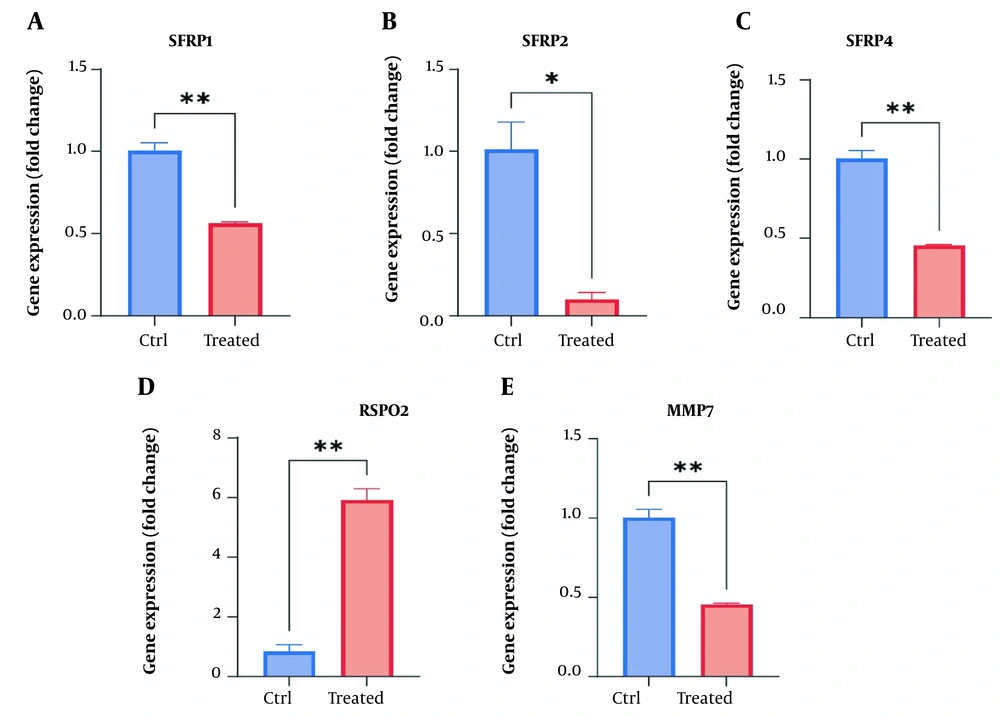
The RT-qPCR data demonstrated that treating the HDF cells with the prepared cocktail effectively decreased the expression levels of SFRP1 (1.76-fold down, P < 0.01), SFRP2 (10-fold down, P < 0.01), SFRP4 (2.1-fold down, P < 0.01), and MMP7 (2.2-fold down, P < 0.01) while increasing the expression of RSPO2 (5.9-fold up, P < 0.01) (Figure 3).
Gene expression levels of SFRP1, SFRP2, SFRP4, MMP7, and RSPO2 in HDF cells in response to cocktail treatment. The Y-axis represents the fold change in the expression of the target gene compared to the untreated group. *: Significant difference compared to the untreated cells (2(−ΔΔCt) = 1 in untreated group). P < 0.01 and P < 0.05, respectively **, * (HDF: Human dermal fibroblast).
4. Discussion
When the body experiences an injury, a series of interconnected processes are triggered, primarily aimed at stopping bleeding and isolating the damaged tissues from the rest of the body to ensure internal balance. The initiation of Wnt signaling seems to constitute an early molecular reaction to such injuries. Research has indicated that Wnt signaling is critical in stimulating fibroblasts, which are responsible for generating collagen and extracellular matrix elements crucial in the wound closure process. Fibroblasts effectively coordinate the entire repair sequence by developing numerous regulatory molecules and establishing communication with other cellular populations engaged in the healing mechanisms (21).
This study investigated the wound healing effects of a cocktail comprising L-carnosine, zinc-sulfate, and B-complex vitamins, targeting the Wnt signaling pathway in normal human fibroblast cells. The expression of six significant genes involved in the Wnt signaling pathway, namely SFRP1, SFRP2, SFRP4, MMP7, and RSPO2, was examined to determine the potential influence of the cocktail on wound healing.
Initially, the cocktail was prepared, and its wound-healing effect on HDF cells was evaluated using the scratch assay. The results indicated that the cells treated with the cocktail exhibited more effective scratch repair and regeneration than the control group.
Zinc, an essential micronutrient in the human body at concentrations below 50 mg/kg, holds particular importance for skin health. Mild deficiencies in zinc might lead to skin roughness and impaired wound healing, given its abundance in the epidermis (11). In this way, a study by Momen-Heravi et al. shows that a 12-week zinc supplementation intervention demonstrated significant benefits for patients with grade 3 diabetic foot ulcers. Their findings suggest that zinc supplementation over 12 weeks can positively impact ulcer size and metabolic profiles in diabetic foot ulcer patients (22).
L-carnosine is a potent anti-oxidant amino acid that accelerates wound healing by regulating signaling pathways (23). Sakae et al. examined the effects of L-carnosine and zinc complex on pressure ulcers in a study involving 42 patients. Both treatment groups (L-carnosine-zinc and L-carnosine alone) showed a significant improvement in wound healing, compared to the control group. The aforementioned study suggested that L-carnosine and zinc could be potential treatments for pressure ulcers, with short-term use providing quicker improvement (10).
In another study, Sakae and Yanagisawa investigated the dietary treatment of pressure ulcers with the L-carnosine and zinc complex. The aforementioned study demonstrated a significant improvement in wound healing after one week of treatment with the complex, with a more pronounced effect by the end of the eighth week. The complex showed potential in accelerating skin cell regeneration and altering serum zinc and copper levels, emphasizing its role as an effective dietary treatment for pressure ulcers (24). Sonamuthu et al. investigated the role of L-carnosine dipeptides hydrogel in treating infected diabetic wounds. The hydrogel-bound L-carnosine-curcumin complex demonstrated anti-oxidant and anti-inflammatory effects, leading to a faster improvement in diabetic skin wounds (25).
Studies have shown that the intake of nutrient supplements, especially vitamin B-complex, can positively impact the processes involved in wound healing (26-28). For example, Rembe et al. focused on the effects of local B vitamins and vitamin C on human skin cells in chronic wounds. The aforementioned study highlighted the significant role of B vitamins and vitamin C in the proliferation and migration of keratinocytes and fibroblasts after 72 hours, which is crucial for accelerating the wound healing process (27). Additionally, Neiva et al. conducted a clinical trial study to investigate the effects of vitamin B-complex supplementation on periodontal wound healing in patients with chronic periodontitis. The vitamin-B group exhibited improvement in both shallow and deep periodontal sites, emphasizing the potential of vitamin-B supplementation in wound healing (28).
Then, we focused on the effect of the prepared cocktail on wound healing by influencing the genes involved in the Wnt signaling pathway. The current gene expression analysis revealed that treating HDF cells with the cocktail led to decreased expression of SFRP1, SFRP2, SFRP4, and MMP7 while increasing the expression of RSPO2. These findings suggest that the cocktail might enhance the Wnt pathway by reducing the inhibitory effects of SFRP1, SFRP2, SFRP4, and MMP7 genes while simultaneously increasing the enhancing effect of the RSPO2 gene.
RSPO2 is a Wnt agonist that enhances Wnt signaling by potentiating the interaction between Wnt ligands and Frizzled receptors (29, 30). Research indicated that introducing recombinant human RSPO2 into a controlled environment led to a notable rise in the growth of fibroblasts. This effect was enhanced in conjunction with Wnt3a, operating through the canonical Wnt/β-catenin pathway. Additionally, when Rspo2 was excessively expressed in regular fibroblasts, a thicker epidermis was observed in contrast to control fibroblasts within a model of skin organotypic culture (31). The findings of the present study align with the findings of previous studies, indicating that the expression of RSPO2 significantly increased during the healing of treated HDF cells.
On the other hand, several proteins have been identified within the Wnt pathway as the negative regulators of the Wnt pathway. One such group is the secreted frizzled-related proteins (SFRPs), including SFRP1, SFRP2, and SFRP4. These groups of proteins bind to Wnt ligands, inhibiting their interaction with Frizzled receptors and thereby attenuating Wnt signaling (32, 33). Studies showed that blocking SFRP1 genes improves palatal healing outcomes in the mouse model (34). The decreased expression of SFRP1 and SFRP2 in proliferative keloid tissue during wound healing was also observed (35).
Furthermore, certain investigations revealed that among humans, the expression of SFRP4 has been linked to skin conditions characterized by fibrosis, including psoriasis and Dupuytren's disease (36). It was also noted that considerable breakdown of SFRP4 by macrophages in areas of immune activity within hidradenitis suppurativa (HS) skin is connected to persistent Wnt activity and fibrogenesis. These are conditions that similarly manifest in Dupuytren's syndrome (37). The present study also demonstrated that in HDF-treated cells, the expression levels of SFRP1, SFRP2, and SFRP4 were decreased, which might activate the Wnt pathway.
Another gene involved in the Wnt pathway and wound healing is matrix metalloproteinase 7 (MMP7). MMP7, which is one of the target genes of the β-catenin-dependent Wnt pathway, is an enzyme that degrades extracellular matrix components and promotes tissue remodeling. Studies have demonstrated a connection between MMPs and effective surgical closure timing in acute traumatic wounds (38). Research has shown that MMP7 levels rise during the initial phases of wound healing and subsequently decrease as the wound progresses toward healing completion. It seems that a certain degree of increasing MMP7 is essential for the standard process of wound closure. Nonetheless, excessive expression of MMP7 might impede or disrupt the typical course of wound healing, particularly when elevated by cytokines found within the inflammatory environment (39-41).
Additionally, researchers have shown that wounded areas with difficulty healing are associated with increased levels of pro-inflammatory MMP2 and MMP7. Therefore, the prognosis of traumatic wounds could be predicted by measuring MMP2, MMP3, and MMP7 levels in serum and effluent (38). The current research findings showed decreased MMP7 levels within fibroblast cells during RNA extraction. This reduction coincided with the cells being in the final phase of wound healing (24 hours post-injury).
4.1. Conclusions
In conclusion, the present study provides compelling evidence for the potent wound-healing properties of the cocktail comprising L-carnosine, zinc sulfate, and B-complex vitamins. The scratch assay demonstrates the cocktail's ability to accelerate wound closure, emphasizing its effectiveness in promoting fibroblast proliferation and migration—the pivotal stages in wound healing. Molecular analyses reveal specific genes within the Wnt pathway that undergo modulation in response to the cocktail, confirming pathway activation and suggesting a sophisticated orchestration of gene expression contributing to enhanced wound repair. Although these findings are encouraging, further research, including in vivo studies and clinical trials, is imperative to validate these promising outcomes for practical applications in wound healing.