1. Background
Wound healing is a remarkable biological process essential for restoring skin integrity after an injury (1). Presently, wound care represents a significant health concern due to the challenges posed by chronic wounds in comorbid conditions, including prolonged hospitalization, loss of mobility, decreased quality of life, and increased risk of nosocomial infections, among other economic burdens (2).
Bee honey has been utilized for medicinal purposes across various cultures since ancient times. Evidence from archaeological findings and literature reveals that ancient Egyptians, Greeks, and Romans used honey for wound treatment (3). Despite its longstanding use, the advent of antibiotics led to a decline in honey's medicinal application (4). However, with the increasing prevalence of antibiotic-resistant bacteria, honey has re-emerged as a viable alternative in clinical medicine for wound treatment (5).
The effectiveness of honey in treating wounds, including those resulting from skin ulcers of various etiologies, is well-documented (6). A review by Jull et al. indicated that, in over 470 cases where honey was applied as a therapeutic agent, only five cases did not achieve satisfactory healing (7).
Honey stands as a potential alternative to current wound care products due to its antibacterial and anti-inflammatory properties and its ability to promote autolytic debridement, deodorization, tissue growth stimulation, pain control, and minimal scarring (8). These benefits are attributed to multiple factors, including moisture, pH level, sugar concentration, the generation of reactive oxygen species (ROS), and the presence of peptides, which collectively aid the four phases of wound healing: Hemostasis, inflammation, proliferation/epithelialization, and tissue remodeling (3).
Inflammation is the immune system's biological response to various stimuli, such as pathogens, damaged cells, and toxic compounds. It is clinically characterized at the tissue level by redness, heat, pain, swelling, and loss of function (9). Normally, the inflammatory response eliminates infectious organisms and facilitates tissue repair. However, prolonged inflammation can alter the cellular composition at the site of injury and potentially hinder the wound healing process (10). Inflammation is a dynamic response that occurs following tissue damage, involving the activation of resident cells, production of pro-inflammatory mediators and cytokines, and recruitment of blood cells to initiate phagocytosis for clearing debris and foreign cells (11).
Despite the availability of numerous anti-inflammatory agents, many are not conducive to wound healing; non-steroidal anti-inflammatory drugs (NSAIDs) exhibit cytotoxicity towards tissues, while corticosteroids can impede epithelial tissue development (12).
The anti-inflammatory activity of honey is linked to phenolic compounds known to suppress the overproduction of inflammatory mediators (13). This effect, combined with honey's antioxidant activity and its capacity to reduce edema, shortens the inflammation phase during wound healing (14).
While all honey possesses wound-healing properties, honey from stingless bees, sourced from a variety of native, often endemic, plant species, stands out (14). The unique practice of these bees storing honey in cerumen pots (a blend of wax and plant resins) enables the infusion of phytochemicals into the honey. This attribute is believed to enhance the therapeutic value of honey from stingless bees over that produced by Apis mellifera (15).
Although numerous reports highlight the therapeutic effects of A. mellifera honey, there has been limited research on the in vivo anti-inflammatory and healing efficacy of Melipona beecheii honey, particularly that produced in the Yucatan Peninsula of Mexico.
In the Yucatan Peninsula, two species of melipona bees, M. beecheii and Melipona yucatanica, are found. The latter is rare, with minimal records of its use in meliponiculture (16) compared to M. beecheii. The cultivation and production of M. beecheii bee honey, known locally as “xunaan-kab,” “colel-kab,” or “pool-kab,” was a widespread practice among the Maya of the Yucatan Peninsula well before the arrival of the Spaniards, marking it as a significant cultural and economic endeavor in the region (17). Its honey has long been a component of traditional Maya medicine (18). Over the past 15 years, there has been a resurgence (19) in interest in this honey due to the increasing demand for natural products with functional properties.
2. Objectives
The aim of this study was to assess the in vivo wound-healing and anti-inflammatory properties of honey produced by M. beecheii bees collected in Yucatan, Mexico.
3. Methods
3.1. Sample Collection
Twelve honey samples were collected from a traditional meliponiculturist who produces honey from M. beecheii bees. These samples came from meliponaries located in various municipalities across Yucatan, Mexico, to ensure a broad representation. The meliponaries were situated in a low deciduous forest, home to native and endemic species of the Yucatan Peninsula, from which the bees gather nectar and pollen.
The collection period spanned from July to October 2020. The honey was transported to the laboratory in polyethylene containers, shielded from light in an isothermal box. It was then transferred into 50 mL Falcon tubes and stored at 4°C until analyzed.
3.2. Animal Model
The study of wound-healing and anti-inflammatory kinetics was conducted on CD1 mice (Mus musculus), male, albino variety, aged 2 - 3 months and weighing 30 - 40 g, sourced from the Biotherium of Universidad Autonoma de Morelos (UAEM). The experimental protocols received prior approval from the Institutional Animal Care Committee of the Faculty of Medicine at UAEM (approval number: 005/2016), adhering to the NOM-062-ZOO-1999 Technical Specifications for the Care and Use of Laboratory Animals and the European Community Council Directive 86/609/EEC guidelines for experimental animal care and use.
3.3. Evaluation of Wound-Healing Activity
The model employed was adapted from Haryanto et al. (20) with modifications. Sixty-five mice were evenly divided into thirteen groups of five each: Twelve for treatments (M1 - M12), with the twelve M. beaches honey samples and one control group treated with pirfenidone (KitosCell®). Mice were housed in individual cages with unlimited access to food and water. They were anesthetized intraperitoneally with sodium pentobarbital (0.05 mg/g, Merck CAS 57-33-0). A circular incision was made on their shaved dorsum using a 5 mm diameter biopsy punch, marking day 0. The wounds received 100 µL of the designated honey treatment initially, followed by 50 µL treatments on days 1, 3, 6, 8, 10, 13, and 15. The control group received a 5 mg application of KitosCell®. Wound photographs were captured with a digital camera attached to a stereo microscope (Stemi DV4, ZeissR), and the wound area was measured using Image J software.
3.4. Evaluation of Anti-Inflammatory Activity
The methodology employed was based on the model reported by Garcia-Argaez et al. (21). Nine M. beecheii honey samples were tested. Sixty male CD1 mice, weighing 25 - 30 g, were divided into nine treatment groups (M1-M9) of five mice each, one negative control group (NC) which received a 2.5 µg solution of 2-O-teradecanoyl-13-acetate (TPA, Sigma) in ethanol (J.T. Baker®), and two positive control groups treated with a solution of indomethacin (Sigma-Aldrich®, St. Louis, MO, USA, CAS 53-861) in acetone at concentrations of 1 mg/mL (Control-I 1) and 30 mg/mL (Control-I 2). The mice were housed in individual cages with unlimited access to food and water. They were anesthetized intraperitoneally with sodium pentobarbital (0.05 mg/g), and 5 µL of TPA solution was topically applied to both the inner and outer sides of the right ear, while the left ear received only 10 µL of ethanol. Ten minutes later, 10 µL of honey was applied in the same manner to the right ear. The control mice were treated solely with the corresponding solvent. Four hours after application, the mice were euthanized by cervical dislocation, and a segment of ear tissue (5 mm in diameter) was excised from both the treated and untreated ears. The difference in weight between the two ear segments served as a measure of the edematous response. The percentage of inhibition relative to the negative control was calculated using the formula:
Where C0 and Ct represent the inflammatory response in the control group (TPA alone) and the treatment groups (TPA plus honey samples), respectively.
3.5. Statistical Analysis
The Kolmogorov-Smirnov test verified the normality of the collected data. A one-way analysis of variance (ANOVA) assessed differences between experimental groups, with Dunnett's test used for multiple mean comparisons against control groups. Results were presented as mean ± standard deviation. The R (version 4.0.2) statistical software (https://rstudio.com/) was utilized for data analysis.
4. Results
4.1. Wound-Healing Activity
In this study, the progress of wound healing was monitored through measurements of wound area reduction, with wound edge contraction indicating a decrease in the original wound area.
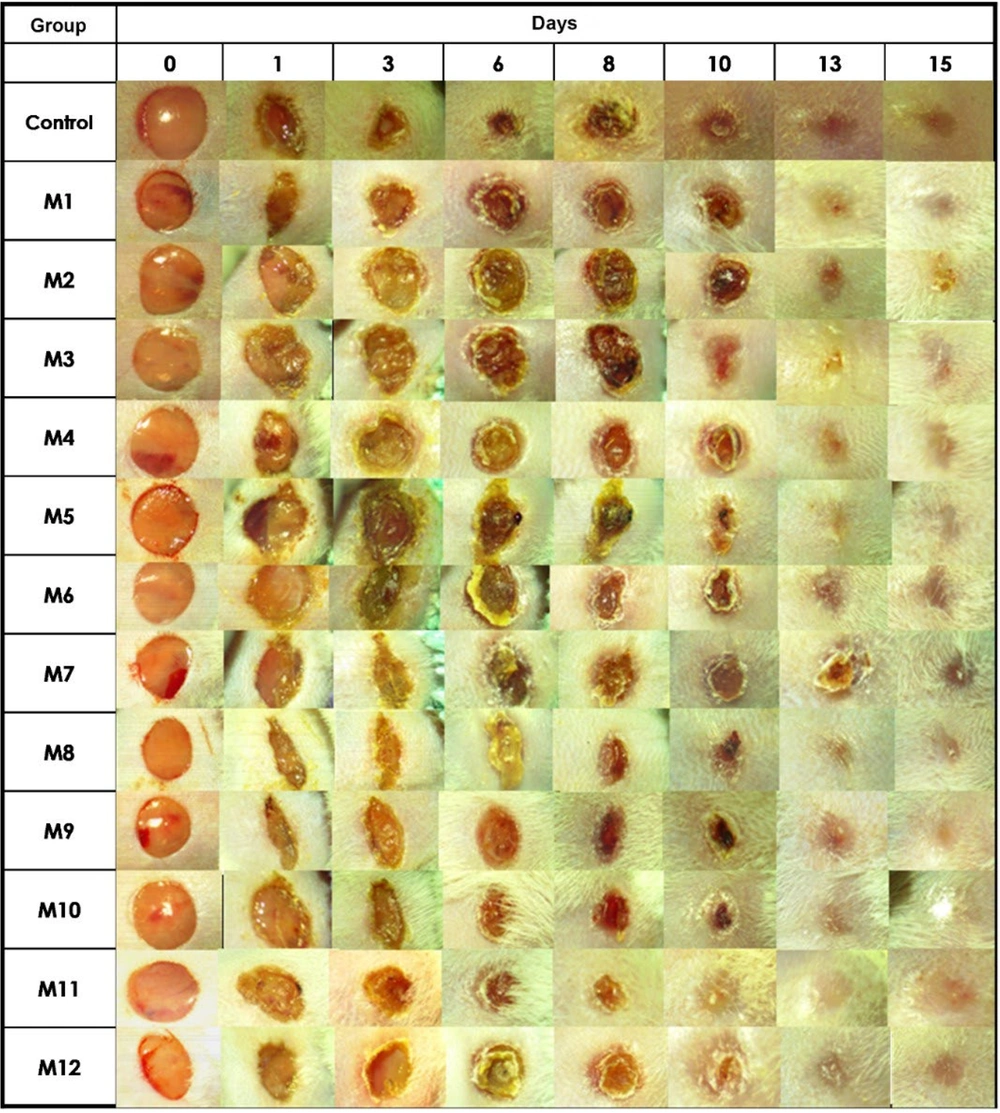
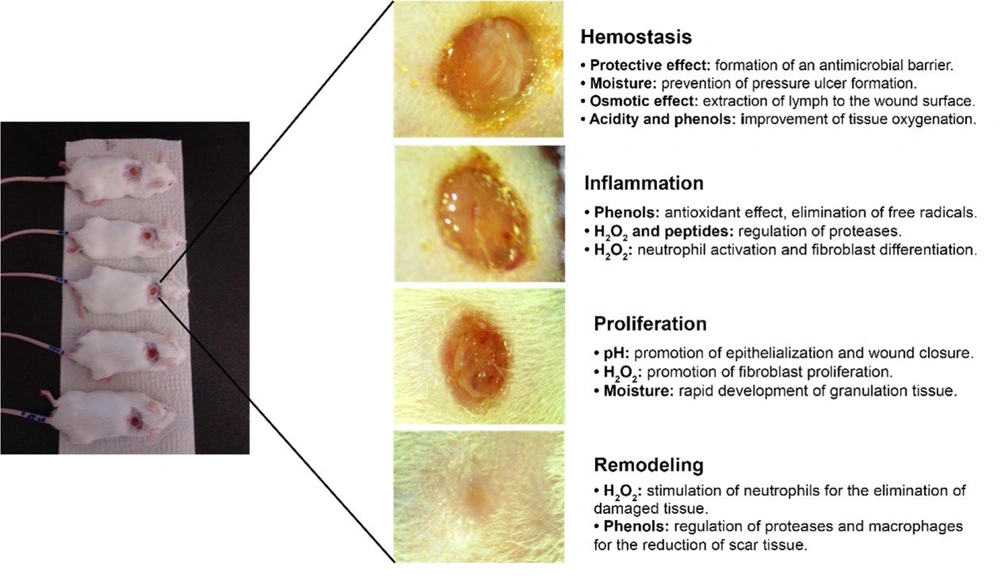
No antigenic reactions to any of the honey treatments were observed in the wounds. From the first day, granulation tissue formation and continuous contraction of the wound edges were noted across all groups (Figure 1). A yellowish residue, likely from unabsorbed honey, was visible on the wound edges of those treated with honey. By day 6, all groups showed complete coverage of the wounds with granulation tissue and epithelialization, although the control group displayed a greater amount of epithelial tissue at the wound edges. By day 15, wounds in all groups had fully epithelialized.
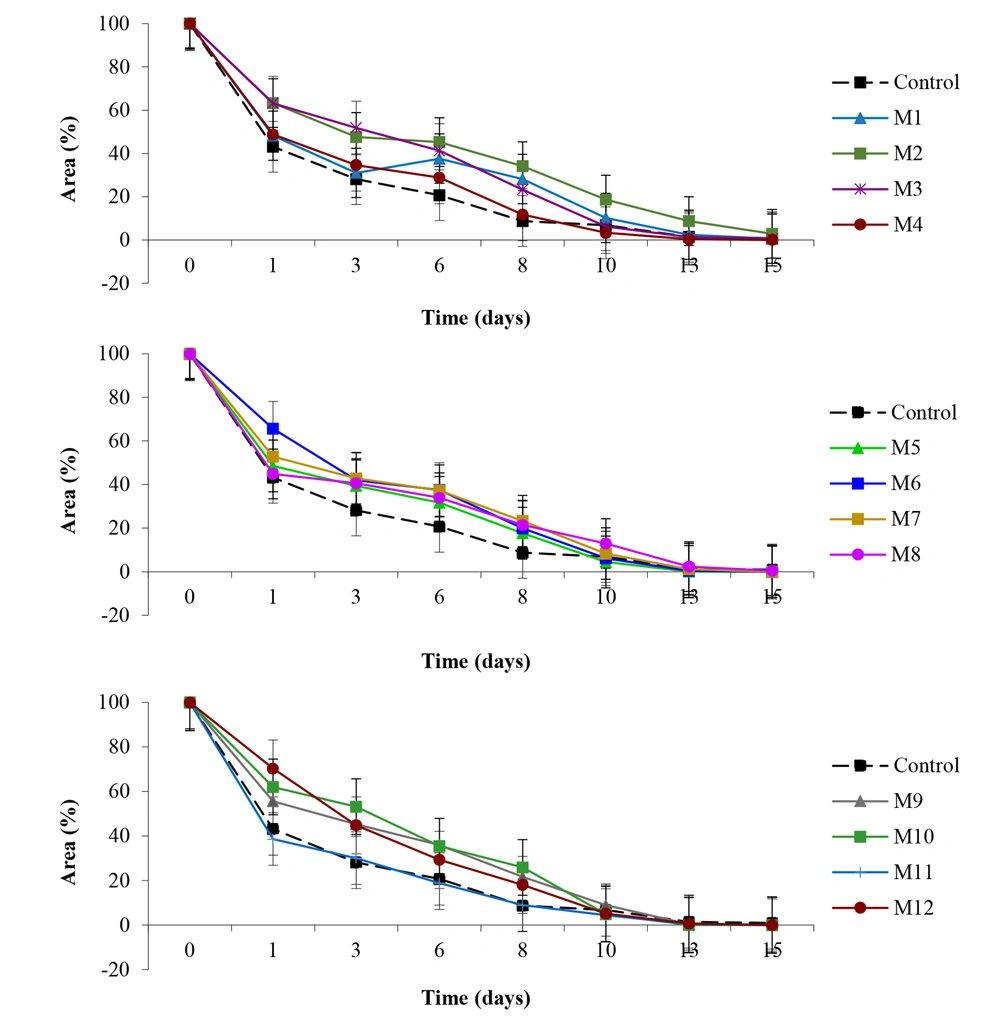
For quantitative analysis, Figure 2 illustrates the wound area reduction over time for different honey treatments compared with the healing agent KitosCell®.
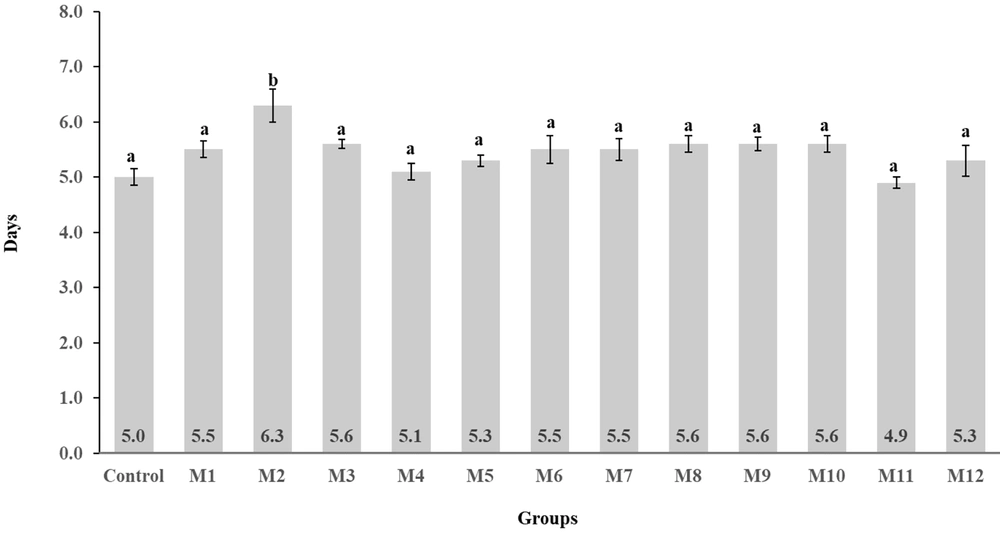
Notably, the M11 group's curve showed a wound reduction percentage comparable to the control group, marking the highest rate of wound area reduction in the healing process among all groups. The timeframe for an 80% wound size reduction (Figure 3) showed that the M2 group was the only one with a statistically significant difference (P < 0.05) compared to the control group, with wounds closing 80% in an average of 6.3 ± 0.9 days (%RSD 14.28).
4.2. Anti-Inflammatory Activity
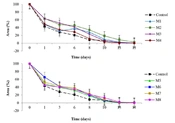
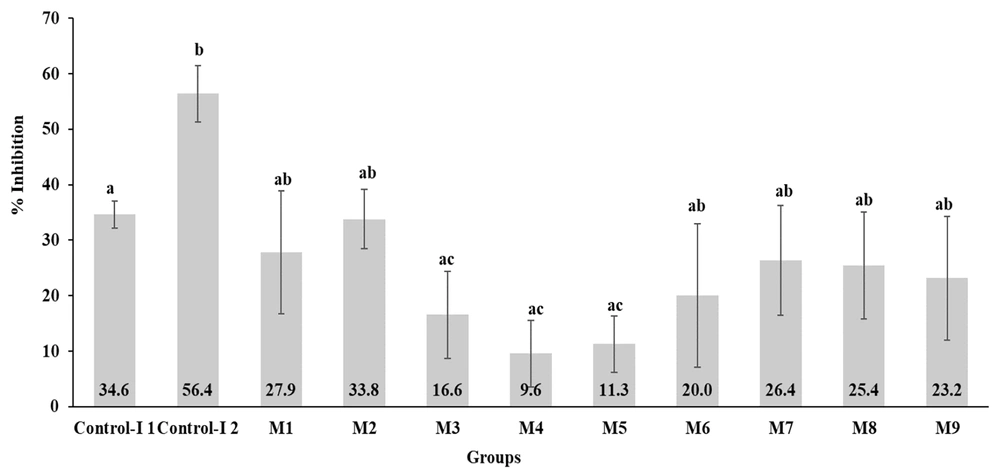
The anti-inflammatory activity assessment revealed no statistical difference (P > 0.05) between the honey treatments and control-I 1 (indomethacin at 1 mg/mL). However, significant differences were noted (P < 0.05) in groups M3, M4, and M5 compared to Control-I 2 (indomethacin at 30 mg/mL), showing lower inhibition percentages (16.6 ± 7.9, RSD 47.59%; 9.6 ± 6.0, RSD 62.5%; 11.13 ± 5.1, RSD 45.82%) (Figure 4).
5. Discussion
The medicinal properties of honey have been recognized for centuries (22), and while bee honey is produced worldwide, its therapeutic efficacy varies significantly. This variation depends on factors such as the bee species (entomological origin), the geographical location, the sources of nectar (botanical origin), and other aspects like the timing of honey extraction, processing, and storage conditions (6).
In this research involving M. beecheii honey, the samples demonstrated efficacy comparable to that of wounds treated with KitosCell®, except for the M2 group, which exhibited a slower wound reduction rate than that of the healing agent. Similar outcomes were reported by Nisbet et al. (23) after assessing A. mellifera honey on rats and rabbits, noting no significant differences between the honey-treated groups and the control regarding wound diameter, granulation tissue formation, and epithelialization.
The study noted an early-phase wound-healing effect of honey comparable to the control drug. Haryanto et al. (20) reported analogous findings using Apis dorsata honey in mouse wounds, with hydrocolloid gel as a control. Mekkaoui et al. (24) observed significant healing of chemically induced wounds in mice treated with Moroccan honey compared to control groups (Vaseline and Madecassol®).
The groups treated with honey displayed healthy granulation tissue formation, which promoted wound contraction, facilitated wound closure, and reduced scarring. This aligns with the conclusions of Jull et al. (7), who found that honey accelerates healing in burns and infected surgical wounds compared to conventional treatments.
Despite honey's anticoagulant properties contributing to the initial stage of wound healing (25), it acts as a barrier that maintains a moist wound environment, preventing eschar formation, reducing dermal necrosis, and encouraging epithelial cell migration to the wound surface (3), as observed during the first week (days 1 and 3) across all honey-treated groups. Other contributing factors might include honey's high acidity, which enhances blood oxygenation (5), and its low water activity, promoting oxygen and nutrient transport from deeper tissues to the wound area (22).
On day 1, the inflammatory phase emerged, likely due to honey's content of phenolic compounds (26, 27), glycopeptides (28), and low molecular weight proteins (29). These components stimulate monocytes and macrophages, enhancing the release of cytokines, chemokines, and enzymes that break down the extracellular matrix. This results in the activation of pro-inflammatory pathways markers, such as interleukins (IL-1, IL-10, and IL6), cyclooxygenases (COX-2), tumor necrosis factor (TNF-α), nuclear factors (NF-κB and IκBκ), transforming growth factor beta (TGF-β), the primary regulator of fibrosis lipoxygenase (LOX), nitric oxide (NO), nitric oxide synthase (iNOS), and prostaglandins (26).
Although inflammation is a critical biological response in the wound-healing process (14), it must be managed to prevent the condition from evolving into a chronic or excessive state (28). Therefore, the anti-inflammatory activity of the honey was evaluated. No significant difference was observed in the inflammation inhibition percentage among any of the treated groups compared to control 1 (1 mg/mL of indomethacin); however, Groups M3, M4, and M5 showed a lower inhibition percentage compared to control 2 (30 mg/mL of indomethacin). The highest inflammation inhibition was seen in group M2 with 33.8 ± 5.3%, which is considerably lower than the 54 ± 5% reported by Borsato et al. (30) for mice topically treated with M. marginata honey. Researchers such as Hussein et al. (13) and Mohammed et al. (31) have reported the in vivo anti-inflammatory activity of different types of honey, but these were administered orally.
The anti-inflammatory activity noted in the honeys studied here could be attributed to various mechanisms identified in previous research. For instance, Biluca et al. (32) demonstrated that meliponid honey's anti-inflammatory activity in vitro stemmed from the inhibition of TNF-α, IL-6, MCP-1, IL-12p70, INF-γ, and IL-10 secretion. Similarly, Ranneh et al. (33) reported a reduction in CRP, TNF-α, IL-1β, IL-6, IL-8, MCP-1, NF-κB, and p38 MAPK in blood levels in rat tissues with LPS-induced inflammation treated with honey from the genus Trigona.
This study successfully demonstrated the proangiogenic activity of honey during the proliferative phase by noting the development of granulation tissues in all groups from day 1 to day 13. Granulation tissues consist of fibroblasts growing in areas rich in capillaries that provide oxygen, spurred by mediators such as TNF-α and IL-1β, which stimulate the release of various growth factors (34). It has also been established that honey's enzymatic regulation of hydrogen peroxide, combined with its antibacterial properties, facilitates calcium (Ca2+) entry into cells (35), promoting the expression of early growth genes essential for wound healing. This process contributes to the modulation of adjacent non-inflammatory cells, such as fibroblasts (28), ultimately enhancing the rate of wound closure (36).
In the remodeling stage, all groups treated with honey in this study showed reduced scabbing and the development of thin scars. This outcome is attributed to the presence of amino acids in honey, such as arginine and glutamic acid, which serve as precursors to proline, a primary amino acid that enhances collagen synthesis. Additionally, the presence of iron, copper, and ascorbic acid in honey aids in the maturation of collagen fibers (36).
Figure 5 illustrates the key components in honey that contribute to the four phases of the wound-healing process: Hemostasis, inflammation, proliferation/epithelialization, and tissue remodeling.
Honey may contain over 200 compounds; its anti-inflammatory and antibacterial effects, which are crucial for wound healing, are mainly due to its antioxidant properties. These properties are significantly influenced by the phenolic compounds' concentration and composition (21, 37), which vary based on factors like the bee species, the botanical sources of nectar, and the timing of honey collection. Variations in phenolic compound content in M. beecheii honey from Yucatan have been documented by Ruiz-Ruiz et al. (27), with 632.2 mg/kg GAE, and by Miranda et al. (38), with 1 317 mg/kg GAE post-harvest and 780 mg/kg GAE during the harvest season.
Although the basic composition of phenolic compounds in different honey varieties is somewhat consistent, differences in the phenolic profiles of honey from stingless bees have been reported by researchers such as Tomás-Barberán et al. (39), Sousa et al. (40), and Ranneh et al. (41). For instance, Ramon-Sierra et al. (42) identified three phenolic acids—chlorogenic, caffeic, and ellagic—and four flavonoids—catechin, myricetin, quercetin, and apigenin—in M. beecheii honey from the Yucatan Peninsula, Mexico. Conversely, Alvarez-Suarez et al. reported the presence of caffeic and coumaric acids, as well as apigenin, quercetin, luteolin, isorhamnetin, and kaempferol in the same type of honey from Cuba (43).
Honey has been shown to possess strong antioxidant activity (44, 45) and may serve as a natural antioxidant remedy, contributing to the prevention of various health issues (46). Stingless bee honey, undergoing natural fermentation during maturation, is a richer source of antioxidants compared to Apis honey, attributed to higher concentrations of aglycones and a greater oxygen radical absorbance capacity (ORAC) (47). Antioxidant activity in A. mellifera honey is reported with DPPH values ranging from 3.17 - 8.79 IC50 mg/mL in Brazilian honey (48), 13.26 - 48.95 IC50 mg/mL in Portuguese honey (49), and 5.24 - 17.51 IC50 mg/mL in Malaysian honey (50). These values are comparable to those found in honeys from native bees like Melipona subnitida, with 10.6 - 12.9 IC50 mg/mL (51), but are lower than those of M. beecheii honey from Yucatan, Mexico, which has 173.0 - 361.8 IC50 mg/mL (38).
This consideration allows us to view each honey as a unique product, explaining the variance in bioactive effects observed in some honey samples analyzed during this research. These differences are due to the concentration of compounds and their degree of isomerization or hydrolysis, which may affect their absorption. Lin et al. (52) observed that aglyconic compounds like quercetin have lower skin absorption compared to its polymethoxylated form (quercetin 3, 5, 7, 3', 4'-pentamethyl ether) in mice.
It's notable that the floral origin and the abundance of flowers throughout the year influence the phenolic content in honey. In this study, the honey samples were collected during the post-harvest period, coinciding with the Yucatan Peninsula's rainy season, which features minimal flowering. This scarcity makes it challenging for bees to forage, leading beekeepers to sometimes feed them with A. mellifera honey or sugar syrup. This supplementary feeding can reduce the phenolic compound content and other elements in the honey (53), possibly contributing to the lack of significant differences in the honey samples.
The scientific community has extensively documented the composition and therapeutic effects of honey (7), including its anti-inflammatory and healing properties (7, 20, 23, 30, 32). The composition and medicinal value of honey vary among bee species, influenced by their foraging behavior and the way they collect nectar and pollen from diverse plants and locations (54). This study evaluated honeys from the native stingless species M. beecheii, adding to the body of knowledge on honeys produced by traditional meliponiculturists in Yucatan. Nonetheless, research is still developing to scientifically validate the ancestral use of this honey. It's crucial to continue characterizing these honeys, assessing their medicinal properties, and correlating them with their physicochemical composition, bioactive compounds, and floral origin.
5.1. Conclusions
The present study found that the wound-healing and anti-inflammatory effects of honey produced by M. beecheii bees in vivo were comparable to those of the positive controls (pirfenidone and indomethacin). This suggests that it could serve as an alternative for topical wound treatment, either directly or as part of a formulation, without inducing the side effects associated with some current medications. However, the varying compositions of different honeys, which influence their therapeutic effectiveness, pose a challenge to standardization. Further research is needed to explore the mechanisms of action in the inflammatory and wound-healing processes prompted by M. beecheii honey, including studies on growth factors, pro-inflammatory cytokines, tissue staining, oxidative enzymes, and the relationship with bioactive compounds in the honey. Continued verification of its use as a topical agent is warranted, as, despite the higher bioactivity related to its antioxidant properties reported in stingless bee honey, significant clinical evaluations in wound healing are yet to be realized.