1. Background
Cyclophosphamide (CP) is a commonly used alkylating agent for treating autoimmune diseases, kidney and bone marrow transplants, and certain tumors. Cyclophosphamide is converted by the liver microsomal cytochrome P450 system into its active metabolites, including phosphoramide mustard and acrolein, which are essential for its therapeutic effects (1, 2). However, CP can cause hepatotoxicity in certain patients (3). The exact mechanism of CP-induced hepatotoxicity is not fully understood. Some evidence suggests that CP induces liver toxicity by promoting ROS production, disrupting the oxidant/antioxidant balance, and reducing antioxidant enzymes such as superoxide dismutase (SOD), glutathione peroxidase (GPx), and glutathione (GSH) (4). Cyclophosphamide also causes a surge in lipid peroxidation, exacerbating the condition (5, 6). Therefore, investigating compounds with antioxidant properties and exploring their mechanisms of action could help reduce organ toxicity.
Sildenafil citrate, a selective phosphodiesterase type 5 inhibitor (PDE5 inhibitor), is primarily used to treat erectile dysfunction but also has a significant role in scavenging free radicals and reducing inflammatory cytokines (7). The effectiveness of sildenafil in preventing organ damage induced by oxidative stress and inflammation has been demonstrated in several studies (8-10). Hameed and Farooq showed that sildenafil reduces oxidative stress by inhibiting the formation of free radicals, including the superoxide anion. Additionally, sildenafil has been shown to effectively attenuate thioacetamide-induced liver fibrosis (11). Further studies have confirmed that sildenafil mitigates hepatotoxicity and renal impairment induced by paracetamol and cisplatin (7, 12). Furthermore, the beneficial effects of vitamin E, as an antioxidant agent, on CP-induced hepatotoxicity have been demonstrated in earlier studies (13). Therefore, vitamin E was also included in this study as an antioxidant agent for comparison with sildenafil.
2. Objectives
Based on the above findings, the purpose of this study was to evaluate the potential effects of sildenafil and vitamin E, both individually and in combination, on CP-induced hepatotoxicity in rats, along with investigating specific underlying mechanisms.
3. Methods
3.1. Chemicals and Reagents
Sildenafil, 2,4,6-Tri (2-pyridyl)-1,3,5-triazine (TPTZ), Sulfanilamide, N- (1-naphthyl) ethylenediamine dihydrochloride, Thiobarbituric acid (TBA), and Trichloroacetic acid (TCA) were purchased from Merck, Germany. Colorimetric kits were obtained from Zist Chem, Tehran, Iran, and vitamin E (alpha-tocopherol) was purchased from Health Burst, Iran.
3.2. Animals
Thirty male Wistar rats (222 - 250 g) were purchased from the Research Center of Yasuj University of Medical Sciences, Iran. The animals were housed in special cages at a temperature of 22 ± 2°C with a relative humidity of 55 - 60%, under a 12-hour dark/light cycle, and were provided a standard diet and water. This research adhered to the protocol of the Animal Laboratory and was approved by the Yasuj University of Medical Sciences Ethics Committee (IR.YUMS.REC.1400.079).
3.3. Experimental Design
The animals were randomly divided into five groups (n = 6): Group 1 (control) received water distillate by gavage. Cyclophosphamide (CP) at 200 mg/kg was administered intraperitoneally (14) to Group 2 to induce hepatotoxicity. Group 3 was administered sildenafil (75 mg/kg) (15) along with CP (200 mg/kg), while Group 4 received vitamin E (500 mg/kg) and CP (200 mg/kg). Group 5 was administered 75 mg/kg of sildenafil, 200 mg/kg of CP, and 500 mg/kg of vitamin E. The animals received treatment for 10 days, with CP administered on the eighth day (1). At the end of the experiment, the animals were sacrificed under deep anesthesia with isoflurane. Blood samples were collected from their hearts and preserved for measuring serum levels of liver enzymes and oxidative stress markers. Liver tissue was excised for the assessment of oxidative stress markers and histological examination.
3.3.1. Assessment of Animal Weight and Liver
The weight of the animals was measured on the first day of the study, and a secondary weight was recorded before sacrifice on the last day of the experiment.
3.4. Biochemical Analysis
Blood samples were centrifuged at 3500 rpm for 10 minutes, and the collected serum was stored at -20°C for biochemical tests. The activity of liver enzymes, including ALT and AST, was measured using diagnostic kits from Zist Chem (Tehran, Iran) and the colorimetric method.
3.5. Oxidative Stress Markers
Liver tissue was homogenized with a homogenizer (IKA Werke Ultra-Turrax T25 basic homogenizer, Germany) in phosphate-buffered saline (10 mmol/L, pH = 7.4). The samples were then centrifuged, and the supernatant was collected to measure oxidative stress markers.
3.6. Determination of Lipid Peroxidation in Serum and Tissue
Malondialdehyde (MDA) levels were measured to evaluate lipid peroxidation. Briefly, 100 microliters of the sample were mixed with a solution containing thiobarbituric acid (TBA) and trichloroacetic acid (TCA) and then placed in a boiling water bath at 96°C for 30 minutes. After centrifugation, the absorbance of the supernatant was read at 535 nm. The amount of MDA was calculated using the molar absorption coefficient of 1.56 × 105 M-1 cm-1 and was expressed in μmol/L (16).
3.7. Determination of Nitric Oxide Metabolite in Tissue
The tissue concentration of nitric oxide (NO) metabolites was measured using the Griess method. A mixture of 100 μL of the sample and 100 μL of Griess reagent (Sulfanilamide and N-1-naphthyl ethylenediamine dihydrochloride in 2.5% phosphoric acid) was transferred to a 96-well plate and incubated at 37°C for 30 minutes. The absorbance of the solution was read at 540 nm (17, 18).
3.8. Determination of Ferric-Reducing Antioxidant Power in Serum
The ferric-reducing antioxidant power (FRAP) was measured using the Benzie and Strain method. In this method, the serum's ability to reduce ferric ions is assessed. When ferric (Fe3+) ions are reduced to ferrous (Fe2+) ions at an acidic pH in the presence of tripyridyl-S-triazine (TPTZ), a blue Fe-TPTZ complex is formed. The intensity of the resulting color was measured by spectrophotometry at 593 nm (19, 20).
3.9. Determination of Antioxidant Enzyme Activity in Tissue
The activity of antioxidant enzymes (CAT, SOD, and GPx) in homogenized tissue was measured using an ELISA kit (ZellBio GmbH, Ulm, Germany) following the manufacturer's instructions (21).
3.10. Histological Evaluation
Liver tissue samples were fixed in 10% neutral-buffered formalin for histological evaluation. After dehydration with alcohol, the tissues were embedded in paraffin. Five-micron-thick sections were then cut using a microtome and stained with hematoxylin-eosin for examination (22).
3.11. Statistical Analysis
Statistical analysis was performed using SPSS version 18 (SPSS Inc, Chicago, IL). Data were reported as mean ± SD, and a P-value of ≤ 0.05 was considered statistically significant. Tukey's post hoc test was used for identifying significant differences between groups.
4. Results
4.1. Effect of Sildenafil and Vitamin E on Body Weight Alterations Due to Cyclophosphamide-induced Hepatotoxicity in Rats
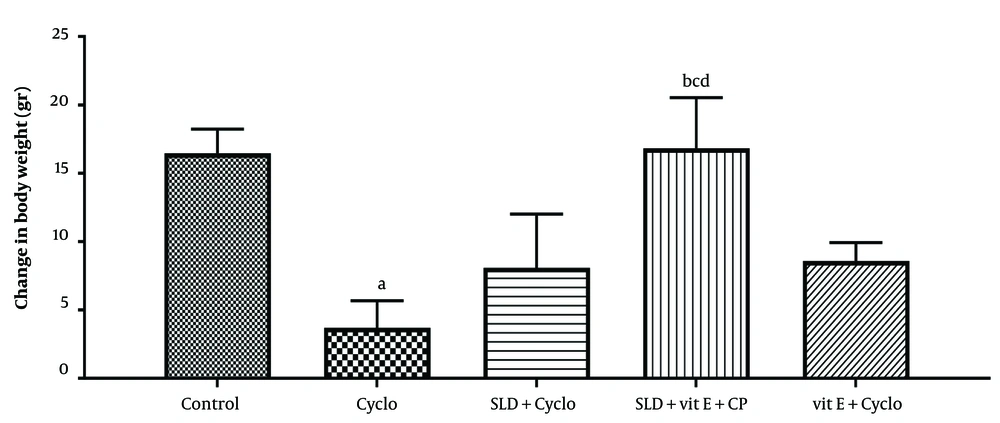
The animals were weighed at the beginning and end of the 10-day experimental period. A marked decrease in body weight was observed in the CP-treated group (P ≤ 0.05). Oral administration of sildenafil (75 mg/kg) and vitamin E (500 mg/kg) prevented CP-induced weight loss, though the effect was not statistically significant. However, simultaneous administration of sildenafil and vitamin E significantly inhibited CP-induced weight reduction in animals compared to the CP-treated group (P ≤ 0.05) (Figure 1).
The effect of sildenafil on changes in body weight resulting from CP-induced hepatotoxicity in rats. Cyclo: 200 mg/kg of cyclophosphamide, SLD: sildenafil (75 mg/kg), Vit E (500 mg/kg). Each value represents the mean ± SD (n = 6). a, significantly different from Control groups; b, significantly different from Cyclo -treated group; c, significantly different from Cyclo + SLD treated group; d, significantly different from Cyclo + Vit E treated group. (P ≤ 0.05).
4.2. Effect of Sildenafil and Vitamin E on Biochemical Parameters in Cyclophosphamide-induced Hepatotoxicity in Rats
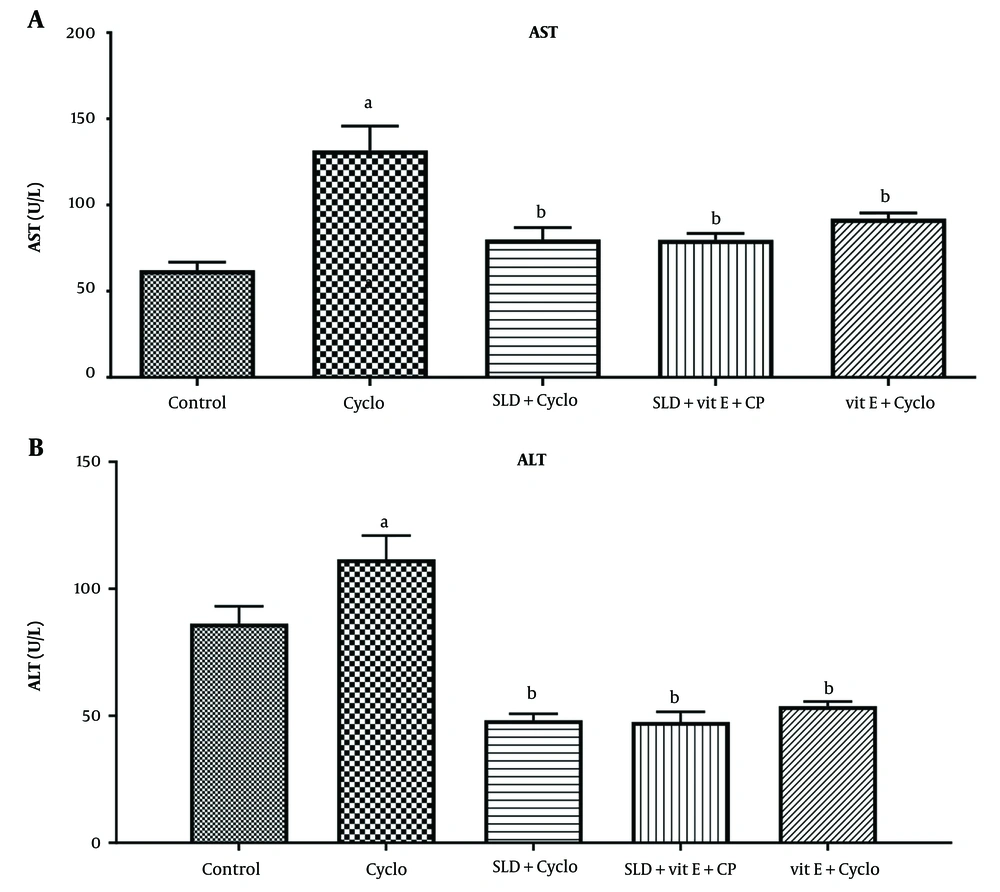
As shown in Figure 2, ALT and AST enzyme activity increased significantly in the CP group compared to the control group (P ≤ 0.05). However, a significant decrease in ALT and AST activity was observed following administration of sildenafil at a dose of 75 mg/kg and vitamin E at 500 mg/kg (P < 0.001). The same result was obtained in the group receiving both vitamin E and sildenafil (P ≤ 0.001).
A, effect of sildenafil on the serum levels of aspartate transaminase (AST); B, alanine aminotransferase (ALT), and total protein in Cyclo-induced hepatotoxicity in rats. Cyclo 200 mg/kg of cyclophosphamide, SLD: Sildenafil (75 mg/kg), Vit E (500 mg/kg). Each value represents the mean ± SD (n = 6). a, significantly different from Control groups; b, significantly different from cyclo-treated group.
4.3. Effect of sildenafil and Vitamin E on Oxidative Stress Markers in Cyclophosphamide-induced Hepatotoxicity in Rats
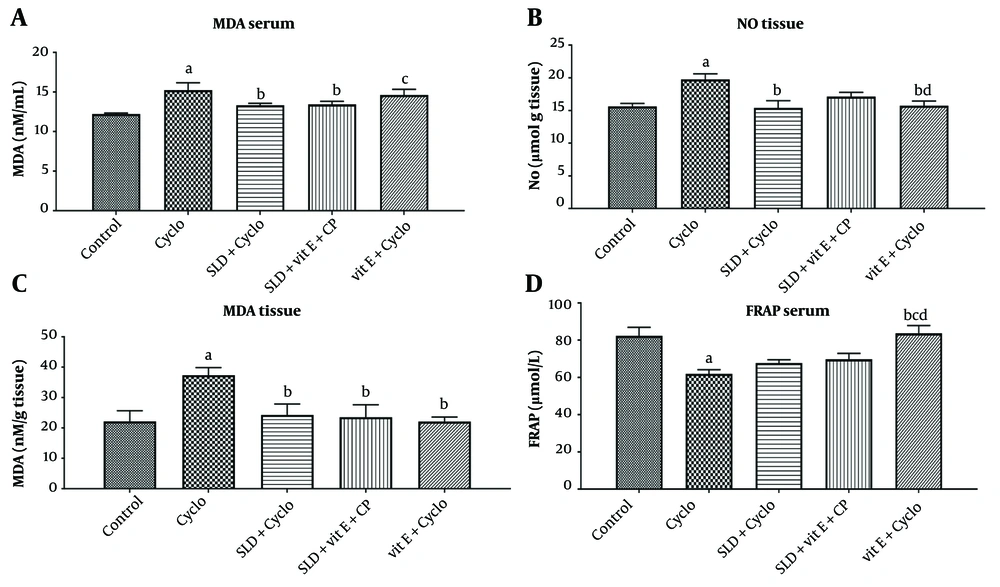
As shown in Figure 3, MDA levels in both liver and serum samples, as well as NO levels in tissue, increased significantly in the CP-treated group compared to the control group (P ≤ 0.05). Oral administration of sildenafil (75 mg/kg) and the combination of sildenafil and vitamin E (500 mg/kg) caused a substantial reduction in these oxidative stress markers (P ≤ 0.05).
The CP injection also resulted in a decreased serum level of FRAP compared to the control group (P ≤ 0.01). Administration of sildenafil and sildenafil plus vitamin E did not significantly affect the reduced FRAP levels in the CP-treated group. However, in the group treated with vitamin E alone, FRAP levels increased significantly compared to the CP group (P ≤ 0.05).
A, effect of sildenafil and Vit E on levels of serum MDA; B, liver markers malondialdehyde (MDA); C, liver No; and D, serum ferric reducing antioxidant power (FRAP) in Cyclo-induced hepatotoxicity in rats. Cyclo 200 mg/kg of cyclophosphamide, SLD: Sildenafil (75 mg/kg), Vit E (500 mg/kg) The value represents the mean ± SD (n=6). a, significantly different from control groups; b, significantly different from cyclo-treated group; c, significantly different from cyclo + SLD treated group; d, significantly different from cyclo + Vit E treated group. (P ≤ 0.05)
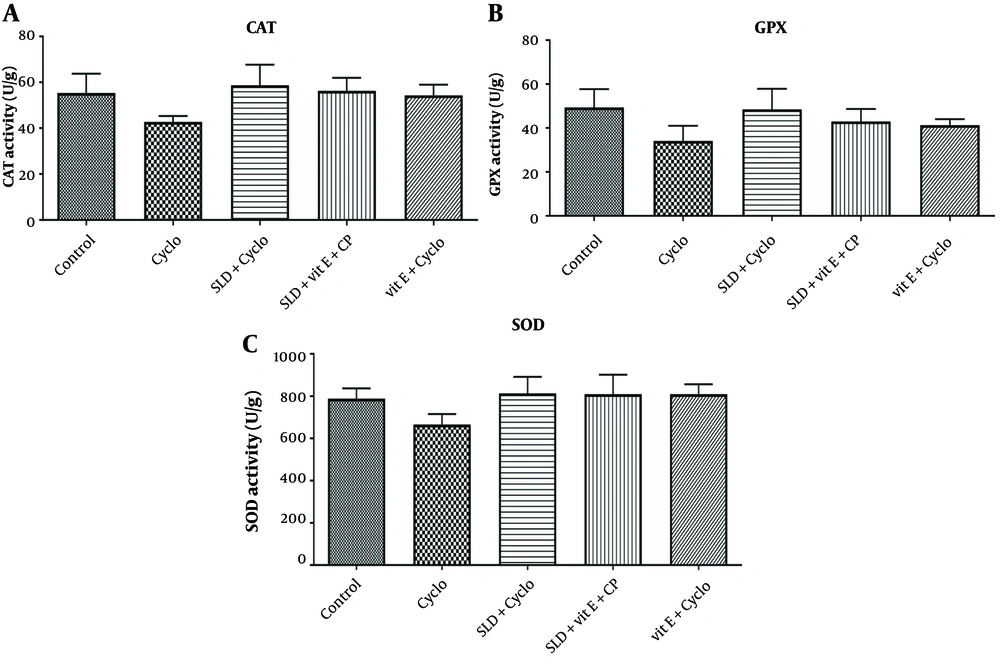
4.4. Effect of sildenafil and Vitamin E on Antioxidant Enzymes in Cyclophosphamide-induced Hepatotoxicity in Rats
As shown in Figure 4, intraperitoneal injection of CP at a dose of 200 mg/kg significantly decreased the activity of antioxidant enzymes CAT, SOD, and GPx in the CP-treated animals compared to the control group (P ≤ 0.05). Treatment with sildenafil (75 mg/kg), vitamin E (500 mg/kg), and the combination of sildenafil (75 mg/kg) and vitamin E (500 mg/kg) significantly increased the activity of these enzymes compared to the CP-treated group (P ≤ 0.05).
A, The effect of sildenafil and Vit E on the activities of CAT; B, glutathione peroxidase (GPx); and C, superoxide dismutase (SOD) against CP-induced hepatotoxicity in rats. Cyclo 200 mg/kg of cyclophosphamide, SLD: Sildenafil (75 mg/kg), Vit E (500 mg/kg), GPx: Glutathione peroxidase, SOD: Superoxide dismutase. Each value represents the mean ± SD (n = 6). a, significantly different from Control groups; b, significantly different from cyclo-treated group.
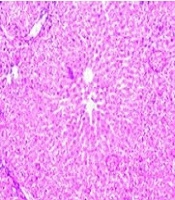
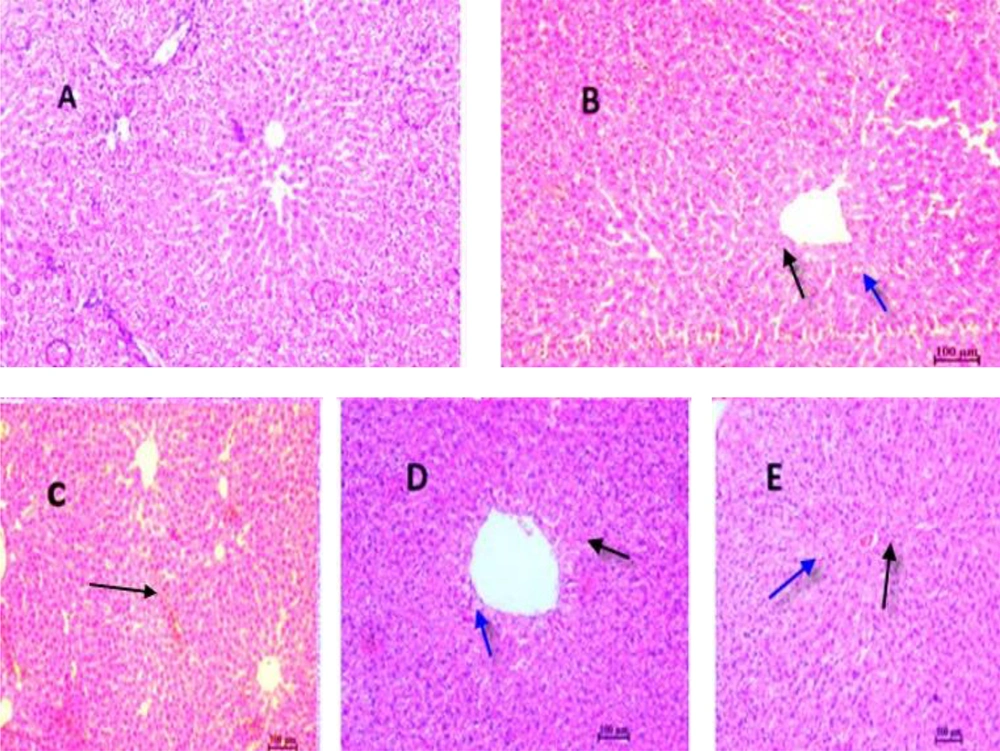
4.5. Effect of Sildenafil and Vitamin E on Histological Changes in CP-Induced Hepatotoxicity in Rats
According to Figure 5, panel A, histopathological analysis revealed a normal cellular structure in the control group. Following CP administration (panel B), various pathological changes were observed, including cell necrosis, neutrophil infiltration, venous congestion, and cell vacuolation. In the groups treated with sildenafil alone (panel C), vitamin E alone, and the combination of sildenafil and vitamin E (panel D), the structure of liver cells and lobules was similar to that of the control group, showing marked improvement compared to the CP-treated group.
Histopathological evaluation of the effect of sildenafil and Vit E on CP-induced hepatotoxicity in rats. A, control group; B, CP (200 mg/kg) group; C, sildenafil (75 mg/kg) + CP group; D, sildenafil (75 mg/kg) + Vit E+ C group, E; CP + Vit E group. Haematoxylin and eosin-stained liver sections (H&E). (10×). A: Normal, B: Black arrow indicates a large amount of apoptosis and also a large increase of blood in the sinusoidal capillaries, C: Widening of sinusoidal capillaries and accumulation in them is observed, D: Closing of sinusoidal capillaries and black arrow indicates apoptosis and blue arrow indicates hepatocyte cell vesicle, E: Black arrow indicates apoptosis and blue arrow indicates hepatocyte cell vesicle.
5. Discussion
Cyclophosphamide is an alkylating agent commonly used to treat certain types of malignancies and autoimmune diseases, such as lupus nephritis and rheumatoid arthritis (23). However, several adverse effects, including liver damage, are typically associated with CP administration in clinical settings (24). Therefore, discovering new or alternative compounds that can protect normal cells and tissues from damage by CP and its metabolites is essential (6, 25). According to the results of the current investigation, sildenafil demonstrated the ability to mitigate CP-induced liver toxicity. This protective effect was evident through a reduction in serum levels of liver function enzymes and decreased oxidative stress. These observations were further confirmed by histological examination, which revealed fewer alterations in liver tissue structure.
Weight loss is one of the most reliable indicators of the toxic effects of drugs, particularly chemotherapeutic agents (26). In the present investigation, CP administration resulted in a significant decrease in animal body weight. This finding aligns with our previous research, which demonstrated that CP administration significantly reduced the body weight of animals. In this study, the combined use of sildenafil and vitamin E mitigated the body weight loss induced by CP toxicity, showing an improvement in weight retention.
Aspartate transaminase and ALT enzymes are widely regarded as essential biochemical markers of acute liver injury (27). The elevation in transaminase levels in CP-induced hepatotoxicity is attributed to liver dysfunction, liver cell damage, and the loss of hepatocyte membrane integrity (1). As a result, these enzymes are released from the cytoplasm into the bloodstream following the disruption of the liver cell membrane (28). Elevated serum levels of AST and ALT due to CP-induced liver damage have been reported in multiple studies (29, 30). Similarly, in the present study, ALT and AST enzyme activities increased significantly in the CP-treated group. However, treatment with sildenafil markedly inhibited the elevation of these enzyme levels, suggesting its potential role in maintaining cell membrane integrity. Consistent with these findings, Baran et al. demonstrated that sildenafil reduces ALT and AST levels in cadmium-induced hepatotoxicity (10).
Oxidative stress is recognized as a key mechanism underlying CP-related hepatotoxicity (31, 32). The conversion of CP to its active metabolites, including phosphoramide mustard and acrolein, by liver cytochrome P450 enzymes leads to the production of reactive oxygen species (ROS), disrupts the oxidant/antioxidant balance, and ultimately results in oxidative stress (33). Cyclophosphamide inhibits the activity of antioxidant enzymes, such as SOD, GPx, and CAT (34). Superoxide dismutase catalyzes the elimination of the superoxide anion (O2-) by converting it to H2O2 and oxygen, after which CAT and GPx detoxify H2O2 into water (30). In this study, CP administration led to a significant decrease in SOD, CAT, and GPx activity, indicating pronounced oxidative stress, which aligns with findings from previous studies (30, 35).
Several studies have confirmed the protective effect of vitamin E against CP-induced hepatotoxicity in rats (36-38). Additionally, sildenafil has been shown to reduce gonadotoxicity associated with CP administration (39). This research demonstrated that sildenafil and vitamin E ameliorate CP-induced hepatotoxicity by reducing oxidative stress and enhancing the liver's antioxidant defenses.
The lipids in cell membranes are highly susceptible to oxidative stress. Cyclophosphamide induces the production of free radicals, leading to lipid peroxidation and stimulating the production of MDA, which is a primary indicator of lipid peroxidation (4, 40). Doustimotlagh et al. observed that CP treatment caused a significant increase in MDA levels along with a reduction in CAT activity in liver tissue, suggesting that these changes disrupt the balance of the antioxidant system in the liver (1). Consistent with these findings, the present study showed that CP elevated MDA levels in the liver and serum of animals.
Moreover, treatment with sildenafil protected the animals from CP-induced lipid peroxidation, as evidenced by a significant decrease in MDA levels. This finding supports the potential free radical scavenging and antioxidant properties of sildenafil. In agreement, Ekor et al. reported that sildenafil reduced MDA levels in paracetamol-induced liver injury in rats (12). Similarly, Cadirci et al. found that sildenafil reduced MDA levels in the lungs and kidneys in a rat model of sepsis (41).
Nitric oxide is a highly reactive mediator with a short half-life that forms the potent oxidant peroxynitrite when it reacts with the superoxide anion. This oxidant can lead to the breakdown of the antioxidant defense system (42). Excessive NO production has been reported in numerous models of inflammation and liver damage, where it acts as a highly reactive oxidant that induces apoptosis and necrosis, thereby promoting hepatotoxicity (12). In line with these findings, research by Doustimotlagh et al. indicated that CP-induced liver toxicity elevates NO levels in liver tissue (1). In the present study, serum and liver levels of NO were reduced by treatment with sildenafil and the combination of sildenafil and vitamin E. Rizk et al. also demonstrated that sildenafil inhibits lipopolysaccharide-induced NO production in N9 and primary microglial cells in rats (7).
Sildenafil maintains cellular cGMP levels by inhibiting PDE5 (43). It has been suggested that an elevated concentration of cGMP within cells may stimulate the production of additional antioxidant enzymes (44). Therefore, it can be proposed that the underlying mechanisms of sildenafil’s protective effects against CP-induced liver injury are associated with its antioxidant potential through its capacity to prevent cGMP degradation.
5.1. Conclusions
These findings indicate that sildenafil alleviates CP-induced hepatotoxicity by inhibiting lipid peroxidation, reducing NO production, and enhancing antioxidant enzyme activity. Consequently, the hepatoprotective effects of sildenafil are likely related to its free radical scavenging and antioxidant properties, paving the way for further clinical research into its potential as a hepatoprotective agent.