1. Background
Acne vulgaris, a prevalent skin disorder, is recognized as a chronic condition due to its prolonged duration and tendency to recur (1). It frequently results in scarring and hyperpigmentation following inflammation, adversely affecting the quality of life of those afflicted (1). Approximately 80% of individuals aged 11 to 30 will experience acne at some point in their lives, with about 40% of them developing scars (2). Acne scars typically arise from delayed or insufficient treatment, although they can also occur despite appropriate medical intervention. Early intervention is advocated to prevent the formation of scars, which is considered a primary objective in acne management (3). It is estimated that 80 to 90% of acne scars are attributable to a decrease in collagen (atrophic scars), whereas the remainder are associated with an increase in collagen (colloidal and hypertrophic scars) (4). Various treatments for acne scars include micro-needling, with or without platelet-rich plasma (PRP), lasers, chemical peels, and dermal fillers, with the choice of treatment being dependent on the scar type specific to each patient (5).
Curcumin, a natural compound extracted from turmeric, has been found effective in managing or treating several inflammatory conditions (6). Research supports the significant role of curcumin and its analogs as antibacterial, antiviral, antifungal, antioxidant, anti-inflammatory, and anti-tumor agents (7-9). Recent studies indicate that curcumin can diminish pro-inflammatory cytokines by interfering with various signaling pathways and transcriptional molecules (10-12). An increasing amount of evidence suggests that curcumin is beneficial in treating a range of skin conditions, including psoriasis, atopic dermatitis, wounds, skin aging, skin cancer, and infections (13). Owing to its antimicrobial and anti-inflammatory properties, curcumin has been explored as a treatment option for acne (14). Moreover, curcumin has shown potential in controlling the overgrowth of Propionibacterium acnes (P. acnes) by targeting the microbial cause of this issue and inhibiting inflammation and the subsequent formation of comedones (15).
Bromelain, a member of the protein-degrading enzyme family, is commercially sourced from the pineapple fruit or its stem (16). Laboratory and clinical studies have demonstrated bromelain's fibrinolytic, anti-edema, anti-thrombotic, and anti-inflammatory properties (16). Predominantly recognized for its anti-inflammatory effects, bromelain has also been identified as having potential anti-cancer and antimicrobial benefits (17). It exerts anti-inflammatory actions by diminishing the synthesis of prostaglandin E2 (PGE2) and COX-2 (18). Bromelain is frequently utilized as a primary remedy for various skin conditions, including burns and infections, and for skin debridement (19).
Considering the diverse range of treatments available for acne scars, the application of herbal medicines presents an opportunity to explore new, effective treatments with reduced side effects. Based on the aforementioned evidence, there exists a potential for bromelain and curcumin to effectively reduce inflammation and post-acne scarring.
2. Objectives
This study was initiated to examine the impact of this herbal combination on the inflammation and scarring associated with acne.
3. Methods
3.1. Study Design
This double-blind, randomized clinical trial was carried out at Bou Ali Sina Hospital in Tehran, Iran. Dermatologists examined patients with acne scars, and those meeting the inclusion and exclusion criteria were enrolled in the study. All participants were treated with Alpha Hydroxy Acid (AHA) exfoliator as a standard treatment for 4 weeks, in addition to being assigned to one of three treatment groups: A supplement containing bromelain and curcumin (300 mg standardized to 95% curcuminoids), micro-encapsulated curcumin (300 mg standardized to 15% curcuminoids), or a placebo.
The study's protocol received approval from the ethics committee of the Islamic Azad University, Tehran Medical Sciences (Ethic code: IR.IAU.PS.REC.1402.100), and the clinical trial was registered in the Iranian Registry of Clinical Trials (IRCT code: IRCT20150706023084N17).
3.2. Participants and Intervention
Seventy-six eligible patients who provided informed consent were randomly divided into three groups (A, B, and C). Group A was administered two capsules of Anaheal Plus supplement daily (produced by Salamat Parmoon Amin Company, Tehran, Iran, containing 150 mg of bromelain plus 300 mg of curcuminoids at 95% concentration), Group B received two capsules of placebo daily, and Group C was given two capsules of bromelain plus micro-encapsulated curcumin daily (also provided by Salamat Parmoon Amin Company, with each capsule containing 150 mg of bromelain and 300 mg of micro-encapsulated curcumin at 15% concentration, equating to 45 mg of curcuminoids). It is important to note that all groups were treated with AHA exfoliator as the standard care throughout the study.
3.3. Endpoints
To evaluate the effectiveness of the study medications compared to the placebo, Goodman and Baron's quantitative scar scale was employed, along with a visual analog scale (VAS) for the physician's assessment of scars. The differences in scoring were analyzed to determine the effect of the medications, focusing on each severity symptom.
3.4. Inclusion and Exclusion Criteria
This study enrolled individuals over 18 years old who were suffering from acne scars and had completed the consent form. Participants who met any of the following exclusion criteria were not included in the study: (1) Allergy to pineapple, celery, carrot, or fennel, (2) Pregnancy or lactation, (3) Severe renal failure (Glomerular filtration rate (GFR) < 30), (4) Severe liver failure (Child-Pugh B, C), (5) Patients with hemophilia, and (6) Patients taking two antiplatelet drugs or one anticoagulant and one antiplatelet drug.
3.5. Sample Size Estimation
Based on the characteristics of the population under study, the sample size was determined to be approximately 45 participants using Cochran's formula, which considers a precision level of 5%, a confidence level of 95%, an estimated proportion of 0.5, and a population size of 50. To minimize error, 50 patients were included in this study.
3.6. Randomization and Blinding
Patients were assigned a code using a random number generator. Patients with code 1 were allocated to group A, those with code 2 to group B, and those with code 3 to group C. Both the participants and the dermatologist assessing the treatment outcomes were blinded to group assignments.
3.7. Statistical Analysis
Data normality was assessed using the Shapiro-Wilk test. The Mann-Whitney U test and Kruskal-Wallis test were utilized to compare independent variables, while the Wilcoxon Signed-Rank test and Friedman's test were applied to compare paired data at different time points.
4. Results
4.1. Study Participants
In this study, 81 patients with acne scars were enrolled and assessed for eligibility. Of these 81 patients, 76 met the inclusion criteria and agreed to participate in the study. During the study period, 19 patients were excluded for the following reasons: (1) Allergic reactions (6 cases), (2) Gastrointestinal complications (4 cases), and (3) Personal reasons (9 cases). Ultimately, 57 patients completed the 4-week study period. The demographic characteristics of the participants are presented in Table 1.
4.2. Goodman and Barron's Scoring
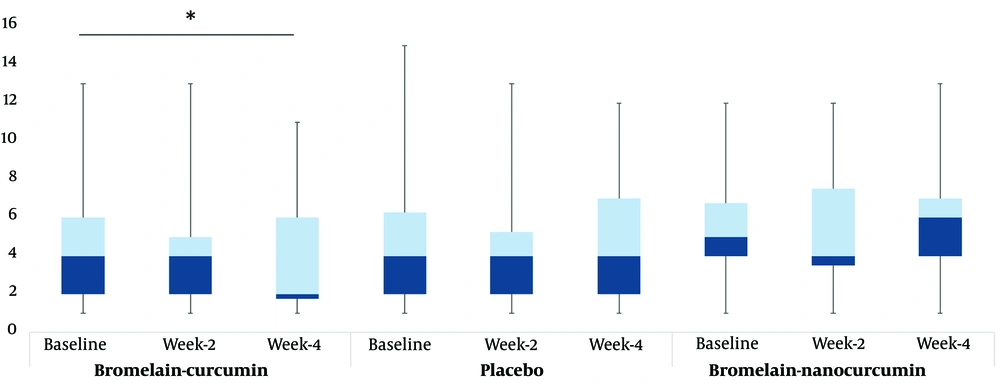
As indicated in Table 2, an examination of Goodman and Barron's qualitative criteria revealed that group A showed significant improvement in the fourth week compared to baseline (Baseline: 4.0 [2 - 6] vs. week 4: 2.0 [1.75 - 6], P < 0.05). However, groups B and C did not demonstrate significant improvement in the fourth week compared to baseline (baseline: 4 [2 - 6.25] vs. week 4: 4 [2 - 7], P > 0.05; baseline: 5 [4 - 6.75] vs. week 4: 6 [4 - 7], P > 0.05, respectively). No significant improvement was observed in any of the three groups at week 2 compared to baseline (Group A: 4 [2 - 5], P > 0.05; Group B: 4 [2 - 5.25], P > 0.05; Group C: 4 [3.5 - 7.5], P > 0.05) (Figure 1).
| Group A | Group B | Group C | P-Value | |
|---|---|---|---|---|
| Baseline | 0.335 a | |||
| Mean ± SD | 4.36 ± 3.3 | 4.6 ± 3.5 | 5.37 ± 2.8 | |
| Median (Q1 - Q3) | 4.0 [2 - 6] | 4.0 [2 - 6.25] | 5.0 [4 - 6.75] | |
| Range | 1 - 13 | 1 - 15 | 1 - 12 | |
| Week 2 | 0.414 | |||
| Mean ± SD | 4.36 ± 3.4 | 4.38 ± 3.2 | 5.46 ± 3.0 | |
| Median (Q1 - Q3) | 4.0 [2 - 5] | 4.0 [2 - 5.25] | 4.0 [3.5 - 7.5] | |
| Range | 1 - 13 | 1 - 13 | 1 - 12 | |
| Week 4 | 0.139 | |||
| Mean ± SD | 3.8 ± 3.3 | 4.7 ± 3.4 | 6.09 ± 2.9 | |
| Median (Q1 - Q3) | 2.0 [1.75 - 6] | 4.0 [2 - 7] | 6 [4 - 7] | |
| Range | 1 - 11 | 1 - 12 | 1 - 13 | |
| Improvement percentage at week 4 | 0.280 b | |||
| Worsen | 0 | 10.5 | 18.2 | |
| Minimal | 100 | 89.5 | 81.8 | |
| Moderate | 0 | 0 | 0 | |
| Good | 0 | 0 | 0 | |
| Very good | 0 | 0 | 0 | |
| Wilcoxon rank test (week 2) c | ||||
| P-value | 1.000 | 1.000 | 0.194 | |
| Wilcoxon rank test (week 4) d | ||||
| P-value | 0.014 | 1.000 | 1.000 |
a Kruskal-Wallis test.
b Chi-square test.
c Comparing week 2 to baseline.
d Comparing week 4 to baseline.
4.3. The Area of Lesions
Table 3 shows that when evaluating the area of scars after two weeks and also after four weeks, no significant changes were observed compared to baseline for any group (Group A, baseline: 3 [2 - 3] vs. week 2: 3 [2 - 3], P > 0.05, week 4: 2 [1.75 - 3], P > 0.05; Group B, baseline: 2 [1 - 3] vs. week 2: 2 [1 - 2], P > 0.05, week 4: 2 [1 - 2], P > 0.05; Group C, baseline: 2.5 [1.25 - 3] vs. week 2: 3 [1.5 - 3.5], week 4: 2 [1 - 4], P > 0.05).
a Values are reported as median [INQ1 - INQ3].
b Kruskal-Wallis test.
c Friedman's test.
4.4. Self-reported Symptoms
Additionally, the number of acne scars reported by the patients did not exhibit a significant change in any of the three study groups after two and four weeks of the study. In Group A, from baseline to week 2 and week 4, the number remained constant (baseline: 2 [1 - 2] vs. week 2: 2 [1 - 2], P > 0.05; and Week 4: 2 [1 - 2], P > 0.05). Similarly, in Group B (baseline: 2 [1 - 2] vs. Week 2: 2 [1 - 2], P > 0.05; and week 4: 2 [1 - 2], P > 0.05) and Group C (baseline: 2 [1 - 2] vs. week 2: 2 [1 - 2], P > 0.05; and week 4: 2 [1 - 2], P > 0.05), no significant changes were observed.
When evaluating the severity of erythema reported by patients, it was observed that Group A experienced a significant reduction after 4 weeks of treatment, whereas no significant changes were seen in Groups B and C (P-values: A: 0.005, B: 0.670, and C: 0.368). A similar outcome was reported in the inflammation scoring by patients (P-values: A: 0.008, B: 0.368, and C: 0.513). The distribution of patients with different scores for each symptom is presented in Table 4.
| Group A | Group B | Group C | Between Group P-Value b | |
|---|---|---|---|---|
| Number of lesions | 0.750/ 0.788/ 0.985 | |||
| 0 - 4 | 36.8/ 47.4/ 35.7 | 31.8/ 38.9/ 42.1 | 31.3/ 35.7/ 36.8 | |
| 5 - 20 | 52.6/ 42.1/ 57.1 | 50.0/ 50.0/ 36.8 | 68.8/ 50.0/ 47.7 | |
| > 20 | 10.5/ 10.5/ 7.1 | 18.2/ 11.1/ 21.1 | 0.0/ 14.3/ 13.6 | |
| Within-group P-value c | 0.368 | 0.368 | 0.368 | |
| Erythema | 0.546/ 0.939/ 0.924 | |||
| No redness | 21.1/ 15.8/ 23.1 | 27.3/ 33.3/ 21.1 | 31.3/ 38.5/ 18.2 | |
| Mild | 5.3/ 47.4/ 38.5 | 22.7/ 16.7/ 36.8 | 0.0/ 15.4/ 45.5 | |
| Moderate | 57.9/ 26.3/ 38.5 | 36.4/ 50.0/ 36.8 | 62.5/ 23.1/ 18.2 | |
| Severe | 15.8/ 10.5/ 0.0 | 13.6/ 0.0/ 5.3 | 6.3/ 23.1/ 18.2 | |
| Within-group P-value c | 0.005 | 0.670 | 0.368 | |
| Inflammation | 0.525/ 0.747/ 0.734 | |||
| No inflammation | 15.8/ 10.5/ 15.4 | 31.8/ 38.9/ 36.8 | 31.3/ 38.5/ 18.2 | |
| Mild | 15.8/ 52.6/ 53.8 | 22.7/ 11.1/ 26.3 | 0.0/ 7.7/ 45.5 | |
| Moderate | 57.9/ 26.3/ 30.8 | 31.8/ 50.0/ 31.6 | 62.5/ 30.8/ 18.2 | |
| Severe | 10.5/ 10.5/ 0.0 | 13.6/ 0.0/ 5.3 | 6.3/ 23.1/ 18.2 | |
| Within-group P-value c | 0.008 | 0.368 | 0.513 |
a Data are reported as a percentage of patients in each group at different time points (% in baseline/% in week 2/% in week 4).
b Kruskal-Wallis test.
c Friedman's test.
5. Discussion
In this study, the efficacy of a combination of two herbal medicines, bromelain, and curcumin, for the treatment of post-acne scarring was investigated. The findings showed that patients receiving the bromelain-curcumin combination experienced significantly lower levels of inflammation and erythema compared to their baseline levels; however, these reductions were not observed in the control group.
In a recent animal study, it was observed that bromelain reduces oxidative and inflammatory parameters while simultaneously increasing antioxidant levels in NaOH-induced corrosive burns (20). The anti-inflammatory and antioxidant properties of bromelain were found to protect the tissues of the esophagus and tongue in corrosive burns. Similarly, a study by Fei et al. in 2023 demonstrated that curcumin reduces scar elevation and collagen deposition in a rabbit ear scar model (21). Gross observation revealed that curcumin accelerated the healing of residual wounds and significantly reduced the width and thickness of the final scars. In line with the current study, the group receiving the bromelain-curcumin combination showed a significant reduction in Goodman-Baron scoring at week 4 of treatment, whereas no significant differences were observed between groups. The group treated with bromelain and microencapsulated curcumin did not show significant improvement over the duration of the study, which could be due to patient variability or a less effective amount of curcumin in the formulation compared to the bromelain-curcumin (300 mg standardized to 95% curcuminoids) group. A larger sample size is necessary to minimize errors related to group variability.
A study conducted in 2009 observed that high doses of curcumin could modulate scar formation due to its effects on fibroblast apoptosis (22). Similarly, another study demonstrated that curcumin exhibits activity against inflammation induced by Cutibacterium acnes (23). Consistent with the current study, patients receiving the bromelain-curcumin supplement had significantly lower inflammation and erythema scores compared to baseline, while no significant differences were observed in the other groups. This result indicates the superiority of the bromelain-curcumin combination over placebo in alleviating symptoms induced by post-acne scarring. However, the lack of significant improvement in the bromelain-microencapsulated curcumin group could be attributed to an insufficient equivalent dose of curcumin compared to the bromelain-curcumin combination alone.
Brochard et al., in 2021, studied the effects of combining curcumin with bromelain and harpagophytum on reducing inflammation in osteoarthritic synovial cells. Their research suggested that the combination might reduce cartilage degradation during the osteoarthritis process. They observed that this combination significantly reduced the gene expression of IL-6 and nerve growth factor (NGF) mRNA expression. It also decreased IL-6 release and the production of PGE2. This indicates that the combination of these three compounds may reduce inflammation and pain (24).
Similarly, the current study investigated the combination of bromelain and curcumin due to the beneficial effects of each component in reducing inflammation, as well as their combined effect in the treatment of acne-induced scarring. The current study did not evaluate curcumin or bromelain alone in order to assess the synergistic effect of their combination.
5.1. Conclusions
Overall, the combination of bromelain with curcumin has shown beneficial effects in the treatment of post-acne scars over a 4-week treatment period. Larger studies with a greater number of patients are needed to further elucidate the beneficial effects of this combination in treating patients with post-acne scars.