1. Background
Inflammatory Bowel Disease (IBD) is a chronic medical condition characterized by inflammation of the gastrointestinal tract, which includes conditions such as Crohn's disease (CD) and ulcerative colitis (UC). UC specifically involves chronic inflammation affecting the colon and rectum, leading to mucosal ulceration. Symptoms include rectal bleeding, increased stool frequency, and abdominal pain (1). The disease alternates between periods of remission and exacerbation, which can occur spontaneously or in response to treatment (2). Its global incidence and prevalence are rising, even in newly industrialized countries (3). Although the exact etiology is unclear, it is believed to involve a complex interplay of genetic, environmental, and immune factors (4).
Given the central role of mucosal injury and inflammation, agents capable of modulating these pathways may hold therapeutic potential in UC. The primary goals in managing UC are to achieve and maintain remission to prevent complications, surgery, and colon cancer (5). Current treatment options for UC include anti-inflammatory drugs such as aminosalicylates, corticosteroids, immunosuppressants, antibiotics, anti-TNFs, and probiotics (6-9). However, a definitive treatment for UC has yet to be identified. Surgery may be necessary in cases of treatment resistance or in surgical emergencies such as colonic perforation or toxic megacolon (10).
The Heracleum genus, comprising over 120 species globally, is one of the largest genera of the Umbelliferae (Apiaceae) family and is widely distributed in Asia (11, 12). Recent scientific investigations into Heracleum species have revealed a wide range of biological and pharmacological attributes. The essential oils obtained from these plants exhibit numerous advantageous characteristics, including anticonvulsant, pain-relieving, anti-inflammatory, and antifungal properties. Moreover, extracts from this genus have demonstrated substantial pharmacological effects, including antioxidative, antibacterial, antiviral, immune-boosting, insecticidal, cytotoxic, gastroprotective, anti-hair loss, infertility-related, and liver-protective properties (13).
Heracleum lasiopetalum (Golpar-e barfi), a plant belonging to the Umbrella family, is found in the humid mountainous areas of Iran, Turkey, and Iraq. In Iranian traditional healing practices, Heracleum lasiopetalum serves various purposes, including its role as an antiseptic, spice, carminative, aid to digestion, flavor enhancer, and food supplement (14). Additionally, several studies have reported the anti-Candida, antibacterial, and antioxidant effects of Heracleum lasiopetalum (15-25). The major terpene constituents (eucalyptol, camphor, pinene) and total phenolics and flavonoids present in Heracleum lasiopetalum are key bioactive components (26). Flavonoids inhibit NF-κB signaling, reducing TNF-α and IL-1β expression (27), while phenolic compounds suppress NF-κB signaling, decreasing TNF-α, IL-1β, and IL-6 production (28). Additionally, flavonoids and phenolic acids possess antioxidant properties, countering oxidative stress in UC pathogenesis and potentially mitigating mucosal damage (27-29).
2. Objectives
While no previous studies have explored the potential benefits of Heracleum lasiopetalum in treating UC, our study aimed to evaluate the efficacy of this plant extract compared to sulfasalazine, a standard UC treatment (30-32). We sought to assess Heracleum lasiopetalum's protective and healing effects on female rats with acetic acid-induced UC. Exploring the potential of natural remedies in managing UC emerges as a promising complementary support to traditional treatment modalities, potentially enhancing treatment outcomes.
3. Methods
3.1. Animals
Healthy adult female Wistar rats (8 - 9 weeks old), weighing an average of 180 ± 15 g, were utilized for this study. The rats were housed in the animal facility at the Faculty of Pharmacy, Kermanshah University of Medical Sciences, under controlled environmental conditions (room temperature: 22 ± 2°C, relative humidity: 50% ± 20%, and a regular day/night cycle). After a one-week acclimation period with unrestricted access to both water and standard laboratory food, the rats were placed in clean Plexiglas cages with wood-shaving bedding (42 × 27 × 15 cm), accommodating a maximum of three animals per cage. Standard laboratory pelleted food and tap water were provided ad libitum throughout the study. The female rats were then randomly assigned to six groups (n = 8 per group) for each of two experiments. All animal procedures were approved by the Ethics Committee of Kermanshah University of Medical Sciences.
3.2. Collection and Identification of the Plant
The plant was collected from the Marivan region in the west of Iran and transported to the Research and Education Center of Agriculture and Natural Resources at Kermanshah. Taxonomic identification was conducted by a qualified botanist, and the plant was officially registered with the herbarium number RANK-8692 for documentation and reference purposes.
3.3. Preparation of Plant Extract
The aerial parts of Heracleum lasiopetalum were harvested and dried under shaded, open-air conditions, maintaining optimal temperature and humidity. After drying, the plant material was partially crushed using an electric mill (LB20ES, Waring, USA). Aqueous extracts were prepared at concentrations of 5%, 10%, and 40% w/v using the following procedure: For the 5% extract, 5 grams of dried plant material were placed in a beaker, and 100 milliliters of water were added. The mixture was heated on a stirrer plate for 45 minutes using a magnetic stirrer (IKA, Staufen, Germany). Subsequently, the filtration process was carried out using a funnel and filter paper. After filtration, the resulting solution was allowed to cool, and extracts were collected. Similar procedures were followed for preparing the 10% and 40% extracts. The obtained extracts were then transferred into 50-milliliter Falcon tubes and used for administration to the rats (33-35).
3.4. Induction of UC in Animals
Female rats underwent anesthesia via intraperitoneal injection of 10% Ketamine (70 mg/kg body weight [BW]) and 2% Xylazine (6 mg/kg BW) sourced from Lab. Alfasan in Worden, Holland (36). A 24-hour fasting period preceded UC induction, which involved stool evacuation and rectal cleansing with normal saline. UC was induced by administering a 1 mL solution containing 4% acetic acid (pH: 2.4) sourced from Sigma-Aldrich in St. Louis, MO, USA, via intra-rectal injection, penetrating 8 cm into the anus. The control group received a 1 mL solution of normal saline intra-rectally. The duration of acetic acid contact with the colon was timed using a stopwatch, and acetic acid evacuation was facilitated by temporarily closing the anus. After precisely 10 minutes of contact, the acetic acid was allowed to drain completely (37). Throughout the examination period, the general health of the rats was carefully monitored, including changes in weight, water and food consumption, alterations in fecal consistency, the presence of blood in the stool, and mortality rates.
3.5. Experimental Design
3.5.1. Protective Effect Assessment
To evaluate protective effects, treatments were administered to 48 adult female Wistar rats, randomly assigned to six groups (n = 8). The groups included a normal control receiving distilled water without UC induction, a negative control (C-) receiving distilled water, a positive control (C+) receiving sulfasalazine (100 mg/kg), and three groups (5%, 10%, and 40% plant extract) receiving corresponding plant extracts. All treatments were given seven days before and continued for three days after acetic acid administration.
3.5.2. Treatment Effect Assessment
For evaluating treatment effects, treatments were administered to 48 adult female Wistar rats, randomly assigned to six groups (n = 8). The groups included a normal control receiving distilled water without UC induction, a negative control (C-) receiving distilled water, a positive control (C+) receiving sulfasalazine (100 mg/kg), and three groups (5%, 10%, and 40% plant extract) receiving corresponding plant extracts. All treatments were initiated three days after acetic acid administration and continued for six days.
3.6. Changes in Weight and Water/Food Consumption
Rat weights were measured using a specialized scale (Ek-4152, Camry) from the study's onset to its conclusion. Daily water and food intake were recorded throughout the study.
3.7. Changes in Colon Weight
On the final day, a seven-centimeter segment of colon tissue was isolated from the rats, and the weights of the colons from different rats were measured.
3.8. Macroscopic Observations
The rats were anesthetized with ether on scheduled days for each group and then euthanized for autopsy. Colon tissue was excised 7 cm from the anus. Photographic records of the biopsies were taken and assessed using the Gerald classification system score (Table 1) (38).
| Criteria of Scoring of IBD Morphological Damage (Gerald Method) | Score |
|---|---|
| No damage | 0 |
| Localized hyperemia, but no ulcers | 1 |
| Linear ulcers with no significant inflammation | 2 |
| Linear ulcers with inflammation at one site | 3 |
| Two or more sites of ulceration and / or inflammation | 4 |
| Two or more major sites of inflammation and ulceration or one major site of Inflammation and ulceration extending > 1 cm along the length of the colon | 5 |
3.9. Microscopic Observations
For histological examination, colon tissues from rats were fixed in 10% buffered formalin. The tissue preparation for hematoxylin and eosin (H&E) staining involved infiltration with paraffin wax, sectioning with a microtome into 5-micron sections, dewaxing in xylene, hydration through graded ethanol concentrations (100%, 95%, 80%, and 70%), followed by water rinsing, hematoxylin staining, water rinsing again, differentiation with mild acid, another rinse, eosin staining, dehydration in ascending ethanol concentrations (70%, 90%, 100%), clearing in xylene, and finally, cover-slipping. Prepared slides were evaluated using a modified Wallace method by an experienced histologist who was blinded to the normal and acetic acid-treated groups (refer to Table 2 for details) (39). Masson's trichrome staining was conducted to assess fibrosis in the tissue sections, which involved the following steps: Deparaffinization and rehydration, staining with hematoxylin for 8 minutes, staining with ponceau acid fuchsine solution for 5 minutes, rinsing in running tap water for 8 minutes, differentiation in phosphomolybdic-phosphotungstic acid for 5 minutes, staining with aniline blue for 5 minutes, further differentiation in 0.2% acetic acid for 2 minutes, dehydration, clearing, and mounting. The sections were photographed using a light microscope (40).
| Variable | Inflammation | Depth of Lesion | Fibrosis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non | Mild | Medium | Severe | Non | Lamina propria | Submucosa | Muscular | Serosa | Non | Mid | Severe | |
| Result | - | + | ++ | +++ | - | + | ++ | +++ | ++++ | - | + | ++ |
3.10. Statistical Analysis
Statistical analyses were performed using SPSS version 27 (SPSS Inc., Chicago, IL, USA). Data are expressed as mean ± standard error of the mean. To evaluate differences between treated groups and the control group, a one-way analysis of variance was used, followed by Duncan's post hoc test, with statistical significance set at P < 0.05. Graphical representation of data was created using Graph Pad Prism version 8 (v8.2.1; Graph Pad Software, Inc., La Jolla, CA, USA).
4. Results
4.1. Changes in Weight, Water, and Food Intake in Protective and Treatment Groups
Table 3 presents the weight changes in the protective groups. No significant differences were observed before acetic acid administration. On the 10th day, only the normal group showed a significant difference. In the treatment groups, significant weight gain occurred in the normal group by the 7th day, surpassing other groups except the positive control. By the 9th day, both the normal and positive control groups differed significantly from the negative control, with no differences noted compared to the extract groups (Table 4).
| Variables | Day 1 | Day 3 | Day 5 | Day 7 | Day 10 |
|---|---|---|---|---|---|
| Normal | 178.00 ± 13.12 a | 180.75 ± 12.36 a | 180.50 ± 12.72 a | 170.25 ± 11.84 a | 186.25 ± 15.20 a |
| C- | 179.80 ± 9.35 a | 183.60 ± 7.83 a | 184.80 ± 5.07 a | 183.20 ± 8.64 a | 155.60 ± 11.28 b |
| C+ | 167.20 ± 8.79 a | 172.20 ± 8.25 a | 178.20 ± 6.98 a | 168.80 ± 6.72 a | 147.80 ± 8.23 b |
| 5% | 171.00 ± 13.47 a | 177.80 ± 12.72 a | 181.80 ± 13.54 a | 169.40 ± 11.72 a | 154.00 ± 16.46 b |
| 10% | 178.20 ± 7.33 a | 183.00 ± 9.38 a | 187.60 ± 8.62 a | 183.60 ± 12.03 a | 157.40 ± 7.73 b |
| 40% | 178.00 ± 10.80 a | 182.20 ± 13.20 a | 185.20 ± 13.14 a | 174.20 ± 11.37 a | 154.60 ± 10.81b |
a C-: Negative control; C+: Positive control; 5%: 5% extract; 10%: 10% extract; 40%: 40% extract.
b Data are expressed as mean ± SEM.
c Distinct letters within each column indicate a significant difference between the groups, with P < 0.05.
| Variables | Day 3 | Day 5 | Day 7 | Day 9 |
|---|---|---|---|---|
| Normal | 205.50 ± 25.20 a | 206.50 ± 23.10 a | 208.50 ± 23.57 a | 209.75 ± 22.87 a |
| C- | 174.75 ± 11.24 b | 175.00 ± 13.95 b | 179.75 ± 17.56 b | 179.00 ± 16.35 b |
| C+ | 183.00 ± 10.10 b | 191.00 ± 9.35 b | 196.40 ± 11.97 a | 206.80 ± 21.96 a |
| 5% | 170.33 ± 6.68 b | 180.17 ± 5.31 b | 190.00 ± 5.93 b | 188.83 ± 7.14 a, b |
| 10% | 172.00 ± 7.40 b | 179.83 ± 6.65 b | 187.67 ± 10.37 b | 193.83 ± 11.62 a, b |
| 40% | 169.00 ± 10.77 b | 180.33 ± 10.75 b | 186.83 ± 9.54 b | 189.83 ± 11.00 a, b |
a C-: Negative control; C+: Positive control; 5%: 5% extract; 10%: 10% extract; 40%: 40% extract.
b Data are expressed as mean ± SEM.
c Distinct letters within each column indicate a significant difference between the groups, with P < 0.05.
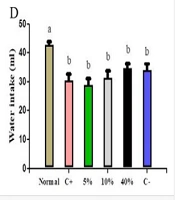
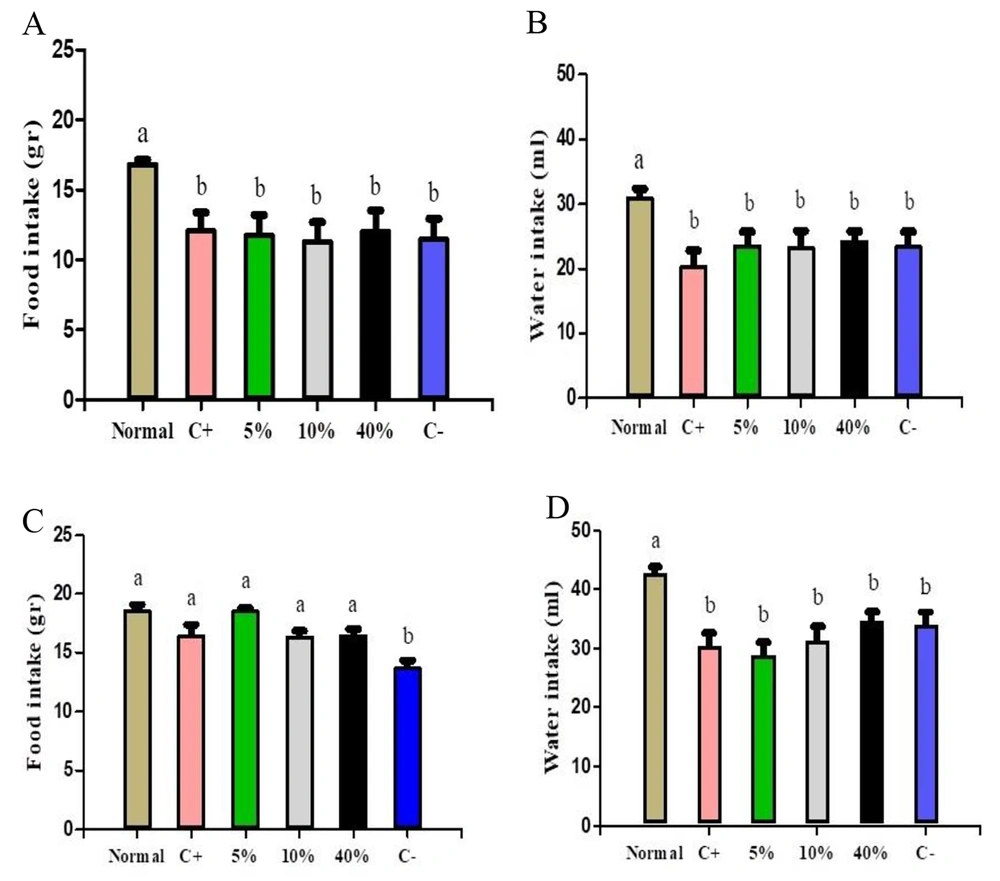
Figure 1 depicts water and food intake in both protective and treatment groups. Notably, only the normal group exhibited significant differences in both intake categories when compared to other protective groups (Figure 1A and B). Additionally, all treatment groups showed significant differences in food consumption compared to the negative control, while only the normal group displayed a significant difference in water consumption compared to the other groups (Figure 1C and D).
A, changes in food consumption; B, water consumption in protective groups; C, changes in food consumption; D, water consumption in treatment groups. Normal, Negative Control (C-), Positive Control (C+), 5% Extract (5%), 10% Extract (10%), 40% Extract (40%). Data are expressed as mean ± SEM. Distinct letters within each column indicate a significant difference between the groups, with P < 0.05.
4.2. Colon Weight Changes in Protective and Treatment Groups
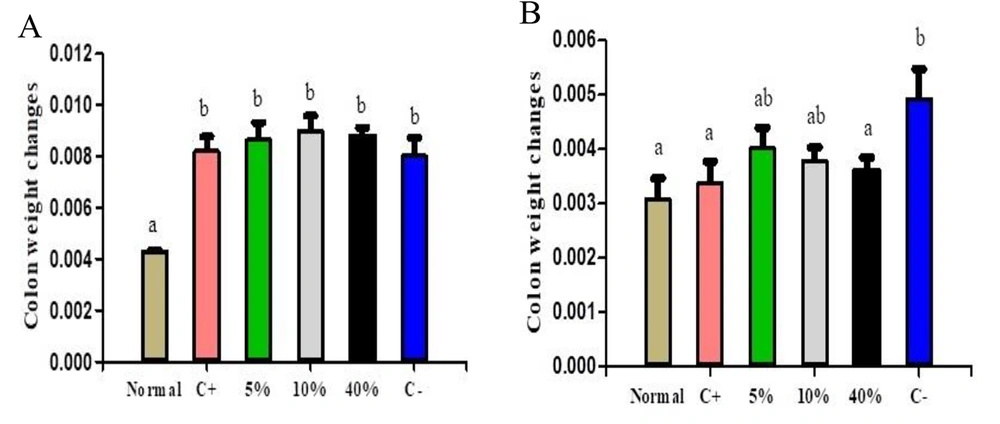
Figure 2 illustrates colon weight changes in both protective and treatment groups. The normal group showed significant differences compared to all other groups. Among the treatment groups, the normal, positive, and 40% extract groups differed significantly from the negative control. However, no significant differences were observed between the 5% and 10% extract groups and the negative control (Figure 2A and B).
Colon weight changes in (A) protective groups and (B) treatment groups. Normal, Negative Control (C-), Positive Control (C+), 5% Extract (5%), 10% Extract (10%), 40% Extract (40%). Data are expressed as mean ± SEM. Distinct letters within each column indicate a significant difference between the groups, with P < 0.05.
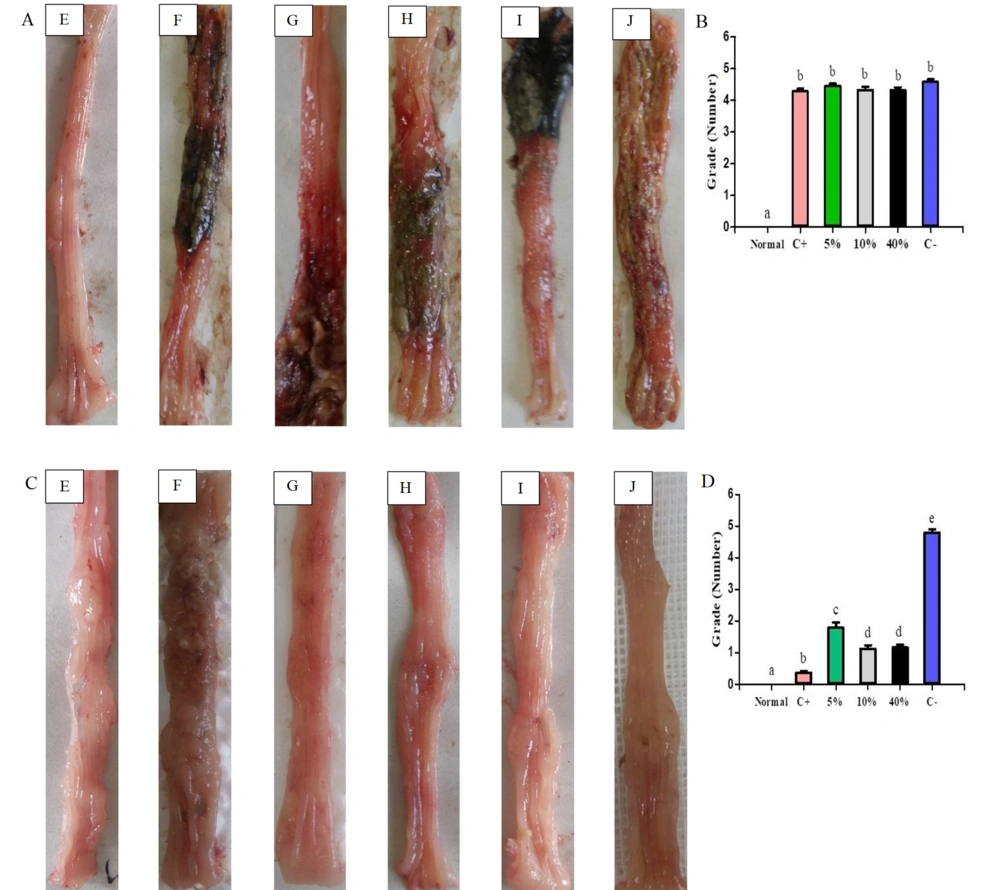
4.3. Macroscopic Observation of Protective and Treatment Groups
In the protective groups, mucosal ulcer, hyperemia, and inflammation were scored. Three days post-UC induction, the normal group showed significant differences compared to other groups (Figure 3A and B). In the treatment groups, scoring revealed the lowest scores nine days after acetic acid administration in the following order: Normal, positive control, and the extract groups (40%, 10%, 5%), with all groups showing significant differences compared to the negative control. Notably, the 10% and 40% plant extract groups demonstrated the most favorable results in reducing inflammation and improving ulcers in rats with UC (Figure 3C and D).
Macroscopic view of colon tissue in A (protective) and C (treatment) groups. Tissue damage scoring based on the Gerald method in B (protective) and D (treatment) groups: E: Normal; F: Negative Control (C-); G: Positive Control (C+); H: 5% extract (5%); I: 10% extract (10%); J: 40% extract (40%). Data are expressed as mean ± SEM. Distinct letters within each column indicate a significant difference between the groups, with P < 0.05.
4.4. Microscopic Observation of Protective and Treatment Groups
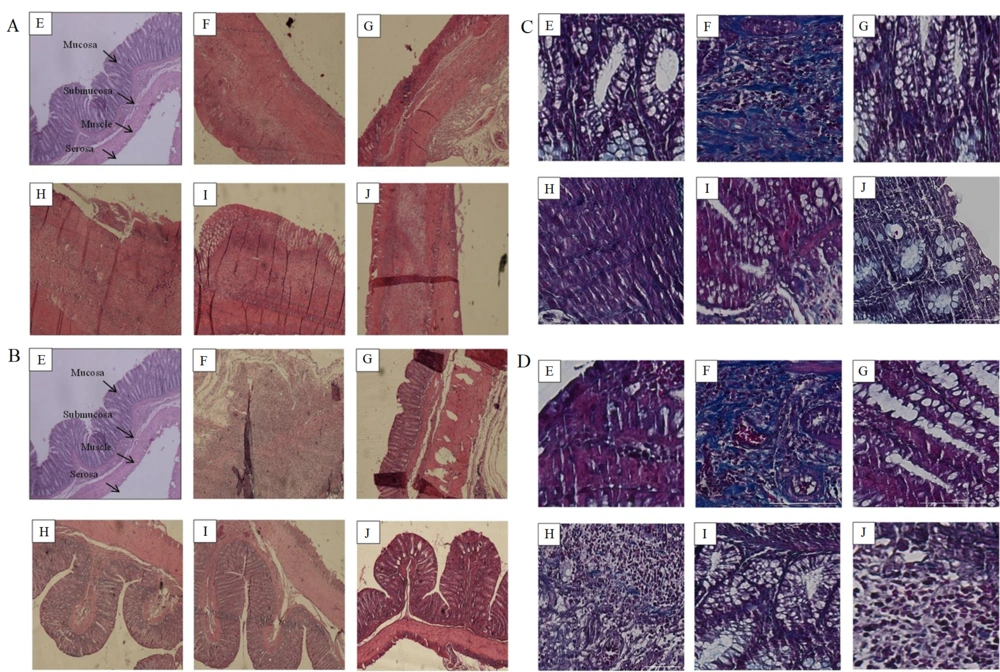
The microscopic study, utilizing the modified Wallace method (Table 2), evaluated inflammation, lesion depth, and fibrosis in the colons of both protective and treatment groups, as depicted in Figure 4A and B. Additionally, tissue fibrosis in acetic acid-induced UC was assessed using Masson's trichrome staining, illustrated in Figure 4C and D. Summarized outcomes are provided in Table 5. Among the protective groups, the normal group exhibited no signs of inflammation, lesions, or fibrosis. Conversely, the negative control group (C-) displayed severe inflammation, deep serosal lesions, and severe fibrosis. The positive control group (C+) showed moderate inflammation and lesion depth, with no fibrosis. Within the plant extract groups, the 5% extract group exhibited severe inflammation, deep serosal lesions, and mid-level fibrosis; the 10% and 40% extract groups both demonstrated mild inflammation, lesions in the lamina propria layer, with the 10% group showing no fibrosis and the 40% group displaying mild fibrosis. Conversely, the negative control group exhibited severe inflammation, serosa lesion depth, and severe fibrosis, while the positive control group displayed no inflammation, lesions, or fibrosis. Notably, the 5% extract group presented mild inflammation, lesions in the lamina propria layer, and mid-level fibrosis; the 10% extract group showed mild inflammation, lesions in the lamina propria layer, and no fibrosis; the 40% extract group had mild inflammation, lesions in the lamina propria layer, and no fibrosis. According to the modified Wallace method, the 10% and 40% plant extract groups demonstrated the most favorable results, indicating their efficacy over the 5% extract.
Photomicrographs of rat colons stained with hematoxylin and eosin (H&E) at × 40 magnification in A (protective) and B (treatment) Groups. Photomicrographs of rat colons stained with Masson's Trichrome at × 200 magnification in C (protective) and D (treatment) Groups. Collagen deposition is revealed by blue staining. E: Normal; F: Negative Control ; G: Positive Control ; H: 5% Extract; I: 10% extract; J: 40% extract (40%).
| Groups | Inflammation | Depth of Lesion | Fibrosis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non | Mild | Medium | Severe | Non | Lamina Propria | Submucosa | Muscular | Serosa | Non | Mid | Severe | |
| Protective | ||||||||||||
| Normal | - | - | - | |||||||||
| C- | +++ | ++++ | ++ | |||||||||
| C+ | ++ | ++++ | - | |||||||||
| 5% | +++ | ++++ | + | |||||||||
| 10% | ++ | ++++ | + | |||||||||
| 40% | +++ | ++++ | + | |||||||||
| Treatment | ||||||||||||
| Normal | - | - | - | |||||||||
| C- | +++ | ++++ | ++ | |||||||||
| C+ | - | - | - | |||||||||
| 5% | + | + | + | |||||||||
| 10% | + | + | - | |||||||||
| 40% | + | + | - | |||||||||
a C-: Negative control; C+: Positive control; 5%: 5% extract; 10%: 10% extract; 40%: 40% extract.
5. Discussion
This study explored the protective and therapeutic effects of Heracleum lasiopetalum extract in female rats with acetic acid-induced UC. Oral administration of the extract resulted in dose-dependent improvements in both macroscopic and microscopic colonic inflammation and injury markers in the animal model. Utilizing female rats allowed the investigation of gender-specific differences in UC, relevant to humans, while also establishing a foundational understanding of the herb's effects in one gender, laying the groundwork for future research. This approach helped minimize variability introduced by gender-related factors. Utilizing animal models is crucial for understanding the mechanisms involved in UC and discovering potential drugs (41).
In different studies, acetic acid concentrations of 3 - 10% have been used to induce UC by applying 1 - 2 milliliters of acetic acid to the colon for a contact time of 15 to 30 seconds. Currently, a common animal model used for inducing UC involves the intrarectal injection of 4% acetic acid with a contact time ranging from 15 to 30 seconds with the colon. The examination of the pharmacological effects of chemical substances occurs within 24 hours after induction, with various treatment periods (42-46). Our previous study identified an optimal model, involving 10 minutes of 4% acetic acid contact, UC confirmation, initiation of treatment 72 hours post-acid administration, and monitoring up to six days post-UC confirmation. This model considers key factors, including acetic acid contact duration, precise UC confirmation timing, treatment period, and non-spontaneous recovery. For protective assessment, administering the extract seven days before UC induction until three days post-acid administration, with UC confirmation on the third day, proved effective. Our rat model, induced with acetic acid, mimics key features of acute inflammation flare-ups seen in human UC, including symptoms like diarrhea, rectal bleeding, and colonic mucosa inflammation/ulceration.
This model provides a platform to study potential therapeutic interventions during this critical phase of the disease (37), laying the groundwork for further exploration of Heracleum lasiopetalum extract as a potential supplement treatment option, which could complement existing therapies for UC.
On the final day of assessing food consumption in treatment groups with plant extract, a notable increase was observed. The rats' weights at the study's end did not significantly differ from the normal and positive control groups, suggesting improved digestive system function and enhanced absorption efficiency. However, no significant improvements in weight or food consumption were noted in the protective extract groups compared to the normal and positive control groups. Examining colon weight in different treatment groups revealed the most significant reduction in the positive control group, followed by the 40% extract treatment group (Figure 2B). In the protective groups, the normal group showed a significant difference from all others, while the remaining protective groups did not differ significantly from each other (Figure 2A). These findings indicate potential benefits of plant extract treatments on food consumption, weight maintenance, and colon weight reduction, suggesting positive effects on digestive system function.
Overall, the diagnosis of UC in patients involves considering several factors. In microscopic examination, the severity of inflammation indicates inflammatory cell infiltration, including neutrophils. Additionally, the degree of inflammation suggests involvement of different parts of the colon wall, such as the mucosa, submucosa, and across the wall (47). In our study, inflammation was examined both macroscopically and microscopically. In protective groups, pretreatment with 5%, 10%, and 40% plant extract for 7 days before UC induction did not significantly reduce inflammation scores compared to the negative control, indicating limited preventive effects. In treatment groups, the plant extract consistently reduced inflammation scores, indicating its suppressive effect on acetic acid-induced inflammation. Photomicrographs H, I, and G in Figure 4B confirmed decreased inflammatory cell infiltration, particularly neutrophils, reinforcing the extract's potential suppressive effect on inflammation. This suggests a possible mechanism involving the inhibition of neutrophil migration and activity. Post-treatment with the plant extract for 6 days after UC induction revealed dose-dependent improvements. The 40% and 10% concentrations demonstrated superior therapeutic efficacy, significantly reducing ulceration, hyperemia, inflammation, and lesion depth compared to untreated controls. In contrast, the 5% dose exhibited lesser effectiveness, underscoring the importance of dose in eliciting pharmacological responses.
To date, understanding the physiological pathways involved in intestinal wound healing remains incomplete. Both acute and chronic intestinal inflammation involve macrophages and neutrophils, which contribute to localized tissue damage through the release of reactive oxygen radicals and tissue-degrading enzymes, triggered by pro-inflammatory cytokines and cell-bound proinflammatory peptides (48). Severe tissue damage prompts the migration of myofibroblasts to the injury site, a critical process involving wound contraction and extracellular matrix (ECM) generation linked to physiological changes in chronic inflammation (49, 50). Importantly, chronic or recurrent inflammation is a prerequisite for the initiation of intestinal fibrosis, a significant complication in patients with IBD (48, 51). In our study, the 40% and 10% treatment extract groups effectively reduced fibrosis (Figure 4D and Table 5), potentially attributed to the plant's impact on reducing inflammation, thus preventing its exacerbation and the occurrence of fibrosis. In the protective groups, fibrosis was not observed in the normal and positive control groups on the third day, while it persisted in the other groups (Figure 4C and Table 5).
The anti-inflammatory effects observed in this study can be attributed to bioactive components in Heracleum lasiopetalum, notably flavonoids and phenolic compounds. Previous research has highlighted their abundant presence in Heracleum lasiopetalum extracts (17, 25). Flavonoids may modulate NF-κB signaling, inhibiting the activation and reducing the expression of downstream pro-inflammatory cytokines like TNF-α and IL-1β (27). Similarly, phenolic compounds in experimental UC models suppress NF-κB signaling, decreasing the production of cytokines such as TNF-α, IL-1β, and IL-6, thereby offering potential as anti-inflammatory agents (28). Additionally, studies indicate alterations in total antioxidant capacity and increased reactive oxygen species levels in patients with IBD (52). Flavonoids (27) and phenolic acids (28) possess antioxidant properties, countering oxidative stress in UC pathogenesis. These compounds may mitigate mucosal damage by inhibiting reactive oxygen species production and enhancing endogenous antioxidants (29). Heracleum lasiopetalum extracts also exhibit broad-spectrum antimicrobial effects against both Gram-positive and Gram-negative bacteria (19-23). This antimicrobial activity could impact the intestinal microenvironment, holding potential significance in the context of UC. Our study has identified several biological effects of Heracleum lasiopetalum Boiss concerning UC. These effects include anti-inflammatory, wound healing, and anti-fibrotic actions, highlighting the plant's potential relevance as a supplementary therapy alongside standard drugs for UC.
5.1. Conclusions
This study provides novel evidence that Heracleum lasiopetalum extract effectively repairs acetic acid-induced colonic injury in female rats. The observed dose-dependent therapeutic potential in experimental UC suggests that Heracleum lasiopetalum may offer complementary support to conventional drugs, enhancing treatment outcomes by ameliorating both macroscopic and microscopic markers of inflammation and injury. While Heracleum lasiopetalum emerges as a promising supplementary phytotherapeutic agent, future research should focus on identifying its active phytochemicals, elucidating underlying mechanisms, and assessing its translational potential for clinical applications.