1. Background
Diabetes mellitus (DM) is a chronic metabolic disease in which hyperglycemia occurs due to the lack of insulin production or insensitivity of the related receptors (1). According to the estimations, there will be 700 million diabetic individuals globally by 2045 (2). Basically, delaying postprandial hyperglycemia in the gastrointestinal canal is one of the substantial therapeutic approaches for treating DM, particularly type 2, that is achieved by decreasing glucose absorption through the inhibition of carbohydrate-hydrolyzing enzymes. Alpha-amylase and α-glucosidase are two primary enzymes located in the brush border of the intestinal villi and are responsible for the breakdown of carbohydrates. Alpha-amylase degrades long-chain carbohydrates; however, starch and disaccharides are converted to glucose by α-glucosidase, leading to an increase in plasma glucose levels and postprandial hyperglycemia. As α-glucosidase is an essential enzyme that catalyzes the final stage of carbohydrate digestion, its inhibitors have been at the center of attention. Acarbose, miglitol, and voglibose are substantial medications for decreasing blood sugar levels in diabetic patients that inhibit α-glucosidase inhibitors (3). However, they have caused gastrointestinal side effects, notably flatulence, diarrhea, abdominal pain, and pneumatosis intestinalis in patients with DM (4). In this respect, the design and development of synthetic (5-7) and natural α-glucosidase inhibitors (8, 9) have received great attention.
Hyperglycemia is associated with various metabolic disorders and oxidative stress. An increase in the level of free radicals in the long term brings up some concerns, such as cancer, cardiovascular, and neurological disorders. Additionally, oxidative stress itself has been linked to the development and progression of diabetes (10). As a result, it is critical to find compounds possessing both antioxidant properties and hypoglycemic effects.
Medicinal herbs have been used traditionally to treat diabetes, and researchers are currently focusing on the bioactive compounds derived from plant extracts or their essential oils (EOs) to develop more potent and safer medications. On the other hand, EOs are high in a variety of biochemicals that have a wide range of activities, such as antioxidant properties (11).
Salvia, the largest genus of the Lamiaceae family, possesses over 900 species of herbaceous aromatic plants and can be found all over the world, especially in Asia. There are 60 species of Salvia in Iran, and among them, 17 species are endemic. Salvia species have been used to treat a variety of diseases, such as colds, aches, infections, bronchitis, Alzheimer’s disease, and hemorrhage (12, 13). Furthermore, extracts and EOs from various Salvia spp. have received great attention in modern medicine. The Salvia sclarea EOs from two different regions of Lebanon were tested for acute and subchronic anti-diabetic effects in alloxan-induced diabetic rats and indicated that the plant collected from Beirut reduced blood glucose levels by 51.70% and 52.00% in acute and subchronic tests (at the concentration of 200 mg/kg), respectively (14). In another study, the S. officinalis EO in the flowering stage was observed to have an α-glucosidase inhibitory activity (half-maximal inhibitory concentration [IC50] = 22.24 ± 0.07 µg/mL, compared to acarbose as a standard [IC50 = 12.31 ± 0.05 µg/mL]) (15).
Salvia mirzayanii (known as “Moor-e-Talkh” in Persian) is a plant native to the southern parts of Iran. Salvia hypoleuca (known as “Boland Maryam-goli” in Persian) is a tall perennial plant that is widespread in Iran, in the northern and central provinces, particularly in Alborz, Tehran, and Mazandaran provinces. They have been used for years in the treatment of various diseases by local individuals living in the aforementioned regions.
2. Objectives
To the best of our knowledge, the α-glucosidase inhibitory activity of S. mirzayanii and S. hypoleuca EOs has not been reported in the literature, and this study aimed to identify the components of the EOs of the plants and evaluate their α-glucosidase inhibitory activity. An attempt was also made to study any synergistic or antagonistic interactions of selected main bioactive compounds on this enzyme.
3. Methods
3.1. Plant Material
The flowering aerial parts of the two Salvia species were collected from different parts of Iran (Fars and Alborz provinces), identified by a botanist, deposited in the Herbarium of the Faculty of Pharmacy, Tehran University of Medical Sciences, and dried in a dark place at room temperature.
3.2. Essential Oil Extraction
Essential oils were extracted by the hydrodistillation of each powdered dried plant (100 g) for 4 hours using the Clevenger apparatus (16). The water residue was removed by the anhydrous sodium sulfate, and EOs were stored in amber vials at refrigerator temperature (4°C).
3.3. Gas Chromatography-Mass Spectroscopy Analysis
Gas chromatography-mass spectroscopy (GC-MS) analysis was conducted on a 7890B Agilent® gas chromatograph, including a DB-5 column (60 cm, 0.25 µ) coupled with a 5977A Agilent® mass spectrometer. One µL of diluted samples (1:100, EO: Ethyl acetate) was used for the corresponding analysis. The oven temperature was programmed to start at 40°C (held for 7 minutes), raised to 140°C by 10°C/minute, and then increased to 250°C at a rate of 3°C/minute, where it was kept for 7 minutes. Helium (99.99%) was used as a carrier gas (flow rate: 1 mL/min), and the ionization voltage of the detector was set at 70 eV. Normal alkanes (C7 - C21) were injected in the same condition to compare calculated retention indices (RI) with references. For more precise identification, the mass spectra of each compound were compared to those in the National Institute of Standards and Technology (NIST) database (https://webbook.nist.gov/chemistry/ accessed 8 January 2022) and Dr. Adams’ book (17).
3.4. Chemicals
Linalool, 1,8-cineole, α-terpineol, α-pinene, β-pinene, caryophyllene oxide, gallic acid, Folin-Ciocalteu reagent, α-glucosidase, p-nitrophenyl α-D-glucopyranoside, and buffers were obtained from Sigma-Aldrich (USA). Solvents and DPPH (2,2-diphenyl-1-picrylhydrazyl) were obtained from Merck (USA).
3.5. Alpha-Glucosidase Inhibitory Assay
The evaluation of α-glucosidase inhibitory activity of EOs and other samples was performed according to the literature (5). Briefly, all chemicals were pre-incubated in a water bath for 10 minutes at 37°C, and different concentrations of each sample were added to 155 µL of the 0.1 U/mL α-glucosidase solution prepared in a sodium phosphate buffer (50 mM). The mixture was then incubated for an additional 10 minutes at 37°C. Then, 25 µL of 4 mM para-nitrophenol-D-glucopyranoside (pNPG) solution was added to the mixture and re-incubated for 20 minutes at 37°C. The absorbance was read at 405 nm, each experiment was repeated three times, and acarbose was used as the positive control. Finally, based on the enzyme activity in the absence of the inhibitor (negative control), results were presented as inhibition percentage or IC50 values.
3.6. Antioxidant Activity: 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Assay
The DPPH assay was used to evaluate the anti-oxidative properties of the EOs with small modifications (18). For this purpose, 100 mg of each EO was diluted with methanol to achieve a concentration of 10 mg/mL. To obtain a 1 mg/mL concentration, 1 mL of the prepared solutions was diluted with 9 mL of methanol. Then, various concentrations were prepared using serial dilution (500, 250, 125, 62.5, and 31.25 µg/mL). Afterward, 2 mL of DPPH solution was added to 1 mL of each concentration of samples, and the mixtures were then placed in a dark room for 30 minutes. Methanol and quercetin were used as a blank and a reference, respectively. At the end, the absorbance of each sample was measured using a spectrophotometer at 517 nm. This procedure was replicated three times for each sample, and then IC50 values were calculated.
3.7. Determination of Total Phenolic Content
The Folin-Ciocalteu assay was used with some modifications to determine the TPC of each EO (19). To achieve a concentration of 1 mg/mL, 10 mg of each EO was diluted with methanol to the final volume of 10 mL. After preparation, 1.5 mL of diluted Folin-Ciocalteu reagent (1 mL of reagent in distilled water up to 10 mL) was added to 1 mL of the prepared EO solution with a concentration of 1 mg/mL and kept in a dark place for 10 minutes. Following that, 1.5 mL of sodium bicarbonate solution (7.5% w/v in water) was added to the initial mixture, and the final solution was kept in a dark place for 30 minutes. Finally, the absorbance of the samples was measured using a spectrophotometer at 765 nm. Gallic acid was used as the reference standard to create a calibration curve. For each sample, these procedures were repeated three times.
4. Results
4.1. Essential Oils Extraction Yield
The EOs extraction yields were calculated as 0.84 %w/w and 0.44 %w/w for S. mirzayanii and S. hypoleuca, respectively (Table 1).
| Species | Collection Site | Collection Date | Voucher No. | Longitude | Latitude | Altitude (m) | Yield (w/w%) |
|---|---|---|---|---|---|---|---|
| Salvia mirzayanii Rech. f. and Esfand. | Mazayjan, Darab, Fars province, Iran | May 2021 | 7112-TEH | 53.80° E | 30.29° N | 850 | 0.84 |
| Salvia hypoleuca Benth. | Gateh Deh, Taleghan, Alborz province, Iran | April 2021 | 7075-TEH | 51.06° E | 36.17° N | 2150 | 0.44 |
4.2. Chemical Composition of Essential Oils
Salvia mirzayanii and S. hypoleuca EOs were analyzed using GC-MS, leading to the identification of 54 and 34 components for each EO, respectively, reported in Table 2.
| Number | Compounds | RI a | RI b | % Relative Peak Area | Class | |
|---|---|---|---|---|---|---|
| S. mirzayanii | S. hypoleuca | |||||
| 1 | α-Pinene | 934 | 934 | 3.74 | 9.04 | MH |
| 2 | Camphene | 952 | 952 | - | 1.63 | MH |
| 3 | Sabinene | 973 | 973 | 0.35 | 2.06 | MH |
| 4 | β-Pinene | 981 | 981 | 3.25 | 8.47 | MH |
| 5 | β-Myrcene | 986 | 986 | 1.31 | - | MH |
| 6 | α-Terpinene | 1018 | 1015 | - | 0.38 | MH |
| 7 | Limonene | 1032 | 1029 | 1.13 | 0.42 | MH |
| 8 | 1,8-Cineole | 1037 | 1034 | 5.20 | 1.14 | OM |
| 9 | cis-β-Ocimene | 1043 | 1040 | 0.62 | - | MH |
| 10 | γ-Terpinene | 1060 | 1057 | 0.65 | 0.31 | MH |
| 11 | Terpinolene | 1090 | 1088 | 0.55 | - | MH |
| 12 | Linalool | 1099 | 1097 | 9.97 | 1.64 | OM |
| 13 | trans-Pinocarveol | 1151 | 1146 | - | 0.29 | OM |
| 14 | δ-Terpineol | 1179 | 1170 | 0.49 | - | OM |
| 15 | Borneol | 1185 | 1176 | - | 0.60 | OM |
| 16 | Terpinen-4-ol | 1191 | 1184 | - | 0.62 | OM |
| 17 | α-Terpineol | 1205 | 1198 | 8.33 | 0.23 | OM |
| 18 | Myrtenol | 1209 | 1202 | 0.14 | - | OM |
| 19 | Nerol | 1228 | 1221 | 0.89 | - | OM |
| 20 | Geraniol | 1249 | 1246 | 2.28 | - | OM |
| 21 | Linalyl acetate | 1254 | 1249 | 1.43 | - | OM |
| 22 | Decanol | 1270 | 1269 | 0.32 | - | Alcohol |
| 23 | Thymol | 1290 | 1286 | 4.36 | 1.53 | PC |
| 24 | Carvacrol | 1296 | 1287 | 2.89 | 1.18 | PC |
| 25 | δ-Elemene | 1346 | 1338 | - | 3.20 | SH |
| 26 | α-Terpinyl acetate | 1353 | 1344 | 8.15 | - | OM |
| 27 | Eugenol | 1359 | 1351 | 0.60 | - | PC |
| 28 | Neryl acetate | 1371 | 1362 | 1.91 | - | OM |
| 29 | α-Copaene | 1389 | 1377 | 0.72 | 1.77 | SH |
| 30 | β-Bourbonene | 1403 | 1388 | - | 1.60 | SH |
| 31 | β-Elemene | 1404 | 1391 | 1.27 | - | SH |
| 32 | α-Gurjunene | 1428 | 1413 | 1.33 | - | SH |
| 33 | trans-β-Caryophyllene | 1441 | 1430 | 1.22 | 14.12 | SH |
| 34 | β-Gurjunene | 1451 | 1436 | - | 0.39 | SH |
| 35 | α-Guaiene | 1459 | 1441 | 1.15 | - | SH |
| 36 | Aromandendrene | 1460 | 1445 | 1.22 | - | SH |
| 37 | cis-Muurola-3,5-diene | 1467 | 1450 | 0.37 | - | SH |
| 38 | α-Humulene | 1476 | 1462 | 0.39 | 1.91 | SH |
| 39 | 9-epi-Caryophyllene | 1482 | 1466 | - | 3.93 | SH |
| 40 | Cadina-1(6),4-diene | 1488 | 1477 | 0.47 | - | SH |
| 41 | γ-Muurolene | 1493 | 1479 | 1.05 | 0.92 | SH |
| 42 | Germacrene D | 1501 | 1485 | 0.47 | 8.69 | SH |
| 43 | β-Selinene | 1503 | 1490 | 0.93 | 0.68 | SH |
| 44 | δ-Selinene | 1509 | 1493 | 1.11 | - | SH |
| 45 | α-Muurolene | 1517 | 1500 | 2.85 | - | SH |
| 46 | Bicyclogermacrene | 1521 | 1500 | 2.19 | 7.46 | SH |
| 47 | γ-Cadinene | 1528 | 1514 | 1.29 | 1.09 | SH |
| 48 | δ-Cadinene | 1532 | 1523 | 3.67 | - | SH |
| 49 | Liguloxide | 1549 | 1536 | 0.33 | - | OS |
| 50 | α-Cadinene | 1551 | 1539 | 0.64 | - | SH |
| 51 | Ledol | 1590 | 1574 | 0.35 | - | OS |
| 52 | Germacrene D-4-ol | 1596 | 1576 | 2.19 | - | OS |
| 53 | Spathulenol | 1597 | 1578 | 3.17 | 6.11 | OS |
| 54 | Caryophyllene oxide | 1605 | 1584 | 0.40 | 12.17 | OS |
| 55 | Viridiflorol | 1613 | 1593 | 0.51 | 0.39 | OS |
| 56 | α-epi-7-epi-5-Eudesmol | 1625 | 1608 | 0.29 | - | OS |
| 57 | 1,10-di-epi-Cubenol | 1638 | 1619 | 0.30 | - | OS |
| 58 | epi-Cubenol | 1644 | 1627 | 0.75 | - | OS |
| 59 | Isospathulenol | 1645 | 1629 | - | 1.48 | OS |
| 60 | Eremoligenol | 1650 | 1631 | 0.39 | - | OS |
| 61 | epi-α -Muurolol | 1660 | 1642 | 0.93 | - | OS |
| 62 | β-Eudesmol | 1674 | 1651 | 1.32 | - | OS |
| 63 | α-Cadinol | 1676 | 1654 | 3.77 | 0.59 | OS |
| 64 | Shyobunol | 1717 | 1691 | 2.87 | - | OS |
| 65 | Sclareoloxide | 1915 | 1906 | - | 1.90 | OS |
| 66 | Phytol | 2100 | 2089 | - | 0.95 | OD |
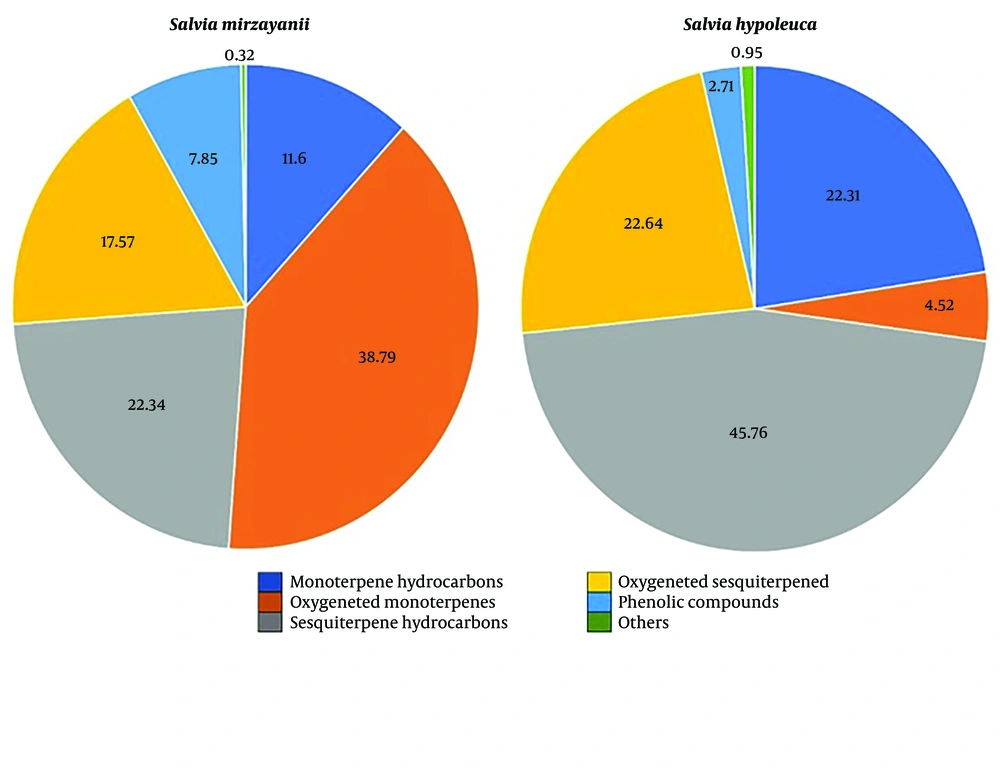
| Monoterpenes | 50.39 | 26.83 | ||||
| Monoterpene hydrocarbons | 11.60 | 22.31 | - | |||
| Oxygenated monoterpenes | 38.79 | 4.52 | - | |||
| Sesquiterpenes | 39.91 | 68.40 | ||||
| Sesquiterpene hydrocarbons | 22.34 | 45.76 | - | |||
| Oxygenated sesquiterpenes | 17.57 | 22.64 | - | |||
| Alcohol | 0.32 | - | - | |||
| Phenolic compounds | 7.85 | 2.71 | - | |||
| Oxygenated diterpene | - | 0.95 | ||||
| Total identified | 98.47 | 98.89 | - | |||
Abbreviations: MH, monoterpene hydrocarbon; OM, oxygenated monoterpene; SH, sesquiterpene hydrocarbon; OS, oxygenated sesquiterpene; PC, phenolic compound; OD, oxygenated diterpene.
a Retention index (calculated)
b Retention index from literature
4.3. Alpha-Glucosidase Inhibitory Activity
The α-glucosidase inhibitory activity of S. mirzayanii and S. hypoleuca EOs and 6 selected standard compounds, including linalool, α-terpineol, 1,8-cineole, caryophyllene oxide, α-pinene, and β-pinene, was reported in Table 3, compared to acarbose as the reference drug. The components were selected based on their abundance in the EOs of different Salvia spp. (20). According to the data in Table 3, S. mirzayanii EO could inhibit the enzyme with an IC50 value of 55.15 ± 1.60 mg/mL, and that of S. hypoleuca showed no activity. Among the tested constituents, α-pinene (IC50 = 17.59 ± 0.19 mg/mL) had the lowest IC50 value followed by caryophyllene oxide (19.94 ± 0.26 mg/mL), linalool (IC50 = 38.00 ± 0.22 mg/mL), 1,8-cineole (IC50 = 47.95 ± 0.23 mg/mL), and α-terpineol (IC50 = 122.10 ± 0.29 mg/mL).
| Sample | IC50 (mg/mL) |
|---|---|
| S. mirzayanii EO | 55.15 ± 1.60 |
| S. hypoleuca EO | Not active |
| Linalool | 38.00 ± 0.22 |
| 1,8-Cineole | 47.95 ± 0.23 |
| α-Terpineol | 122.10 ± 0.29 |
| α-Pinene | 17.59 ± 0.19 |
| β-Pinene | nd a |
| Caryophyllene oxide | 19.94 ± 0.26 |
| Acarbose | 0.10 ± 0.00 |
Abbreviations: IC50, half-maximal inhibitory concentration; EO, essential oil.
a Not determined due to precipitation in buffer moiety.
4.4. Antioxidant Activity and Total Phenolic Content
Antioxidant capacity based on the DPPH assay and TPC of the EOs were measured, and the results are shown in Table 4. Salvia mirzayanii EO showed higher antioxidant activity (IC50 = 0.778 ± 0.00 mg/mL) than S. hypoleuca EO (IC50 > 1 mg/mL). As expected, S. mirzayanii showed higher TPC (mg gallic acid equivalent [GAE]/g EO) than that of S. hypoleuca (Table 4).
| Sample | IC50 (DPPH Assay, mg/mL) | TPC (mg GAE/g EO) |
|---|---|---|
| S. mirzayanii | 0.77 ± 0.00 | 78.26 ± 1.26 |
| S. hypoleuca | > 1 | 49.43 ± 1.13 |
| Quercetin | 0.25 ± 0.00 | - |
Abbreviations: IC50, half-maximal inhibitory concentration; EO, essential oil; GAE, gallic acid equivalent; TPC, total phenolic content.
5. Discussion
In the present study, S. mirzayanii EO mainly contained monoterpenes (50.39%); however, sesquiterpenes accounted for 39.91% of the constituents. It also consisted of linalool (9.97%), α-terpineol (8.33%), α-terpinyl acetate (8.15%), 1,8-cineole (5.2%), and thymol (4.36%). In the S. hypoleuca EO, sesquiterpenes and monoterpenes accounted for 68.4% and 26.83%, respectively. Among different classes of compounds, sesquiterpene hydrocarbons were observed to be the most abundant components (45.76%). Moreover, it mainly included trans-β-caryophyllene (14.12%), caryophyllene oxide (12.17%), α-pinene (9.04%), germacrene D (8.69%), and β-pinene (8.47%). Comparing the EOs components (Figure 1) revealed that the amounts of monoterpenes and phenolic compounds in S. mirzayanii are higher than those of S. hypoleuca; however, it contained higher amounts of sesquiterpene hydrocarbons. It merits mentioning that sesquiterpene hydrocarbons constituted the larger portion of sesquiterpenes in both EOs (Figure 1).
As reported in Table 5, the components of two EOs were compared to those documented in the literature. In a study reported by Hasheminya and Dehghannya, EO constituents of S. mirzayanii collected from Kerman, Iran, were identified, indicating the presence of monoterpene hydrocarbons (5.48%), and the main compounds also belonged to the oxygenated monoterpenes (34.34%) (21). In another study, the EO components of S. mirzayanii from Lamerd, Fars province, Iran, were classified as sesquiterpene hydrocarbons (26.10%), oxygenated monoterpenes (18.40%), oxygenated sesquiterpenes (11.00%), and monoterpene hydrocarbons (1.00%) (22). Furthermore, the results from the analysis of S. mirzayanii EO collected from Jahrom in Fars province, Iran, are in line with the data of the current study as oxygenated monoterpenes (56.50%) and monoterpene hydrocarbons (7.00%) were observed to be the highest and lowest abundant components (23).
| Species | Major Compounds | Ref. | ||
|---|---|---|---|---|
| In This Study | Literature | Region | ||
| S. mirzayanii | Linalool (9.97%), α-Terpineol (8.33%), α-Terpinyl acetate (8.15%), 1,8-Cineole (5.20%), Thymol (4.36%) | 1,8-Cineole (11.54%), Spathulenol (10.34%), α-Terpinyl acetate (10.32%), Bicyclogermacrene (6.34%), γ-Cadinene (5.67%), and Linalool (4.23%) | Kerman province, Iran | (21) |
| γ-Cadinene (12.5%), Caryophyllene oxide (8.50%), Bicyclogermacrene (7.70%), α-Terpinyl acetate (6.70%), Linalool (3.6%), 1,8-Cineole (2.60%) | Lamerd, Fars province, Iran | (22) | ||
| α-Terpinyl acetate (19.7%), Linalyl acetate (13.50%), Eudesm-7(11)-en-4-ol (9.10%), Linalool (7.40%), 1,8-Cineole (6.00%), δ-Cadinene (4.80), Bicyclogermacrene (4.70%), α-Terpineol (4.10%) | Jahrom, Fars province, Iran | (23) | ||
| S. hypoleuca | Trans-β-caryophyllene (14.12%), caryophyllene oxide (12.17%), α-pinene (9.04%), germacrene D (8.69%), β-pinene (8.47%) | Bicyclogermacrene (15.30%), trans- β-Caryophyllene (14.60%), Viridiflorol (13.30%), Spathulenol (12.50%), δ-Elemene (7.70%), β-Pinene (7.20%), α-Pinene (5.90%) | Haraz, Mazandaran province, Iran | (24) |
| Caryophyllene oxide (21.30%), Bicyclogermacrene (10.30%), trans-β-Caryophyllene (13.00%), β-Pinene (9.80%), α-Pinene (9.70%) | Mazandaran province, Iran | (25) | ||
| α-pinene (20.60%), β-pinene (19.70%), bicyclogermacrene (16.10%), trans-β-caryophyllene (11.50%), germacrene D (8.60%) | Alborz province, Iran | (26) | ||
According to the study of Nickavar et al., sesquiterpene hydrocarbons (44.90%) were identified as the most abundant components in the S. hypoleuca EO, collected from Haraz, Tehran province, Iran, in comparison to the other compounds (oxygenated sesquiterpenes [28.20%], hydrocarbon monoterpenes [15.50%], and oxygenated monoterpenes [3.40%]) (24). The EO components of S. hypoleuca were collected from Mazandaran province, Iran, in three different vegetative, flowering, and fruiting stages. More specifically, in the flowering stage, sesquiterpene hydrocarbons and oxygenated sesquiterpenes were the most abundant compounds, with 32.90% and 28.80%, respectively (25). In another study, the analysis of EOs components of 21 populations of S. hypoleuca collected from different sites of Alborz, Tehran, and Mazandaran provinces in Iran demonstrated that sesquiterpene hydrocarbons constituted 39.70%; nevertheless, oxygenated monoterpenes comprised 3.00% of the EO compounds of the sample collected from Alborz province, Chalos Road, Dizin, that are in alignment with two previous studies and the present study (26).
It is critical to identify efficient compounds in inducing desired biological activity. It is important to answer the question of whether major compounds are responsible for the biological activity (27) or whether a synergistic effect occurs. In this regard, the synergistic interactions of three major constituents of S. mirzayanii and S. hypoleuca EOs were investigated, as reported in Table 6. These combinations were provided based on their natural proportions in Salvia spp. EOs. The mixture of linalool, α-terpineol, and 1,8-cineole (40:35:25) exhibited α-glucosidase inhibitory activity with percentage inhibition of 72.5 ± 0.8; nonetheless, the mixture of caryophyllene oxide, α-pinene, and β-pinene (50:25:25) demonstrated a weaker inhibition (14.0 ± 1.8%).
Abbreviation: CI, combination index.
a Same as the ratio in the essential oils
The combination effect of components can be characterized by the combination index (CI), which is defined as:
Where Cx,n is the concentration of the compoundn alone that inhibits the enzyme (x%). Cn is the concentration of the compoundn in combination with other compounds which inhibits the enzyme (x%). When the CI is equal to, less than, or greater than 1, the combination effect would be additive, synergistic, or antagonistic, respectively (28). According to the calculated CI values, the constituents of the first mixture seem to interact with one another synergistically (CI < 1); in the meantime, the components of the second mixture have an antagonistic impact on one another (CI > 1).
The S. mirzayanii EO was also observed to have higher antioxidant activity than the S. hypoleuca EO. To the best of our knowledge, no study reported the DPPH radical scavenging capacity of S. mirzayanii EO. However, in a study conducted in 2016, the antioxidant activity of S. hypoleuca EO at different growth stages was assessed by DPPH assay. The EO at the flowering stage showed the best radical scavenging capacity with IC50 = 25 mg/mL among the two other stages (positive control: Butylated hydroxytoluene [BHT] with an IC50 value of 0.4 mg/mL) (25). Various compounds, such as 1,8-cineole, α-pinene, camphor (29), carvacrol, and thymol (30), were reported to have antioxidant activity. Because the S. mirzayanii EO contains substantially more of the compounds than that of S. hypoleuca, it has shown greater antioxidant activity. Additionally, the results of the present study showed that S. mirzayanii EO has a higher phenolic content (TPC) (78.3 ± 1.3 mg GAE/g EO) than S. hypoleuca EO (49.4 ± 1.1 mg GAE/g EO) (Table 4). Total phenolic content and the antioxidant activity based on the DPPH assay were observed to be directly related, and the higher TPC of S. mirzayanii EO resulted in a lower DPPH IC50 value that can be explained by the fact that phenolic compounds neutralize free radicals by transferring the hydrogen of their hydroxyl group to them (31).
5.1. Conclusions
This study was designed to develop natural compounds for the treatment of type 2 diabetes based on their α-glucosidase inhibitory and antioxidant activities. In this study, S. mirzayanii and S. hypoleuca EOs were selected, leading to the desired activity of S. mirzayanii. Moreover, the evaluation of α-glucosidase inhibitory of selected pure components that have been identified in the corresponding EOs revealed that caryophyllene oxide, α-pinene, and linalool were more potent than others. It should be noted that the enzyme inhibitory activity of S. mirzayanii was observed to be induced through a synergistic effect. It seems that the S. mirzayanii EO can be considered a promising lead for the discovery of new natural anti-diabetic agents or supplements.