1. Background
Parkinson's disease (PD) is a progressive and irreversible neurological disease that is more common in people aged over 65 years and is characterized by unintended or uncontrollable movements such as rigidity, slowness, and tremor. As PD progresses, symptoms generally begin gradually and worsen over time, leading to difficulties in walking and talking (1). The available therapeutic options involve pharmacologic approaches (levodopa) and non-pharmacologic approaches (exercises) that offer good control of motor symptoms but do not modify the evolution of the disease. Therefore, it will be very important to know the effective treatments (2).
Throughout history, cultures around the world have learned how to use herbal medicine to maintain and improve health. The importance of natural products derived from plant sources for disease treatment is exponentially increasing due to the increased issues relating to adverse drug reactions (3). Despite the wide variety of plants in the world, a limited number of them have been studied for anti-PD activity. Traditional Persian medicine (TPM) herbs have gained popularity as a source of potential new drugs for the prevention and treatment of PD and other neurodegenerative diseases due to their low toxicity and limited side effects (4). Among these herbs, Ricinus communis Linn, commonly known as the castor oil plant, is a species of flowering plant in the Euphorbiaceae family. This soft-wooded shrub is widespread throughout tropical regions. The main phytochemical compounds of the plant are alkaloids, flavonoids, tannins, sterols, and terpenes. The castor oil plant has many uses in traditional and modern medicine if the dose is maintained correctly. It is used as a traditional natural remedy or folkloric herb for the control and treatment of a wide range of diseases around the globe due to significant pharmacological activities, including anti-fungal and anti-microbial, hepatoprotective, anti-diabetic, anti-fertility, anti-nociceptive, antioxidant, larvicidal, insecticidal, anti-inflammatory, free radical scavenging, anti-cancer, wound healing, and bone regenerative properties (5-7).
Although the laxative effects have been extensively studied, other effects of this plant mentioned in traditional medicine texts remain largely unexplored (8). In R. communis, leaves have been traditionally used in India for the treatment of pain and central nervous system disorders. In Russia, the oil extracted from this plant is used as a motor activity stimulant. Senegalese people living in Walo and Cayor prescribe the seeds of R. communis to treat nervous and mental illnesses. Locals in Algeria use the oil topically to alleviate limb paralysis. During the tenth century, Abu Mansur used this oil to treat facial paralysis (9). The castor oil plant, known as khorve in TPM medicine texts, has been used as a therapeutic agent for numerous health issues in traditional medicine for over 4000 years due to its laxative properties. In his book Makhzan al-Adveieh, Aghili Khorasani, a 12th-century physician, mentioned that this plant has remarkable effects on brain diseases and is beneficial in treating symptoms such as paralysis and tremors. These characteristics are reminiscent of the symptoms of PD (10). Castor oil (CO), derived from the R. communis, is a pale yellow oil with a subtle scent. Its composition primarily consists of fatty acids and neutral lipids (triglycerides). Additionally, the oil contains various minor biologically active compounds, including unsaponifiable fractions such as carotenoids, phenolics, phospholipids, phytochemicals, phytosterols, tocopherols, and tocotrienols. The relative proportion of fatty acids composition in castor oil can be influenced by factors such as the type of cultivars, the geographical origin of the crop, or the time of harvest, similar to other oilseed crops (Appendix 1 in the Supplementary File) (11).
2. Objectives
According to the above explanations and referring to TPM books, it seems very necessary to investigate the effect of this plant on PD. The present study determined and compared the anti-oxidant and anti-inflammatory effects of different doses of castor oil against an animal model of malathion-induced PD.
3. Methods
3.1. Chemicals
Malathion (purity 96%, Ariashimi Factory, Zahedan, Iran), levodopa (Ramopharmin, Iran), Noshad edible castor oil (Ganjeh Osareh Nature Pharmaceutical Company), acetylthiocholine iodide, 5, 5-dithiobis-2- nitrobenzoic acid (DTNB), malondialdehyde bis dimethyl acetal, phenylacetate, 2- thiobarbituric acid (TBA), were purchased from Sigma-Aldrich (USA), and all the other chemicals were purchased from Merck (Germany).
3.2. Animals and Treatments
Male Wistar rats, weighing 220 ± 20 g and aged 70 days, were acquired from the Faculty of Medical Sciences at Tarbiat Modares University. The rats were acclimated in polystyrene cages for 1 week prior to the study. They were kept under a 12-hour light/dark cycle at a room temperature of 20 – 25°C, with a relative humidity of 50%. Food and water were provided ad libitum. The animals were allowed to adapt to their environment before the start of the experiment.
A total of 48 male rats were randomly divided into 8 groups, each consisting of 6 animals. The groups were as follows: (1) Control group (received normal saline); (2) malathion-treated group (100 mg/kg); (3) malathion + castor oil (0.01 mg/kg) treated group; (4) malathion + castor oil (0.05 mg/kg) treated group; (5) malathion + castor oil (0.5 mL/kg) treated group; (6) malathion + levodopa (10 mg/kg) treated group; (7) castor oil (0.5 mg/kg) group; and (8) polyethylene glycol (PEG) group (vehicle for levodopa) (12).
3.3. Open-Field Test
The open-field apparatus used in this study was made of white wood and had a floor measuring 100 × 100 cm, divided into 25 equal units of 20 × 20 cm by red lines. The walls of the apparatus were painted white and were 50 cm high. The test room was illuminated at the same intensity as the colony room to maintain consistency. The apparatus was thoroughly cleaned with a wet sponge and a dry paper towel after each test to minimize the influence of other animals. Each rat was placed individually in the center of the open field, and its behavior was observed for a period of 5 minutes. The parameters evaluated in this test included the total number of squares crossed (indicating total locomotion) (TL), the number of outer squares crossed (indicating peripheral locomotion) (PL), and the number of inner squares crossed (indicating central locomotion) (CL). The open-field test was conducted on the first day and repeated after the last treatment (13).
3.4. Rotarod Test
An accelerating rotarod test was performed 24 hours after the final treatments. We first trained each rat on the morning prior to the test to remain for 30 s on the rotarod apparatus that revolved at 5 rpm. Subsequently, we placed the rats on a rotarod rotating at 10 rpm. We measured the duration (seconds) the rat remained on the rod, with a cut-off of 180 s (12).
3.5. Catalepsy
The bar test was used to measure catalepsy (failure to correct an imposed posture resulting from muscular rigidity) according to the method of Costall and Naylor (1974). Briefly, the rodent is located with both forelimbs on a horizontal thin metal bar (9 cm) above and parallel from the base, and the time it takes for a rodent to remove one or both of its forelimbs from a bar is measured (14).
3.6. Determination of Acetylcholinesterase (AChE) Activity
Animals were killed by decapitation using a guillotine, and their serum was used to measure the plasma AChE activity according to Ellman's spectrophotometrical method. Briefly, sample serum (20 µL) and 5% (w/v) acetylthiocholine iodide solution (100 µL) was added to 3 mL Elman reagent ((5, 5’-dithiol-bis-[2-nitrobenzoic acid]). The absorbance of different samples was measured at 0.5 min intervals for 2 min at 405 nm. The enzyme inhibition (%) was calculated according to Equation (1):(1):
Inhibition (%) = (Abs control−Abs sample/Abs control) × 100 (13).
3.7. Measurement of Malondialdehyde in the Striatum Tissue
Brains were removed after decapitation, and striatum tissue was dissected on ice immediately. The striatum was homogenized for 2 min at 4°C in 1.15% potassium chloride (KCl) to determine the levels of the measurement of malondialdehyde (MDA) (an end-product generated by the decomposition of arachidonic acid and larger PUFAs) described previously (15).
3.8. Measurement of Glutathione in the Striatum Tissue
One ml of homogenate of the striatum was precipitated with 1 ml of 10% trichloroacetic acid (TCA), and the precipitate was removed by centrifugation at 3000g for 5 min. To 0.5 mL of supernatant, 2 mL of DTNB (5,50-dithiobis(2- nitrobenzoic acid) was added, and the total volume was prepared up to 3 mL with phosphate buffer (0.2M, pH 8.0). The absorbance was read at 412 nm. Measurement of glutathione (GSH) contents of tissues were expressed as nmol/g tissue (16).
3.9. Detection of TNFα and IL-6 in the Striatum Tissue
The frozen striatum was homogenized in 1 mL of homogenization buffer through mechanical disruption and then centrifuged at 10,000 g for 10 minutes at 4°C. The resulting supernatant was separated, and the protein concentration was determined using a Bio-Rad protein assay kit. Subsequently, the levels of tumor necrosis factor-alpha (TNFα) and interleukin-6 (IL-6) in the supernatants were measured using a rat-specific ELISA kit (Invitrogen, USA) according to the manufacturer's protocol (17).
3.10. Statistical Analysis
All data were presented as the mean ± standard deviation (SD) and were analyzed using one-way analysis of variance (ANOVA), followed by Tukey's multiple comparison test using PRISM, v. 6.00 (GraphPad Software Inc., San Diego, CA, USA). P < 0.05 was considered statistically significantly different.
4. Results
4.1. Effect of Malathion and Castor Oil on Locomotor Activity using the Open-Field Test
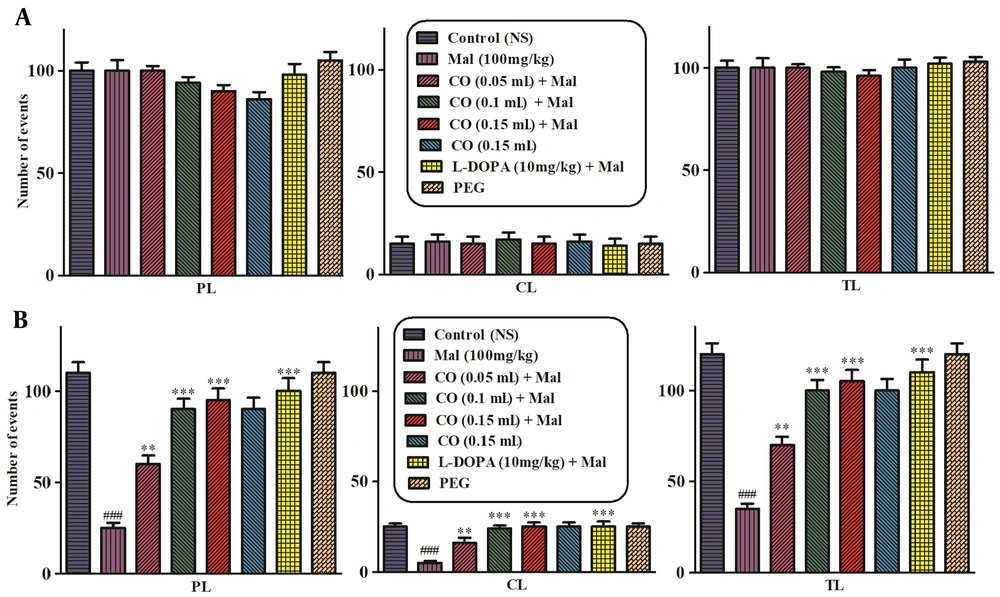
The open-field test was performed in the first and final of the experiment to assess the locomotor activity. On the 1st day, treatment with malathion alone or plus castor oil and levodopa did not have any considerable effect on TL (Figure 1A), and on the final day, malathion (100 mg/kg, IP) decreased the PL, central CL, and TL in comparison to the normal saline group. Castor oil and levodopa plus malathion markedly increased the PL and TL (Figure 1B). In addition, castor oil (10 mg/kg) markedly enhanced CL compared to the malathion group (P < 0.001).
Effects of castor oil on total locomotion (TL), central locomotion (CL), and peripheral locomotion (PL) in the open-field test on the 1st day of the experiment (A) and 28th day of the experiment (B). Data are presented as mean ± SD (n = 5). **P < 0.01 and ***P < 0.001 compared to normal saline and ###P < 0.001 compared to the malathion group, Tukey-Kramer test, Mal (malathion), CO (castor oil ), PEG (polyethylene glycol).
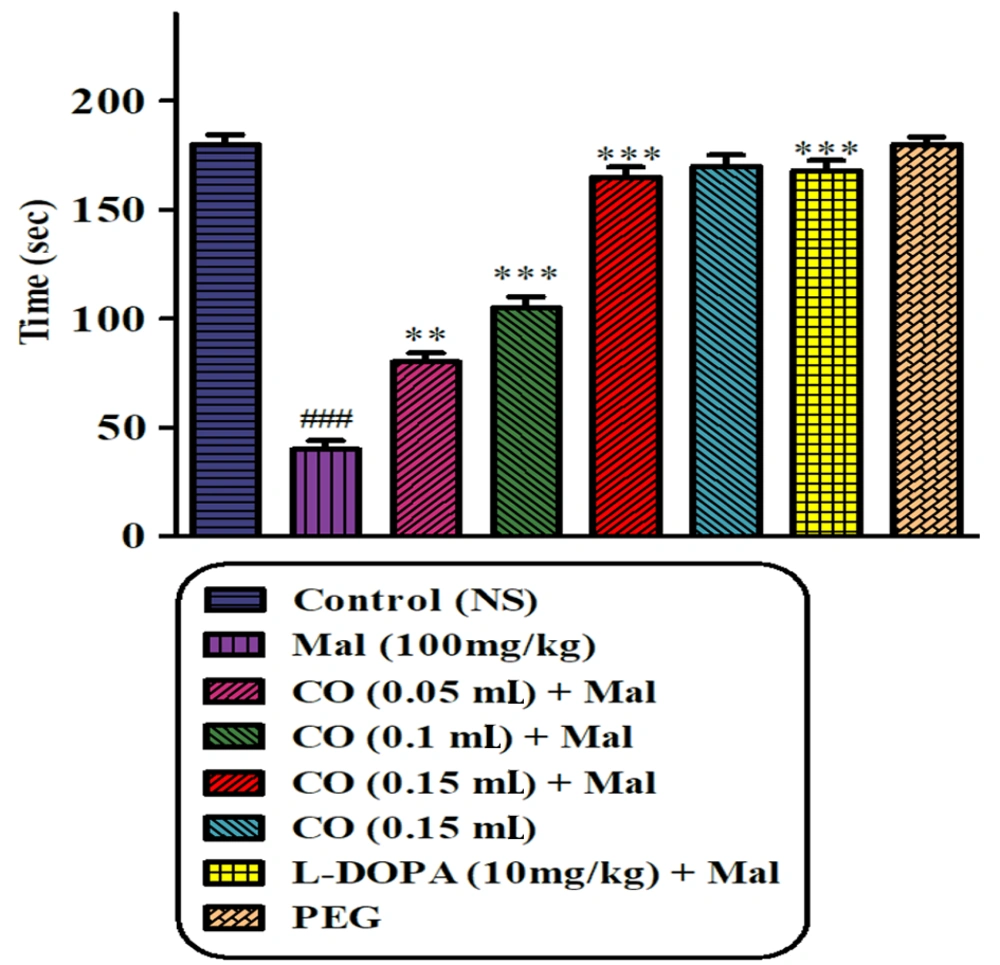
4.2. Rotarod Test
Results of the rotarod test represented an obvious decline in the latency before the falling in the malathion (100 mg/kg)-treated animal when compared to the normal saline group (P < 0.001). The malathion group showed a significant reduction in muscle coordination compared to the normal saline group. Castor oil (0.05, 0.1, and 0.15 mL) and levodopa, in combination with malathion, were able to partially recover muscular incoordination. The best effect was observed in animals receiving a dose of 0.15 mL. No significant difference was observed between castor oil (0.15 mL) alone and the normal saline group (Figure 2).
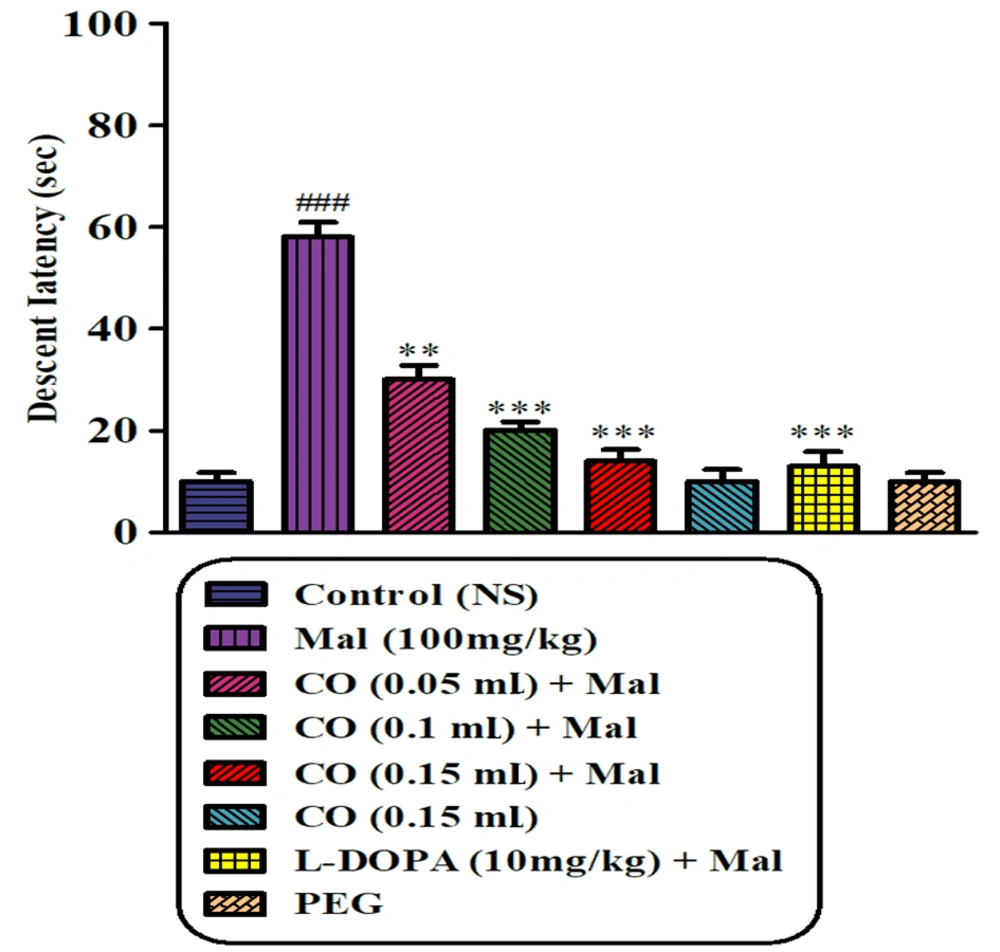
4.3. Catalepsy
The results of the bar test demonstrated that sub-chronic exposure to malathion led to a significant induction of cataleptic immobilization compared to the normal saline group (P < 0.001). However, the administration of castor oil (at 3 different doses) and levodopa in combination with malathion significantly reduced the intensity of malathion-induced catalepsy (P < 0.001). Furthermore, there were no significant differences in the elapsed time on the bar test between the groups treated with PEG or castor oil alone and the normal saline group (Figure 3).
Effects of castor oil on malathion-induced catalepsy determined in the bar test. Data are presented as mean ± SD (n = 5). **P < 0.01 and ***P < 0.001 compared to normal saline and ###P < 0.001 compared to malathion group, Tukey-Kramer test, Mal (malathion), CO (castor oil), PEG (polyethylene glycol).
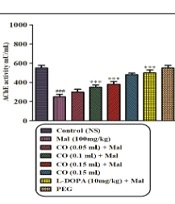
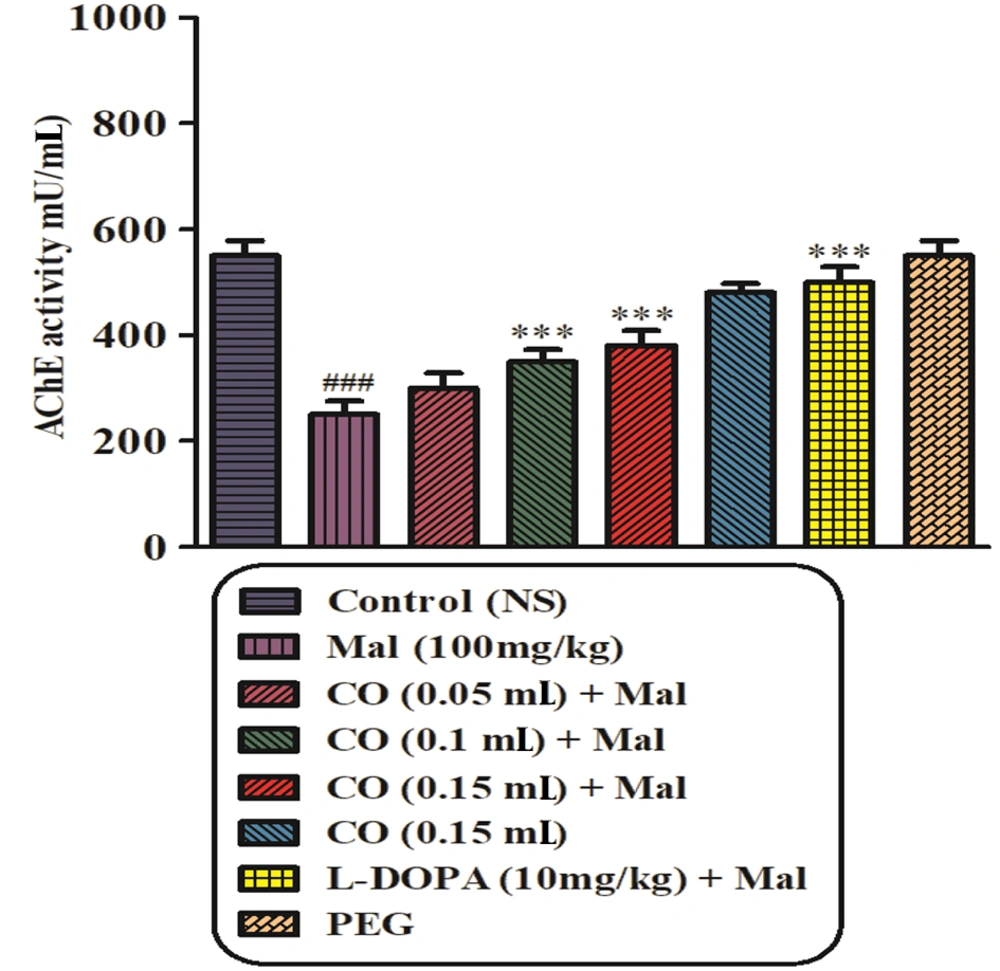
4.4. AChE Activity
As depicted in Figure 4, treatment of rats with malathion (100 mg/kg) resulted in a significant decrease in plasma AChE activity after 28 days compared to the normal saline group (P < 0.001). However, no statistically significant changes in AChE activity were observed between the malathion plus castor oil groups (0.05 mL) and the malathion-treated groups. On the other hand, the malathion plus castor oil groups (0.1 and 0.15 mL) and the levodopa group exhibited a significant increase in AChE activity compared to the malathion group (P < 0.001).
4.5. Effect of Malathion and Castor Oil on Oxidative Stress Markers
4.5.1. Measurement of MDA
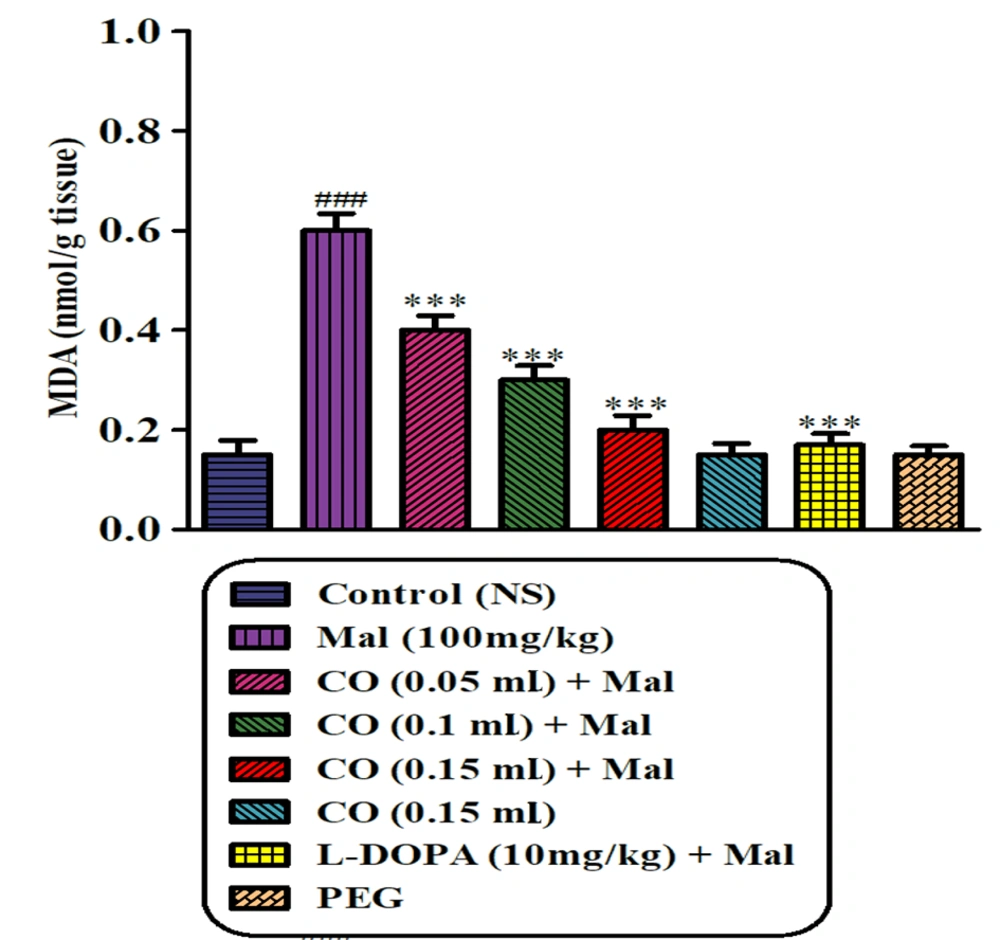
Sub-chronic exposure to malathion resulted in a significant elevation in the MDA level in the striatum of rats compared to the normal saline group (P < 0.001). However, co-administration of castor oil (at 3 different doses) and levodopa with malathion effectively reduced the MDA level in the rat striatum compared to the malathion group (P < 0.001) (Figure 5).
Effect of castor oil on lipid peroxidation induced by malathion in the striatum of the rats. Data are expressed as mean ± SD (n = 6). ***P < 0.001 compared to normal saline, ###P < 0.001 compared to malathion group, Tukey-Kramer test, Mal (malathion), MDA (malondialdehyde), CO (castor oil), PEG (polyethylene glycol)
4.5.2. Measurement of GSH
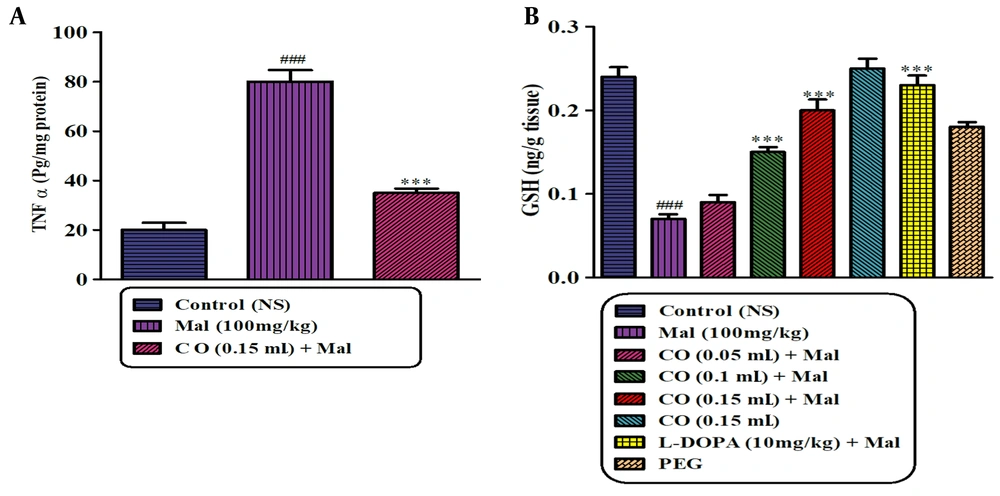
Castor oil (0.1 and 0.15 mL) and levodopa plus malathion significantly enhanced the content of GSH in rat striatum in comparison with malathion alone (P < 0.001) (Figure 6).
Effect of castor oil on GSH content following exposure to malathion in the striatum of the rats. Data are expressed as mean ± SD (n = 6). ***P < 0.001 compared to normal saline and ###P < 0.001 and #P < 0.05 compared to the malathion group, Tukey-Kramer test, Mal (malathion), CO (castor oil), GSH (glutathione), PEG (polyethylene glycol)
4.5.3. Effect of Malathion and Castor Oil on Cytokine Production
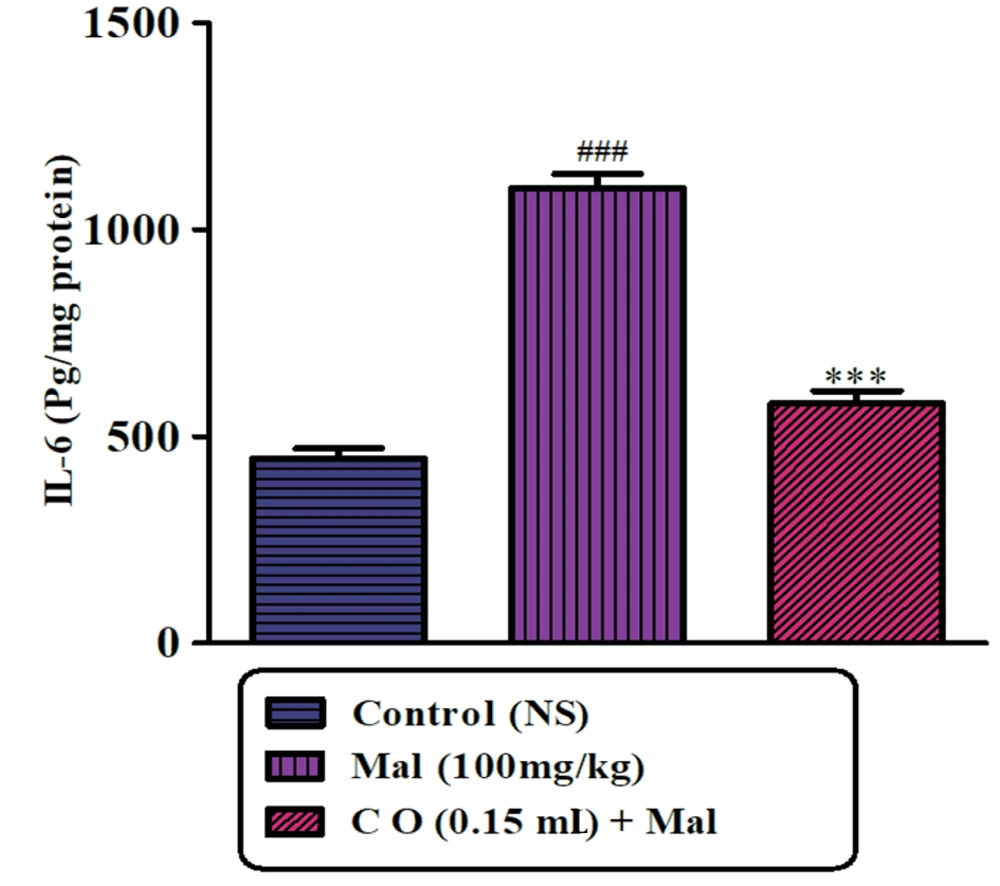
As shown in Figure 7A and B, the levels of TNFα and IL-6 in the striatum were markedly increased in rats treated with malathion compared to the control group. However, treatment with castor oil led to a significant reduction in TNFα and IL-6 levels compared to the malathion group. Administration of castor oil alone did not show any significant changes in TNFα and IL-6 levels compared to the control rats.
5. Discussion
In the present study, our objective was to evaluate the protective effect of castor oil on malathion-induced PD-like behavior in rats using behavioral tests. Our findings revealed that malathion reduced locomotor activity and time spent on the rotarod apparatus while increasing catalepsy and castor oil improved these responses. To assess motor dysfunction, we utilized a rotarod test, which is influenced by several factors such as incoordination, bradykinesia, and stiffness and has been widely used to evaluate motor skills in rodent models of PD (18, 19). The findings confirmed that malathion treatment for 28 days decreased the time spent on the rotarod in comparison to the control group. This result indicates an impaired muscular coordination caused by malathion. Besides, the castor oil and malathion-treated animals demonstrated significantly better grip strength and muscle coordination. At the end of the treatment, there was also a significant rise in postural instability and catalepsy in the group treated with malathion compared to the control group. These variations in postural instability and cataleptic behavior due to the malathion treatment were significantly reversed in the castor oil-receiving versus the malathion-receiving group. Catalepsy is a common symptom of PD and is often used as a marker to measure the extent of nigrostriatal neurodegeneration. The standard bar test technique is commonly used to induce catalepsy in animal models, and it involves placing an animal on a horizontal bar and measuring the time it takes the animal to remove its forepaws from the bar. Neurotoxins such as 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and 6-hydroxydopamine (6-OHDA) have been shown to induce catalepsy in animal models, as have organophosphorus pesticides like dichlorvos. These models are useful for studying the underlying mechanisms of PD and for evaluating potential treatments. In fact, cataleptic behavior in rats due to some neurotoxins, such as malathion in our study, is linked to dopaminergic nigrostriatal pathway degeneration. The open-field experiment showed that injecting malathion reduced total locomotion. Castor oil was a significant factor in the protection of these events (12, 20). The study's results demonstrated that malathion-induced PD-like behavior deficits in rats were ameliorated by the administration of castor oil, highlighting its potential as a neuroprotective agent for PD treatment. These results are in agreement with the ethnobotanical uses of R. communis in TPM (4). Levodopa (10 mg/kg) was also found to improve the behavioral impairments caused by malathion, consistent with previous studies indicating that levodopa treatment can reverse behavior deficits related to nigrostriatal dopamine system degeneration (21).
Acetylcholine is a crucial neurotransmitter that plays a critical role in various physiological processes, such as muscle contraction, memory, and attention. AChE is an enzyme responsible for breaking down acetylcholine, thereby regulating its concentration. Inhibiting AChE activity can cause the accumulation of acetylcholine, leading to overstimulation of cholinergic receptors and subsequent neurotoxicity. Thus, inhibiting AChE activity serves as a marker for OP neurotoxicity in acute and chronic exposure. OPs, including malathion, primarily exert their pharmacological and toxicological effects by inhibiting AChE. Studies have reported that administering subacute doses of malathion (50 and 150 mg/kg, IP) significantly reduces AChE activity in rats. In this research, we observed that plasma AChE activity significantly decreased in malathion-induced rats, which is consistent with prior studies. Interestingly, our results also indicate that castor oil could enhance AChE activity and alleviate neurobehavioral impairments caused by malathion exposure. These findings imply that cholinergic stimulation could play a role in the protective impact of castor oil against neurobehavioral toxicity induced by malathion (22, 23).
The etiopathology of PD is not fully understood. However, based on various observations in animal models of PD and human brains affected by PD, it is believed that oxidative stress in the substantia nigra (SN) plays a crucial role in the hypothesis of PD's etiopathology (24, 25). Results from this study showed that free radical generation and oxidative damage are responsible for neural abnormalities in malathion-induced PD behavior. Malathion exposure causes oxidative stress by disrupting the balance between reactive oxygen species and antioxidants, leading to an increase in MDA levels. Malathion's lipophilic nature allows it to interact with brain membranes and impair antioxidant defenses, making the CNS vulnerable to oxidative damage. Malathion has been shown to affect antioxidant enzyme activity and reduce GSH levels while increasing glutathione disulfide (GSSG) levels, resulting in a lower GSH/GSSG ratio. This reduction in GSH concentration is similar to what is observed in PD, highlighting malathion's neurotoxicity through a pro-oxidative mechanism (26, 27). The antioxidant effect of castor oil against malathion-induced oxidative stress was examined to study its anti-parkinsonian effect. Glutathione is an important antioxidant that can neutralize MDA and other reactive oxygen species. Figures 6 and 5 show that treatment with castor oil significantly reduced the malathion-induced MDA production and increased GSH concentration. These results suggest that castor oil ameliorated reduced oxidative esters and aided in maintaining redox homeostasis (Ihekuna et al., 2019; Iqbal et al., 2012). On the other hand, elevated MDA levels can serve as a marker for oxidative stress, resulting in the secretion of pro-inflammatory cytokines by macrophages. These cytokines then stimulate immune cells and vascular endothelial cells to release inflammatory cytokines, including IL-6 and TNFα (28). In this study, the levels of TNFα and IL-6 in the striatum were markedly increased in rats treated with malathion. However, treatment with castor oil led to a significant reduction in IL-6 levels. Furthermore, NFα and IL-6 are known to have various effects on the central nervous system, including the modulation of neurotransmitter systems. Several studies have demonstrated that these cytokines can influence the activity of AChE, an enzyme responsible for the breakdown of the neurotransmitter acetylcholine. In conditions associated with neuro-inflammation, such as neurodegenerative diseases or brain injury, the levels of TNFα and IL-6 can be elevated. This inflammatory environment can affect AChE activity, potentially influencing cholinergic neurotransmission and cognitive function (29-31).
Flavonoid molecules are important antioxidant components responsible for deactivating free radicals by donating hydrogen atoms to them. Lee et al.'s study showed that kaempferol-3-O-β-D-xylopyranoside, kaempferol-3-O-β-D-glucopyranoside, kaempferol-3-O-β-rutinoside, quercetin-3-O-rutinoside, quercetin-3-O-β-D-xylopyranoside, quercetin, and rutin are the major flavonoids present in the castor oil plant: Therefore, the antioxidant effect of this plant is likely due to the presence of these compounds (32, 33).
Several pro-inflammatory cytokines, including IL-1, IL-2, IL-6, and TNFα, have been found to be elevated in PD. TNFα, a central cytokine involved in inflammation, immunity, and cellular organization, plays a crucial role in the inflammatory response. Meanwhile, IL-6 is a key player in inflammation and tissue damage with diverse humoral and cellular immune responses (34, 35). The results of the study revealed that malathion treatment led to an increase in the levels of pro-inflammatory cytokines such as IL-6 and TNFα in striatal tissue, suggesting that inflammation may play a part in the pathogenesis of malathion-induced neurotoxicity. Interestingly, the addition of castor oil supplementation significantly decreased the levels of both IL-6 and TNFα. Castor oil has been extensively used in medicine and other health-related fields due to its anti-inflammatory properties. This is attributed to the presence of bioactive compounds such as polyphenols, phytosterols, and tocopherols in castor oilseeds. These compounds exhibit antioxidant properties that protect against oxidation, thereby prolonging the shelf life of the oil. Several studies have demonstrated the anti-inflammatory and anti-proliferative properties of tocopherols, which are natural phytochemicals present in castor oil. These properties make castor oil a potential therapeutic agent for various diseases that involve inflammation and cell proliferation (11).
5.1. Conclusions
In this study, we demonstrated that oral administration of castor oil could alleviate PD-like behaviors induced by malathion. This effect is attributed to the reduction of oxidative stress and pro-inflammatory cytokines. Our findings indicate that castor oil could serve as a novel and practical approach to prevent PD-like symptoms caused by exposure to pesticides. The extensive use of castor oil in both traditional and modern medicine further highlights its potential as a valuable natural remedy. However, additional research is necessary to comprehensively elucidate its mechanisms of action and therapeutic potential.
5.2. List of Abbreviations
1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP); 6-hydroxydopamine (6-OHDA); Acetylcholinesterase (AChE); Castor oil (CO); Central locomotion (CL); Glutathione (GSH); Glutathione disulfide (GSSG); Intraperitoneal injection (IP); Malathion (Mal); Malondialdehyde (MDA); Parkinson's disease (PD); Peripheral locomotion (PL); Polyethyleneglycol (PEG); Potassium chloride (KCl); Standard deviation (SD); Traditional Persian medicine (TPM); Total locomotion (TL); Tumor necrosis factor alpha (TNFα).